| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Original Article
Volume 11, Number 4, April 2019, pages 267-274
Renoprotective Effects of Additional SGLT2 inhibitor Therapy in Patients With Type 2 Diabetes Mellitus and Chronic Kidney Disease Stages 3b-4: A Real World Report From A Japanese Specialized Diabetes Care Center
Seigo Sugiyamaa, b, k, l, Hideaki Jinnouchia, b, c, k, Akira Yoshidaa, d, Kunio Hieshimaa, e, Noboru Kurinamia, f, Katsunori Jinnouchia, g, h, Motoko Tanakai, Tomoko Suzukia, b, Fumio Miyamotoa, j, Keizo Kajiwaraa, b, f, Tomio Jinnouchia, b, f
aDiabetes Care Center, Jinnouchi Hospital, Kumamoto, Japan
bCardiovascular Division, Diabetes Care Center, Jinnouchi Hospital, Kumamoto, Japan
cDivision of Preventive Cardiology, Department of Cardiovascular Medicine, Kumamoto University Hospital, Kumamoto, Japan
dPharmacology Division, Diabetes Care Center, Jinnouchi Hospital, Kumamoto, Japan
eInfectious Disease Division, Diabetes Care Center, Jinnouchi Hospital, Kumamoto, Japan
fObesity Treatment Division, Diabetes Care Center, Jinnouchi Hospital, Kumamoto, Japan
gGastroenterology Division, Diabetes Care Center, Jinnouchi Hospital, Kumamoto, Japan
hHemodialysis Division, Diabetes Care Center, Jinnouchi Hospital, Kumamoto, Japan
iDepartment of Nephrology, Akebono Clinic, Kumamoto, Japan
jOphthalmology Division, Diabetes Care Center, Jinnouchi Hospital, Kumamoto, Japan
kThey contributed equally to this study.
lCorresponding Author: Seigo Sugiyama, Cardiovascular Division, Diabetes Care Center, Jinnouchi Hospital, 6-2-3 Kuhonji, Chuo-ku, Kumamoto City 862-0976, Japan
Manuscript submitted January 21, 2019, accepted February 22, 2019
Short title: Renal Effects of SGLT2-Inhibitors in DM and CKD
doi: https://doi.org/10.14740/jocmr3761
| Abstract | ▴Top |
Background: Large randomized clinical trials of patients with type 2 diabetes mellitus (T2DM) and at high risk for cardiovascular disease revealed that sodium-glucose cotransporter 2 (SGLT2) inhibitors significantly reduced renal events. However, the trials included small numbers of patients with moderate-to-severe chronic kidney disease (CKD). Therefore, the renoprotective effects of SGLT2 inhibitors remain unknown in T2DM patients complicated with impaired renal function. We examined if SGLT2 inhibitors conferred beneficial effects on kidney function in T2DM patients with CKD.
Methods: We retrospectively recruited T2DM patients who were newly treated with add-on of SGLT2 inhibitors and suffered from moderate-to-severe renal impairment with CKD stages 3b-4 (15 < estimated glomerular filtration rate (eGFR) < 45 mL/min/1.73 m2), at initiation of SGLT2 inhibitor therapy. We analyzed T2DM patients with moderate-to-severe renal impairment who continued to use SGLT2 inhibitors for at least 1 year. We investigated the effects of SGLT2 inhibitor therapy on 1-year changes in eGFR and urinary protein excretion before and after the treatment.
Results: We analyzed 42 T2DM patients with median eGFR of 40.4 mL/min/1.73 m2. One-year SGLT2 inhibitor therapy lowered median hemoglobin A1c (HbA1c) levels from 7.6% to 7.5% (not significant). Body weight and blood pressure were significantly decreased, and hemoglobin was significantly increased. The median value of eGFR after 1 year of SGLT2 inhibitor therapy was 41.0 mL/min/1.73 m2, with no significant difference compared with baseline. The annual decline in eGFR improved significantly after SGLT2 inhibitor therapy (eGFR: (median), pre: -3.8, vs. post: 0.1 mL/min/1.73 m2 per year, P < 0.01). We also found a significant decrease in urinary protein excretion after SGLT2 inhibitor therapy (urinary protein-to-creatinine ratio: (median), pre: 0.36, vs. post: 0.23 g/g creatinine, n = 35, P < 0.01).
Conclusions: This study revealed the promising observations that add-on treatment with SGLT2 inhibitors exerted significant renoprotective effects, culminating in improvements in annual decline in eGFR and urinary protein excretion in T2DM patients with CKD stages 3b-4, but did not significantly reduce HbA1c. Further prospective clinical trials are warranted to fully elucidate the effects of SGLT2 inhibitors on glycemic control and renal function in T2DM patients with moderate-to-severe renal impairment.
