| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Original Article
Volume 11, Number 1, January 2019, pages 21-25
Obstructive Sleep Apnea Screening in Patients With Atrial Fibrillation: Missed Opportunities for Early Diagnosis
Asif Khana, c, Jay Patelb, Dikshya Sharmaa, Saleha Riaza, Seleshi Demissiea, Anita Szerszena
aDepartment of Internal Medicine, Staten Island University Hospital Staten Island, 475 Seaview Ave, Staten Island, NY 10305, USA
bWest Virginia University School of Medicine, Morgantown, WV, USA
cCorresponding Author: Asif Khan, Department of Internal Medicine, Staten Island University Hospital Staten Island, 475 Seaview Ave, Staten Island, NY 10305, USA
Manuscript submitted October 7, 2018, accepted October 22, 2018
Short title: OSA Screening in AF Patients
doi: https://doi.org/10.14740/jocmr3635
| Abstract | ▴Top |
Background: “There is a high prevalence of obstructive sleep apnea (OSA) among patients with atrial fibrillation (AF). There is also strong evidence that proper OSA management can reduce AF recurrence.” Polysomnography is the gold standard for OSA diagnosis, but screening tests, such as STOP-BANG, have been successful in identifying patients at risk for OSA. Our study assesses screening rates for OSA in patients with persistent AF, and willingness of patients at increased risk for OSA towards further diagnostic evaluation.
Methods: A total of 254 persistent AF patients were surveyed regarding prior screening for OSA, and if previously unscreened, assessed with STOP-BANG. Prior cardioversions and willingness to undergo further workup was also recorded. Patients at risk for OSA were given educational brochures. Subjects with diagnosis of OSA were asked about their compliance with positive airway pressure therapy.
Results: Sixty-six percent of AF patients were never screened for OSA; 75% unscreened participants (95% CI: 68-81%) were at high risk for OSA. Patients with previous hospitalizations or electrical cardioversions were more frequently screened for OSA (P = 0.02, P = 0.03, respectively). Forty-three percent of high-risk individuals had a BMI < 30. Among patients at risk for OSA (score ≥ 3), the majority (n = 99, 79%) were interested in follow-up with a sleep study (n = 93, 74%).
Conclusions: Although there is a strong OSA-associated risk for AF, which is amenable to intervention, most patients with persistent AF are not assessed for OSA. Simple to use screening questionnaires are sensitive and can reliably identify patients at high risk for OSA, reserving costlier and somewhat inconvenient nocturnal polysomnography to only those at risk. We hope our study will help to push the AF and OSA connection into the spotlight in the primary care of patients with AF.
Keywords: Atrial fibrillation; Obstructive sleep apnea; STOP-BANG; Obstructive sleep apnea screening; Polysomnography
| Introduction | ▴Top |
Obstructive sleep apnea (OSA) is a risk factor for the onset and recurrence of atrial fibrillation (AF) [1]; it carries greater risk than age, gender, obesity, or hypertension (HTN) [2]. AF is the most common arrhythmia in adults, and is associated with significant morbidity and mortality [1, 2]. AF accounts for over 750,000 hospitalizations and 130,000 deaths each year in the USA [3]. An estimated 2.7 - 6.1 million people in the USA are affected, with 2% prevalence in people less than 65 years old to 9% of those 65 and over [3]. Analysis of the Framingham Heart Study showed an odds ratio for death of 1.5 in men and 1.9 in women diagnosed with AF [4].
Untreated OSA may underline the failure of anti-arrhythmic therapy in AF. OSA is relatively common, with clinically apparent disease affecting 3-7% of adult men and 2-5% of adult women, respectively [5]. OSA prevalence increases to 50% in patients with poorly controlled AF [6]. The two diseases share many risk factors including older age, male gender, obesity, and HTN [7-10]. There is a stronger association between AF and OSA, however, than other traditional OSA risk factors, such as obesity, HTN, or diabetes [11].
The pathophysiology of this link is not yet completely understood. It is thought that during apneic spells the induction of hypoxemia causes increased oxidative stress and surging of catecholamines [12-14]. This heightened sympathetic activity leads to HTN and an increased risk of cardiovascular disease. Abrupt increases in intra-thoracic pressure during apneic episodes can also lead to excess venous return, atrial enlargement, and remodeling [13, 15]. The evidence of association is strengthened by the fact that treatment of OSA with continuous positive airway pressure (CPAP) has been shown to be effective in reducing recurrence of AF [16-18]. A recent meta-analysis showed that CPAP was associated with a 42% relative risk reduction in recurrence of AF [19].