Keywords: Sodium-glucose co-transporter 2 inhibitors; Estimated glomerular filtration rate; Kidney; Urine protein-to-creatinine ratio; Type 2 diabetes mellitus; Renoprotection; Annual eGFR decline; Rapid eGFR decliner
| Introduction | ▴Top |
The incidence of type 2 diabetes mellitus (T2DM) is increasing worldwide [1]. Diabetic nephropathy/diabetic kidney disease is considered a serious complication of T2DM that remains unsolved. It is the leading cause of end stage renal disease (ESRD) and requires treatment with hemodialysis. It therefore represents an important issue in clinical practice and public health [1, 2]. Regarding practical approaches to treating diabetic nephropathy/diabetic kidney disease, clinical assessment of renal damage through measurement of the urine protein-to-creatinine ratio (UPCR) and estimated glomerular filtration rate (eGFR) is recommended to promote active interventions with renoprotective effects [3]. The annual decline in eGFR is a clinically useful biomarker that reflects dynamic and time-dependent changes in renal function [4]. Patients with higher 1-year rates of decline in eGFR (over 7.5%) are considered “rapid eGFR decliners” and are associated with poor renal prognosis in patients with T2DM [5]. Increased attention has been paid to investigating and developing practical strategies for the clinical preservation and improvement of renal function [6, 7]. Patients with chronic kidney disease (CKD) stages 3b-4 are at high risk of developing ESRD, although they are asymptomatic to worsening renal function and progressive kidney disease [7]. In patients with severely impaired kidney function, treatment options for hyperglycemia are limited because of drug metabolic pathways, side effects, and the risk of hypoglycemia [8, 9]. Glucose-lowering therapies that confer renoprotective effects are considered to have additional clinically important effects and advantages for the comprehensive management of T2DM [6, 10, 11].
Recently, we reported that 6-month treatment with dapagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor used globally, provided protective effects from glomerular damage and tubulointerstitial injury in patients with insufficiently controlled T2DM [12]. Large randomized clinical trials conducted in patients with high risk of atherosclerotic cardiovascular disease (ASCVD) revealed that SGLT2 inhibition had protective effects on renal events [13-15]. However, the trials included small numbers of patients with moderate-to-severe CKD [16]. Compared with patients with normal kidney function, the glucose-lowering effect of SGLT2 inhibitors in advanced CKD patients was diminished [17]. Based on this lack of efficacy, SGLT2 inhibitors are not clinically recommended for the treatment of T2DM patients with impaired renal function (eGFR < 45 mL/min/1.73 m2) [16, 18]. Therefore, the clinical and renoprotective effects of SGLT2 inhibitors have not yet been fully elucidated in T2DM patients with severely impaired renal function.
In the present study, we retrospectively examined whether SGLT2 inhibitor therapy could exert beneficial effects on renal function in T2DM patients with CKD stages 3b-4.
| Materials and Methods | ▴Top |
Study population and study protocol
Based on clinical information from hospital medical records, we retrospectively identified Japanese outpatients with T2DM who were newly treated with SGLT2 inhibitors between April 2014 and November 2018 in the Diabetes Care Center at Jinnouchi Hospital, one of the largest and most comprehensive private diabetes care centers in Kumamoto, Japan. Among them, we selected patients who also suffered from moderate-to-severe renal impairment defined as CKD stages 3b-4 (15 < eGFR < 45 mL/min/1.73 m2) at the initiation of SGLT2 inhibitor therapy. Finally, we selected patients with T2DM and moderate-to-severe renal impairment who continued to use SGLT2 inhibitors for at least 1 year. The exclusion criteria were as follows: type 1 DM, unstable cardiovascular diseases, active inflammation, autoimmune diseases, infectious diseases, severe liver disease, dementia and cancer. Patients with newly diagnosed DM who were not undergoing any form of treatment and those with ketosis were also excluded.
This cohort consisted of patient data obtained from a database at Jinnouchi Hospital. We investigated annual changes in eGFR before and after SGLT2 inhibitor therapy. The primary outcome was a quantitative assessment of annual changes in eGFR (mL/min/1.73 m2 per year). We analyzed eGFR at 1 year before and 1 year after SGLT2 inhibitor therapy. This was a retrospective, observational, single-arm, and single-center study. The study was conducted in accordance with the Declaration of Helsinki and was approved by the Human Ethics Review Committee of Jinnouchi Hospital (2018-4-①). Signed informed consent was obtained from each patient. The study was registered under the UMIN protocol registration system (ID: UMIN000035278).
Assessments and measurement of clinical parameters
We collected clinical information pertaining to medical treatments, complications, and past history. We also collected data on body weight, height, blood pressure, and pulse rate. Analyses of blood and urine were conducted in the hospital laboratory to measure hemoglobin, hematocrit, blood glucose, hemoglobin A1c (HbA1c), creatinine, blood urea nitrogen (BUN), uric acid (UA), urinary protein, and urinary creatinine. For the semiquantitative assessments of urinary protein excretion (negative, borderline, mild, moderate, and severe), we also examined the results of dipstick urine test. The eGFR (mL/min/1.73 m2) was calculated using the formula from the Japanese Society of Nephrology [19] before SGLT2 inhibitor therapy (1 year and 6 months before), at the initiation of SGLT2 inhibitor therapy, and after therapy (6 months and 1 year after). We calculated the annual changes in eGFR by the following formula:
Pre-treatment annual eGFR change = (eGFR at starting SGLT2-inhibitor therapy) - (eGFR at 1-year ago from starting SGLT2-inhibitor therapy)
Post-treatment annual eGFR change = (eGFR after 1-year SGLT2-inhibitor therapy) - (eGFR at starting SGLT2-inhibitor therapy)
Statistical analyses
The primary endpoint of the present study was treatment-induced annual changes in eGFR. We sought to analyze significant improvements in annual changes in eGFR before and after SGLT2 inhibitor therapy. Based on a preliminary examination for our hospital, a power analysis indicated that enrollment of over 22 patients was required to detect a mean difference in annual changes in eGFR of -3.0 mL/min/1.73 m2 per year before SGLT2 inhibitor therapy and -1.0 mL/min/1.73 m2 per year after SGLT2 inhibitor therapy, with a power of 80%, and a two-sided alpha value of 0.05. Data pertaining to normally distributed continuous variables (determined by the Shapiro-Wilk test) are presented as the mean (standard deviation), while those of continuous variables with a skewed distribution are presented as median values (interquartile range). To investigate the effects of SGLT2 inhibitor therapy on body weight, body mass index (BMI), blood pressure, and pulse rate, we compared these indicators at the initiation of SGLT2 inhibitor therapy and at 1 year after therapy. Either a paired Student’s t-test or Wilcoxon’s test was used to analyze the effects of SGLT2 inhibitor therapy. To determine the relationships between changes in annual eGFR decline and changes in HbA1c (an indicator of blood glucose control), correlations between variables of interest were analyzed using Spearman’s rank correlation coefficient. A P-value < 0.05 was considered statistically significant. Statistical analyses were performed using the Statistical Package for Social Sciences software program, version 23 (SPSS Inc., IBM, Tokyo, Japan).
| Results | ▴Top |
Baseline clinical parameters of patients
A total of 1,187 Japanese stable T2DM patients who were newly treated with SGLT2 inhibitors were initially identified, 46 (3.9%) of whom suffered from moderate-to-severe CKD (stages 3b-4) at the initiation of SGLT2 inhibitor therapy. Four patients were excluded because they were not treated with SGLT2 inhibitors for over 1 year at November 2018. We analyzed a final cohort of 42 patients, whose baseline clinical parameters are shown in Table 1. Mean age was 64.8 years, 71.4% were male, mean duration of T2DM was 18 years, and mean BMI was 28.2 kg/m2. In total, 95.2% of patients were complicated with hypertension and dyslipidemia, 23.8% of whom had a past history of cerebrovascular/cardiovascular diseases. The median HbA1c value was 7.6%, and the patients were treated with an average of three antidiabetic drugs including insulin. Overall, 50.0% of patients were treated with metformin and 71.4% were treated with insulin. The most frequently used SGLT2 inhibitor was dapagliflozin (33.0%). The attending physicians at Jinnouchi Hospital started SGLT2-inhibitors by own discretion to improve glycemic control; and the starting treatment doses of SGLT2-inhibitor were regularly established in Japanese clinical practice (dapagliflozin: 5 mg/day; empagliflozin: 10 mg/day; canagliflozin: 100 mg/day; Luseogliflozin: 2.5 mg/day and tofogliflozin: 20 mg/day). Median eGFR was 40.4 mL/min/1.73 m2 at the initiation of SGLT2 inhibitor therapy. In total, 9.5% of patients had CKD stage 4, while 35.7% of patients had diabetic kidney disease without proteinuria. Data pertaining to quantitative urinary protein excretion were available in 35 patients. Urinary albumin was not measured in the present study. No patient was treated by erythropoietin in the present study.
 Click to view | Table 1. Baseline Clinical Parameters |
Changes in clinical and laboratory parameters
The effects of 1-year treatment with SGLT2 inhibitors compared with baseline values are shown in Table 2. Regarding glycemic control, the levels of HbA1c were improved in 50% of patients and decreased from 7.6% to 7.5% in all subjects, although the decline was not statistically significant (P = 0.17). The patients demonstrated a significant decrease in body weight, BMI, systolic blood pressure, and diastolic blood pressure. As shown in Table 2, hematocrit and hemoglobin levels were significantly increased. SGLT2 inhibitor therapy decreased serum creatinine levels from 1.38 mg/dL to 1.35 mg/dL, although the change was not statistically significant. No patient developed ESRD (eGFR less than 15 mL/min/1.73 m2) after SGLT2-inhibitor therapy in the present study. We did not find any marked changes in BUN or UA. SGLT2 inhibitor therapy significantly decreased urinary protein excretion assessed by UPCR as shown in Figure 1 (n = 35).