Despite the importance of identifying and treating OSA in AF patients, under-diagnosis of OSA remains a national issue [20-25]. Nocturnal polysomnography (also known as a sleep study) is the gold standard for the diagnosis of OSA [26], but it is expensive and inconvenient. As a result, alternate easy-to-use methods of screening for OSA such as STOP-BANG (used in our study) and Berlin questionnaires have been developed [27, 28]. STOP-BANG scores correlate well with apnea-hypopnea index (AHI) and OSA severity [29-31]. The screen score (0 - 8) is based on yes/no answers to eight questions pertaining to the clinical presentation of sleep apnea, with score 3 or more indicating an increased risk for OSA. The sensitivity for moderate to severe OSA (AHI > 15) is 93% with negative predictive value of 90%. The sensitivity to detect severe OSA (AHI > 30) is 100% [32].
Although USPSTF does not recommend screening for OSA in the general population, the American College of Physicians’ guidelines suggests screening for OSA in symptomatic individuals (unexplained daytime sleepiness) [32]. Furthermore, the American Academy of Sleep Medicine recommends screening in high-risk patients such as those with AF [33, 34]. To what extent is this screening being practiced in a clinical setting is not well documented. The primary objectives of our study were to assess the rate of OSA screening in hospitalized patients with persistent AF as well as their willingness to undergo further evaluation, including sleep study, after being identified as moderate to high risk for OSA.
| Materials and Methods | ▴Top |
Study population
In this prospective single-center cohort study, randomly selected subjects were patients admitted to the Staten Island University Hospital-Northwell Health and diagnosed with persistent AF. A minimum target of 200 subjects completing the study was set.
Inclusion criteria included the ability to speak English, age 18 years or older, and diagnosis of persistent AF as defined by American Heart Association (AHA) guidelines [35]. Patients were excluded if unable to complete the survey such as those with history of dementia, delirium or hemodynamic instability. No protected health information was collected for the study. The study was approved by the Northwell Health Institutional Review Board.
Screening procedure
Subjects who met the inclusion criteria were asked to complete a STOP-BANG questionnaire. Pertinent demographics, OSA-associated conditions such as HTN and heart failure (HF), history of prior screenings for OSA and past cardioversions were recorded. The investigator measured the subject’s height, weight, and neck circumference. Subjects who tested positive (≥ 3) on the STOP-BANG questionnaire were informed of their result and given an educational brochure on OSA. They were then asked about their willingness to undergo further testing with a sleep study. Regardless of the answer, patients were advised to follow up with their primary care physician.
Informed consent
No identifiable protected health information was collected for the purposes of this study. A waiver of documentation of informed consent and HIPAA was submitted to the IRB.
Statistical analysis
Summary statistics are provided for all demographic and baseline characteristics. Continuous variables are summarized with descriptive statistics. Frequency counts and percentage of patients within each category are provided for categorical data.
The prevalence of suspected OSA among the study population was estimated using the simple asymptotic method based on the normal approximation to the binomial distribution. The 95% confidence limits for the true prevalence rate are also presented. Differences between groups in continuous variables were examined using independent-sample t-test, nonparametric Mann-Whitney U-test, or Kruskal-Wallis test, as appropriate. Associations between discrete variables were evaluated using Chi-squared test or Fisher’s exact test and odds ratios.
All statistical tests of significance were two-sided and conducted at the 0.05 level of significance. All analyses were conducted using SAS (Statistical Analysis System) software Version 9.3.
Sample size determination
Assuming that the expected prevalence of OSA among persistent AF patient population is 50%, a sample size of approximately 265 patients will provide us with a two-sided 95% confidence interval for the true prevalence that will extend 6.0% from the observed prevalence.
| Results | ▴Top |
Among 254 participants, 34% (95% CI: 28-40%) were screened for OSA (Fig. 1, Table 1). There was no difference in screening by age, nor in the likelihood of being screened among those older than 60 (RR: 0.99, 95% CI: 0.79 - 1.2). There was a significant difference in screening rates among obese patients (P < 0.001) and those with HTN (P = 0.03). Patients with previous hospitalizations or electrical cardioversions were more frequently screened for OSA (P = 0.02, P = 0.03, respectively).
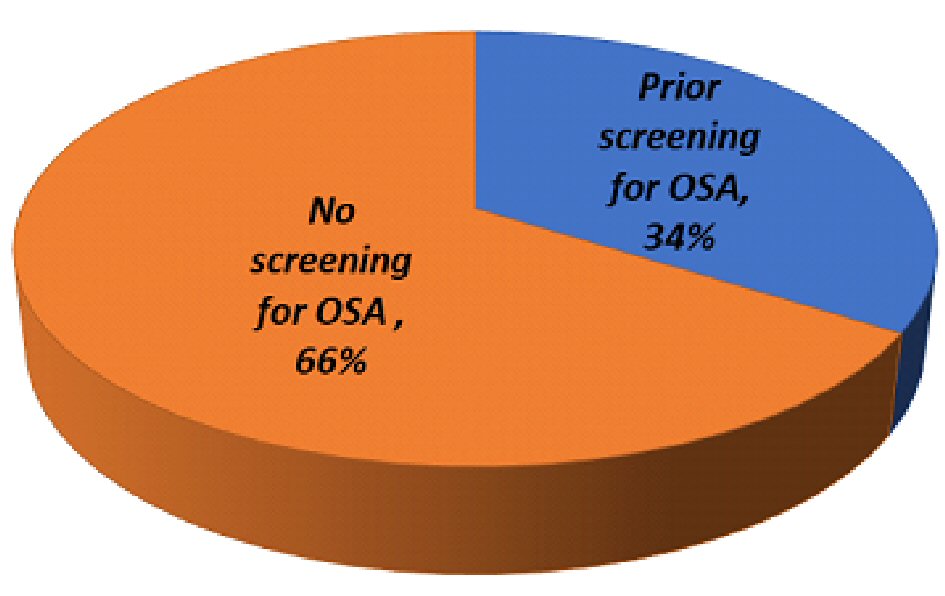 Click for large image | Figure 1. Breakdown of study participants’ OSA screening status. |
 Click to view | Table 1. Characteristics of Study Participants |
On multivariable analysis, BMI was the only independent correlate for OSA screening among patients with AF (Table 2). For a unit increase in BMI above 25 kg/m2, the odds of being screened were increased by 10%.
 Click to view | Table 2. Multivariable Logistic Regression Analysis of OSA Screening Among AF Patients |
Seventy-five percent unscreened participants (95% CI: 68-81%) were at high risk for OSA (average STOP-BANG: 3.6) (Fig. 2, Table 1); 43% of high-risk individuals had a BMI < 30. Among patients at risk for OSA (score ≥ 3), the majority (n = 99, 79%) were interested in follow-up with a sleep study (n = 93, 74%).
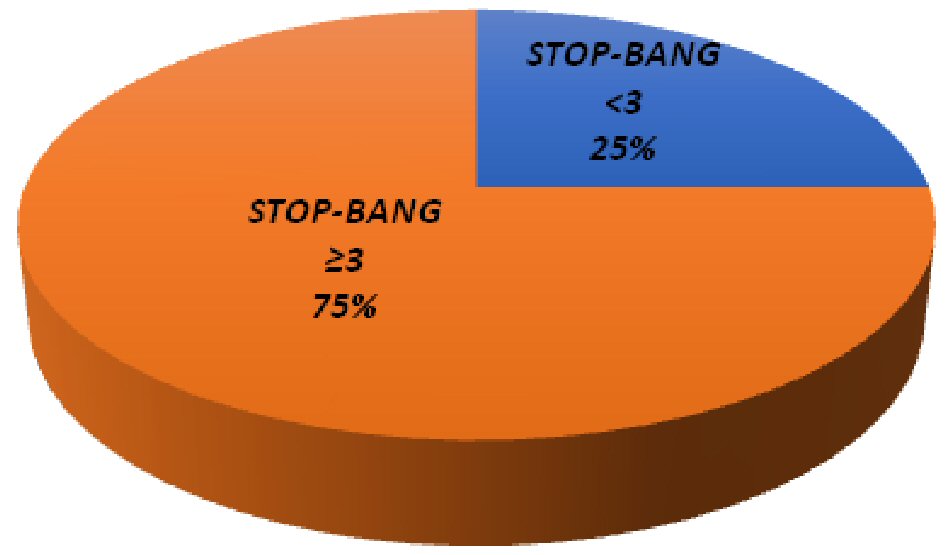 Click for large image | Figure 2. Comparison of study participants’ STOP-BANG scores. |
| Discussion | ▴Top |
OSA is an important modifiable risk factor for AF, and therefore an additional target for therapeutic intervention. Yet an estimated 70-80% of OSA cases in the general population remain undiagnosed [5, 25]. Our study emphasizes the fact that a large number of patients with underlying persistent AF have undiagnosed OSA. Although the USPSTF recently concluded that there is insufficient evidence for routine screening in asymptomatic individuals, other guidelines recommend screening for OSA in patients with high-risk conditions including AF, obesity, HTN and HF, stroke, diabetes mellitus, and pulmonary hypertension [36]. Identifying and treating OSA is especially important in patients with AF because OSA actively contributes to the onset and progression of AF [14]. In addition, OSA may be a predictor of AF in patients undergoing cardiac surgery, cardioversion, ablation or having HF [16, 37].
In our study, the majority (66%) of patients with persistent AF were not screened for OSA even though most of them also had other commonly recognized risk factors such as obesity, HTN, and HF. We call attention not only to low screening rates in patients with AF in the outpatient setting, but also in hospitalized patients with underlying risk factors for OSA, recurrent hospital admissions and prior cardioversions. This implies that even patients with poorly controlled or symptomatic AF are not routinely screened for underlying contributing factors and represents missed opportunity for not only potentially improving AF management, but also possible healthcare cost savings [38].