 Click to view | Table 2. Changes in Glucose Metabolic Parameters and Clinical Variables Before and After SGLT2 Inhibitor Therapy |
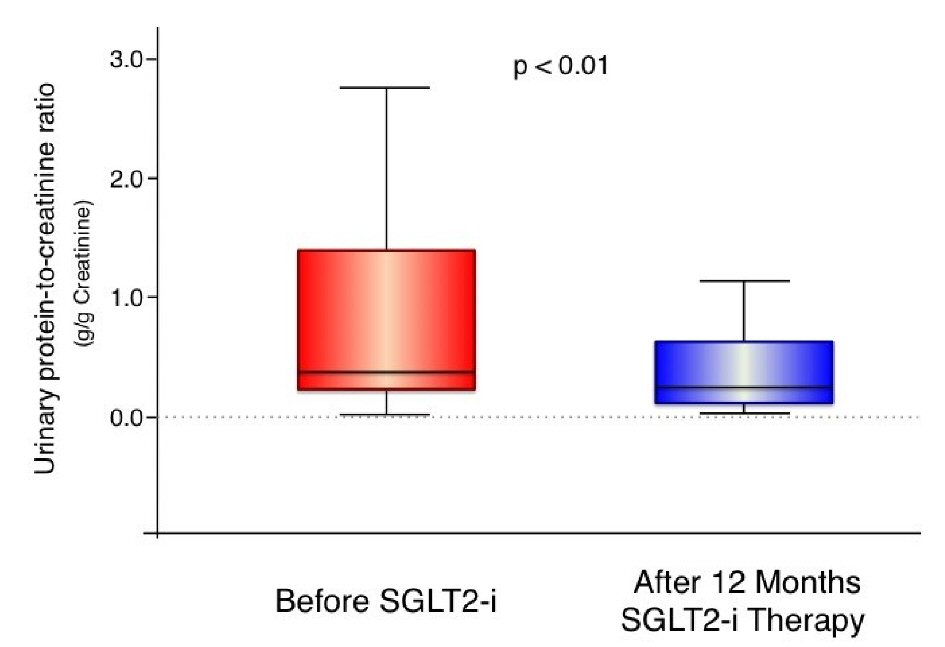 Click for large image | Figure 1. Urinary protein excretion (urinary protein-to-creatinine ratio) before and after 1-year treatment with sodium glucose cotransporter 2 inhibitors. In the box-and-whisker plots, lines within boxes represent median values; the top and bottom lines of the boxes represent the 25th and 75th percentiles, respectively; the top and bottom bars outside the boxes represent the 90th and 10th percentiles, respectively. N = 35, SGLT2i: sodium glucose cotransporter 2 inhibitor. |
Annual changes in eGFR before and after SGLT2 inhibitor therapy
Figure 2 shows the serial values of eGFR before and after SGLT2 inhibitor therapy. We found a significant decrease in 1-year eGFR in the pretreatment period of SGLT2 inhibitor therapy (P < 0.01), but not in the post-treatment period (P = 0.18). At 6 months and 1 year after SGLT2 inhibitor therapy, the levels of eGFR were not significantly different compared with baseline values. Figure 3 and Table 3 show the annual changes in eGFR before and after SGLT2 inhibitor therapy. The slope of eGFR decline was significantly attenuated with the addition of SGLT2 inhibitor therapy (Fig. 2). We found a significant improvement in the annual decline in eGFR after SGLT2 inhibitor therapy compared with pretreatment values.
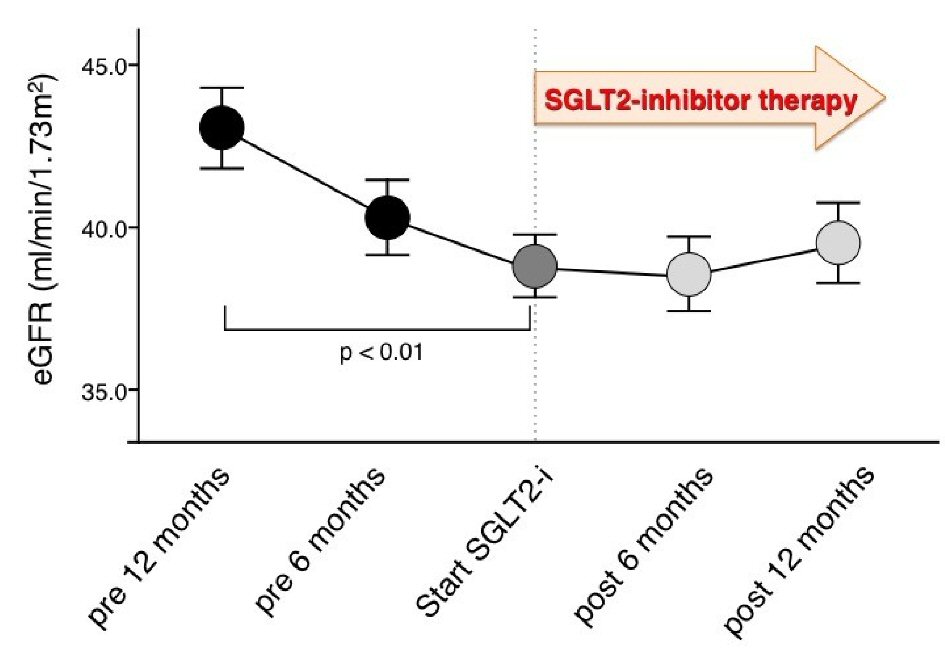 Click for large image | Figure 2. Serial changes in estimated glomerular filtration rate before and after treatment with sodium glucose cotransporter 2 inhibitors. SGLT2i: sodium glucose cotransporter 2 inhibitor; eGFR: estimated glomerular filtration rate; mean ± standard error. |
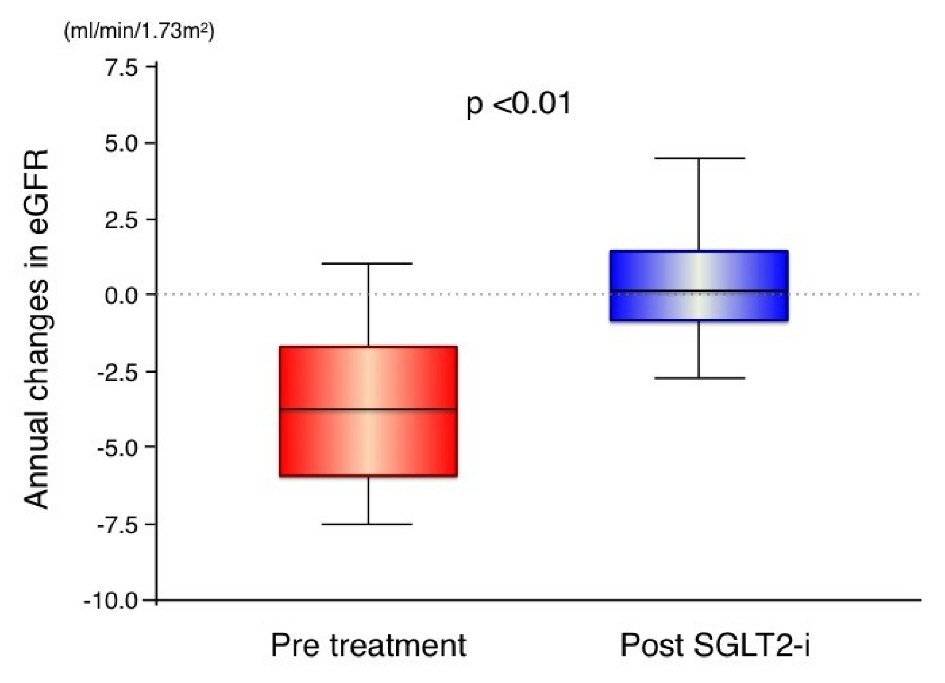 Click for large image | Figure 3. Annual changes in estimated glomerular filtration rate before and after treatment with sodium glucose cotransporter 2 inhibitors. In the box-and-whisker plots, lines within boxes represent median values; the top and bottom lines of the boxes represent the 25th and 75th percentiles, respectively; the top and bottom bars outside the boxes represent the 90th and 10th percentiles, respectively. SGLT2i: sodium glucose cotransporter 2 inhibitor; eGFR: estimated glomerular filtration rate. |
 Click to view | Table 3. Annual Changes in Estimated Glomerular Filtration Rate Before and After Sodium Glucose Cotransporter 2 Inhibitor Therapy |
To determine the possible association between the changes in renal function and glycemic control during the treatment period, we performed Spearman’s rank correlation coefficient analysis between the changes in annual eGFR decline and the changes in HbA1c before and after SGLT2 inhibitor therapy. The changes in annual decline in eGFR were not significantly correlated with the changes in HbA1c (Spearman’s ρ = 0.063, P = 0.69).
Subgroup analyses of annual changes in eGFR before and after SGLT2 inhibitor therapy
In patients without baseline proteinuria who were clinically recognized as having diabetic kidney disease but not typical diabetic nephropathy (n = 15) [11], we found that SGLT2 inhibitor therapy successfully resulted in significant improvement of the annual decline in eGFR (pre: -3.6 (-6.2 - -2.1), vs. post: 0.3 (-0.6 - 3.1) mL/min/1.73 m2 per year, P < 0.01).
This study included four patients with severely impaired CKD (stage 4) (15 < eGFR < 30 mL/min/1.73 m2). In these patients, baseline eGFR values were 23.7 ± 5.7 mL/min/1.73 m2. Annual changes in eGFR before SGLT2 inhibitor therapy were -3.8 ± 3.2 mL/min/1.73 m2 per year and those after therapy were -0.7 ± 2.3 mL/min/1.73 m2 per year. The effects of SGLT2 inhibitor therapy on annual changes in eGFR were consistent with the effects of SGLT2 inhibitors in the entire study population, but the difference was not statistically significant (P = 0.13).
| Discussion | ▴Top |
The present study demonstrated that add-on treatment with SGLT2 inhibitors for over 1 year resulted in clinically relevant improvements in the annual decline in eGFR and in urinary protein excretion associated with non-significant decreases in HbA1c in T2DM patients with moderate-to-severe CKD. These findings are promising, and demonstrate the clinical usefulness of SGLT2 inhibitor therapy for renal protection, which may be advantageous in treating T2DM patients complicated with advanced CKD to prevent the progression to ESRD.
The incidence of T2DM is increasing globally [1]. Diabetic nephropathy/diabetic kidney disease is considered an unsolved complication of T2DM [2]. Therefore, increased attention has been paid to investigating and developing practical strategies for preventing and improving renal function [3, 6, 7]. We reported that dapagliflozin had protective effects from glomerular damage and tubulointerstitial injury in patients with T2DM [12]. Recently, large randomized clinical trials conducted in patients at high risk for ASCVD revealed that SGLT2 inhibition reduced renal events [13-15]. However, the trials included small numbers of patients with moderate-to-severe CKD [16]. Therefore, the renoprotective effects of SGLT2 inhibitors are not completely understood in T2DM patients with severely impaired renal function. Herein, we demonstrated that the add-on of SGLT2 inhibitor therapy resulted in clinically significant renoprotective effects such as improvements in the annual decline in eGFR, and in urinary protein excretion, in T2DM patients with concomitant CKD stages 3b-4.
Patients with CKD stages 3b-4 are at high risk of progressing to ESRD, but are asymptomatic to the worsening of renal function [7]. It is difficult for both patients and physicians to recognize the true severity of ongoing kidney dysfunction. In patients with severely impaired kidney function, treatment options for hyperglycemia are limited because of drug metabolic pathways, side effects, and the risk of hypoglycemia [8, 9]. Glucose-lowering therapies that have renoprotective effects are considered to have important additional clinical effects and advantages regarding the comprehensive management of T2DM [6, 10, 11]. A recent randomized placebo-controlled trial showed that the risk of acute kidney injury does not increase during SGLT2 inhibition [15], suggesting a good safety profile for kidney function with this therapy. The annual decline in eGFR is a clinically useful biomarker that reflects dynamic and time-dependent changes in renal function [4]. Compared with the general population, T2DM patients have been shown to present with increased rates of annual decline in eGFR [4]. Additionally, patients with higher 1-year rates of decline in eGFR (over 7.5%) are considered “rapid eGFR decliners”, and are associated with poor renal prognosis in patients with T2DM [5]. In the present study, patients with CKD stages 3b-4 had elevated rates of annual decline in eGFR (-3.8 mL/min/1.73 m2 per year), consistent with the “rapid eGFR decliners” during the pretreatment period of SGLT2 inhibitor therapy. Our results showed that the add-on of SGLT2 inhibitor therapy significantly flattened the slope of decline in eGFR, suggesting it has potential renoprotective effects even in patients complicated with severely impaired renal function. Because SGLT2 inhibitor therapy held the eGFR almost constant for 1 year, the time period before renal replacement therapy can be extended. When advanced renal dysfunction such as in CKD stages 3b-4 is initially detected clinically, we believe it may not be too late to intervene in the preservation and improvement of kidney function with the addition of SGLT2 inhibitors. Within the next decade, it may be possible to preserve eGFR levels following the initiation of SGLT2 inhibitor therapy, potentially avoiding or delaying the progression to ESRD and the need for hemodialysis.
It is well established that higher amounts of urinary protein excretion reflecting glomerular injury have been associated with the rapid development of progressive kidney dysfunction in patients with CKD [7, 20]. The present results demonstrated that SGLT2 inhibitor therapy significantly decreased urinary protein excretion in patients with T2DM and CKD stages 3b-4, demonstrating the ability of SGLT2 inhibitors to improve pre-existing glomerular damage. Recent studies suggested potential mechanisms for the renoprotective effects of SGLT2 inhibitors such as through improvement of podocyte damage [21] and mitochondrial function [22], and reduction of oxidative stress [23] and inflammation [24] in the kidney. Dapagliflozin was also shown to ameliorate microvascular endothelial dysfunction [25]. Furthermore, it is widely believed that SGLT2 inhibitors may be beneficial for renal protection in both patients with T2DM and non-DM-CKD, as demonstrated from the ongoing renal outcomes study (NCT03036150; DAPA-CKD). Ideally, future studies will reveal new applications and clinical effects of SGLT2 inhibitors on renal function and kidney diseases.
Among the patients with T2DM in the present study, there were 15 patients with CKD stages 3b-4 and no proteinuria (35.7%). These patients were recognized as having diabetic kidney disease with atypical features, who were in the process of developing classical diabetic kidney dysfunction (diabetic nephropathy) associated with increased albuminuria and proteinuria [11, 26]. Interestingly, according to the subgroup analysis, we found that SGLT2 inhibitor therapy resulted in a significant improvement in the annual decline in eGFR in the patients without proteinuria. This suggests SGLT2 inhibitors exert protective effects on glomerular function while preserving eGFR through mechanisms independent of decreasing urinary protein excretion.
Compared with patients with normal kidney function, the glucose-lowering effect of SGLT2 inhibitors in advanced CKD patients was decreased [17]. Based on this lower efficacy, SGLT2 inhibitors are not clinically recommended for the treatment of T2DM patients with severely impaired renal function (eGFR < 45 mL/min/1.73 m2) [16, 18]. Therefore, the clinical effects of these inhibitors, including their ability to preserve renal function, remain incompletely understood in T2DM patients with advanced CKD. In the present study, we found that the glucose-lowering effects of SGLT2 inhibitors as assessed by changes in HbA1c levels were attenuated. Moreover, the effects of SGLT2 inhibitor therapy on renal function, body weight, hemoglobin, hematocrit, and blood pressure persisted in T2DM patients with severe CKD, suggesting the presence of additional renoprotective mechanisms independent from glycemic control that are likely to be involved in SGLT2 inhibitor therapy in T2DM patients with severely impaired renal function. We propose that SGLT2 inhibitors should be considered and used primarily for their renoprotective effects, rather than for lowering blood glucose levels in T2DM patients with CKD.
This study had several limitations including the small sample-size and relatively short study period in the single center. There is selection bias in this study because the proportion of patients with SGLT2-inhibitor relative to all diabetic patients was extremely low and patients undergoing proteinuria measurement at the follow-up further decreased. The present study was retrospectively designed and the study protocol was not prospectively controlled. Thus, the evidence level of this study is not enough. Further prospective, detailed, large, and longer studies focusing primarily on patients with advanced CKD (stage 4) with a multicenter design are required to validate the effects of SGLT2 inhibitor therapy on the improvement of renal function as shown herein. The detailed molecular mechanisms underlying SGLT2 inhibitor-induced improvements in renal function could not be determined in the present clinical study and should be investigated in future studies.
Conclusions
This study provided promising results for the use of SGLT2 inhibitors as add-on treatment in patients with T2DM with renal function impairment. The treatment had significant renoprotective effects, notably improvements in annual decline in eGFR and urinary protein excretion. However, the therapy did not sufficiently reduce HbA1c levels in T2DM patients complicated with moderate-to-severe CKD. Further prospective clinical trials are warranted to fully elucidate the clinical effects of SGLT2 inhibitors on glycemic control and renal function in T2DM patients with renal impairment.
Acknowledgments
We thank Richard Robins, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Grant Support
None.
Conflict of Interest
Dr. Seigo Sugiyama is on the Speaker’s Bureau of MSD, Inc., and AstraZeneca Pharmaceuticals LP, Ono Pharmaceutical CO., LTD, and Bayer Yakuhin Ltd. Dr. Hideaki Jinnouchi has received consultant fees from Sanofi U.S., Novo Nordisk, Inc., and Eli Lilly Japan K.K. Dr. Hideaki Jinnouchi is also on the Speaker’s Bureau of MSD, Inc., Astellas Pharma US, Inc., Sanofi U.S., Novo Nordisk Pharma, Ltd., Taisho Toyama Pharmaceutical, Co., Ltd. Daiichi-Sankyo Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Eli Lilly Japan K.K., Boehringer Ingelheim Pharmaceuticals, Inc., Takeda Pharmaceutical Company Limited, and AstraZeneca Pharmaceuticals LP. All other authors declare that they have no conflicts of interest.
| References | ▴Top |
- Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, Malanda B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271-281.
doi pubmed - Duru OK, Middleton T, Tewari MK, Norris K. The landscape of diabetic kidney disease in the United States. Curr Diab Rep. 2018;18(3):14.
doi pubmed - Doshi SM, Friedman AN. Diagnosis and Management of Type 2 Diabetic Kidney Disease. Clin J Am Soc Nephrol. 2017;12(8):1366-1373.
doi pubmed - Levey AS, Inker LA, Matsushita K, Greene T, Willis K, Lewis E, de Zeeuw D, et al. GFR decline as an end point for clinical trials in CKD: a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis. 2014;64(6):821-835.
doi pubmed - Nojima J, Meguro S, Ohkawa N, Furukoshi M, Kawai T, Itoh H. One-year eGFR decline rate is a good predictor of prognosis of renal failure in patients with type 2 diabetes. Proc Jpn Acad Ser B Phys Biol Sci. 2017;93(9):746-754.
doi pubmed - Tsimihodimos V, Filippatos TD, Filippas-Ntekouan S, Elisaf M. Renoprotective effects of SGLT2 inhibitors: beyond glucose reabsorption inhibition. Curr Vasc Pharmacol. 2017;15(2):96-102.
doi pubmed - Webster AC, Nagler EV, Morton RL, Masson P. Chronic kidney disease. Lancet. 2017;389(10075):1238-1252.
doi - Moen MF, Zhan M, Hsu VD, Walker LD, Einhorn LM, Seliger SL, Fink JC. Frequency of hypoglycemia and its significance in chronic kidney disease. Clin J Am Soc Nephrol. 2009;4(6):1121-1127.
doi pubmed - Davies M, Chatterjee S, Khunti K. The treatment of type 2 diabetes in the presence of renal impairment: what we should know about newer therapies. Clin Pharmacol. 2016;8:61-81.
doi - Neumiller JJ, Alicic RZ, Tuttle KR. Therapeutic considerations for antihyperglycemic agents in diabetic kidney disease. J Am Soc Nephrol. 2017;28(8):2263-2274.
doi pubmed - Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017;12(12):2032-2045.
doi pubmed - Sugiyama S, Jinnouchi H, Kurinami N, Hieshima K, Yoshida A, Jinnouchi K, Tanaka M, et al. Impact of dapagliflozin therapy on renal protection and kidney morphology in patients with uncontrolled type 2 diabetes mellitus. J Clin Med Res. 2018;10(6):466-477.
doi pubmed - Cherney DZI, Zinman B, Inzucchi SE, Koitka-Weber A, Mattheus M, von Eynatten M, Wanner C. Effects of empagliflozin on the urinary albumin-to-creatinine ratio in patients with type 2 diabetes and established cardiovascular disease: an exploratory analysis from the EMPA-REG OUTCOME randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017;5(8):610-621.
doi - Perkovic V, de Zeeuw D, Mahaffey KW, Fulcher G, Erondu N, Shaw W, Barrett TD, et al. Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS Program randomised clinical trials. Lancet Diabetes Endocrinol. 2018;6(9):691-704.
doi - Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347-357.
doi pubmed - Kelly MS, Lewis J, Huntsberry AM, Dea L, Portillo I. Efficacy and renal outcomes of SGLT2 inhibitors in patients with type 2 diabetes and chronic kidney disease. Postgrad Med. 2019;131(1):31-42.
doi pubmed - Dekkers CCJ, Wheeler DC, Sjostrom CD, Stefansson BV, Cain V, Heerspink HJL. Effects of the sodium-glucose co-transporter 2 inhibitor dapagliflozin in patients with type 2 diabetes and Stages 3b-4 chronic kidney disease. Nephrol Dial Transplant. 2018;33(11):2005-2011.
doi pubmed - Davies MJ, D'Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, Rossing P, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41(12):2669-2701.
doi pubmed - Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53(6):982-992.
doi pubmed - Heerspink HJ, Kropelin TF, Hoekman J, de Zeeuw D, Reducing Albuminuria as Surrogate Endpoint C. Drug-induced reduction in albuminuria is associated with subsequent renoprotection: a meta-analysis. J Am Soc Nephrol. 2015;26(8):2055-2064.
doi pubmed - Cassis P, Locatelli M, Cerullo D, Corna D, Buelli S, Zanchi C, Villa S, et al. SGLT2 inhibitor dapagliflozin limits podocyte damage in proteinuric nondiabetic nephropathy. JCI Insight. 2018;3(15).
doi pubmed - Takagi S, Li J, Takagaki Y, Kitada M, Nitta K, Takasu T, Kanasaki K, et al. Ipragliflozin improves mitochondrial abnormalities in renal tubules induced by a high-fat diet. J Diabetes Investig. 2018;9(5):1025-1032.
doi pubmed - Tanaka S, Sugiura Y, Saito H, Sugahara M, Higashijima Y, Yamaguchi J, Inagi R, et al. Sodium-glucose cotransporter 2 inhibition normalizes glucose metabolism and suppresses oxidative stress in the kidneys of diabetic mice. Kidney Int. 2018;94(5):912-925.
doi pubmed - Yaribeygi H, Butler AE, Atkin SL, Katsiki N, Sahebkar A. Sodium-glucose cotransporter 2 inhibitors and inflammation in chronic kidney disease: Possible molecular pathways. J Cell Physiol. 2018;234(1):223-230.
doi pubmed - Sugiyama S, Jinnouchi H, Kurinami N, Hieshima K, Yoshida A, Jinnouchi K, Nishimura H, et al. The SGLT2 inhibitor dapagliflozin significantly improves the peripheral microvascular endothelial function in patients with uncontrolled type 2 diabetes mellitus. Intern Med. 2018;57(15):2147-2156.
doi pubmed - Hirakawa Y, Tanaka T, Nangaku M. Mechanisms of metabolic memory and renal hypoxia as a therapeutic target in diabetic kidney disease. J Diabetes Investig. 2017;8(3):261-271.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