Furthermore, studies show that patients who are female and those with low BMI are particularly susceptible to being overlooked for screening [22]. Interestingly, we found that 43% of the unscreened individuals at high risk for OSA had a BMI < 30. Another reason may be an unawareness of the OSA and AF association due to lack of education in screening for sleep disorders [20, 39]. Many clinicians may also believe the only way to screen for OSA is by expensive and time-consuming polysomnography. They may not be familiar with the validated, easy to administer, and patient-friendly screening tools available for OSA screening. Home screening technology is now also increasingly employed for patients’ convenience.
As many as 50% of patients with OSA are not adherent to positive airway pressure therapy, and 20-30% of patients do not accept therapy after initial diagnosis [40]. However, although CPAP is a standard treatment for OSA, there are new FDA-approved devices, which include Night Shift (by Advanced Brain Monitoring), Winx (by ApniCure), or Provent Sleep Apnea Technology (by Theravent) [41-43]. They may be used for those who refuse or cannot tolerate CPAP and may provide an alternative to oral surgical intervention. At the very least, the patients found to be at high risk for OSA may be started on aggressive weight management therapy that can be very effective, even curative option for those who are overweight or obese [19]. Furthermore, educating patients with AF about the risks of untreated OSA may increase compliance with recommended therapy.
To address our study limitations, a broader patient group should be assessed to extrapolate our results to the general population. It is also important to evaluate whether screening and educating patients on their risk for OSA by STOP-BANG questionnaire leads to improved nocturnal polysomnography completion rates and a subsequent adherence to recommended therapy.
In conclusion, our study showed that although there is a strong OSA-associated risk for AF, which is amenable to intervention, most patients with persistent AF are not assessed for OSA. This suggests there is a pressing need to increase awareness of the link between AF and OSA among both clinicians and patients. This awareness becomes especially significant in pre-surgical evaluations of AF patients and those with multiple hospitalizations or undergoing ablative therapies. Simple to use screening questionnaires are sensitive and can reliably identify patients at high risk for OSA, reserving costlier and somewhat inconvenient nocturnal polysomnography to only those at risk. We hope our study will help to push the AF and OSA connection into the spotlight in the primary care of patients with AF.
| References | ▴Top |
- ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation - executive summary. Circulation. 2006;114(7):700-752.
doi - Chow GV, Marine JE, Fleg JL. Epidemiology of arrhythmias and conduction disorders in older adults. Clin Geriatr Med. 2012;28(4):539-553.
doi pubmed - National center for chronic disease prevention and health promotion. Atrial Fibrillation [Fact Sheet]. https://www.cdc.gov/dhdsp/data_statistics/fact_sheets/fs_atrial_fibrillation.htm.
- Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98(10):946-952.
doi pubmed - Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):136-143.
doi pubmed - Todd K, McIntyre WF, Baranchuk A. Obstructive sleep apnea and atrial fibrillation. Nat Sci Sleep. 2010;2:39-45.
pubmed - Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. JAMA. 2004;291(16):2013-2016.
doi pubmed - Krahn AD, Manfreda J, Tate RB, Mathewson FA, Cuddy TE. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow-Up Study. Am J Med. 1995;98(5):476-484.
doi - Arias MA, Alonso-Fernandez A, Garcia-Rio F, Pagola C. Association between obesity and obstructive sleep apnoea. Eur Heart J. 2005;26(24):2744-2745.
doi pubmed - Wang TJ, Parise H, Levy D, D'Agostino RB, Sr., Wolf PA, Vasan RS, Benjamin EJ. Obesity and the risk of new-onset atrial fibrillation. JAMA. 2004;292(20):2471-2477.
doi pubmed - Miller JD, Aronis KN, Chrispin J, Patil KD, Marine JE, Martin SS, Blaha MJ, et al. Obesity, Exercise, Obstructive Sleep Apnea, and Modifiable Atherosclerotic Cardiovascular Disease Risk Factors in Atrial Fibrillation. J Am Coll Cardiol. 2015;66(25):2899-2906.
doi pubmed - Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96(4):1897-1904.
doi pubmed - Latina JM, Estes NA, 3rd, Garlitski AC. The relationship between obstructive sleep apnea and atrial fibrillation: a complex interplay. Pulm Med. 2013;2013:621736.
doi pubmed - Goyal SK, Sharma A. Atrial fibrillation in obstructive sleep apnea. World J Cardiol. 2013;5(6):157-163.
doi pubmed - Caples SM, Somers VK. Sleep-disordered breathing and atrial fibrillation. Prog Cardiovasc Dis. 2009;51(5):411-415.
doi pubmed - Kanagala R, Murali NS, Friedman PA, Ammash NM, Gersh BJ, Ballman KV, Shamsuzzaman AS, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003;107(20):2589-2594.
doi pubmed - Tang RB, Dong JZ, Liu XP, Kang JP, Ding SF, Wang L, Long DY, et al. Obstructive sleep apnoea risk profile and the risk of recurrence of atrial fibrillation after catheter ablation. Europace. 2009;11(1):100-105.
doi pubmed - Szymanski FM, Filipiak KJ, Platek AE, Hrynkiewicz-Szymanska A, Kotkowski M, Kozluk E, Kiliszek M, et al. Presence and severity of obstructive sleep apnea and remote outcomes of atrial fibrillation ablations - a long-term prospective, cross-sectional cohort study. Sleep Breath. 2015;19(3):849-856.
doi pubmed - Shukla A, Aizer A, Holmes D, Fowler S, Park DS, Bernstein S, Bernstein N, et al. Effect of obstructive sleep apnea treatment on atrial fibrillation recurrence: a meta-analysis. JACC Clin Electrophysiol. 2015;1(1-2):41-51.
doi pubmed - Kramer NR, Cook TE, Carlisle CC, Corwin RW, Millman RP. The role of the primary care physician in recognizing obstructive sleep apnea. Arch Intern Med. 1999;159(9):965-968.
doi pubmed - Costa LE, Uchoa CH, Harmon RR, Bortolotto LA, Lorenzi-Filho G, Drager LF. Potential underdiagnosis of obstructive sleep apnoea in the cardiology outpatient setting. Heart. 2015;101(16):1288-1292.
doi pubmed - Kapur V, Strohl KP, Redline S, Iber C, O'Connor G, Nieto J. Underdiagnosis of sleep apnea syndrome in U.S. communities. Sleep Breath. 2002;6(2):49-54.
doi pubmed - Rasmussen JJ, Fuller WD, Ali MR. Sleep apnea syndrome is significantly underdiagnosed in bariatric surgical patients. Surg Obes Relat Dis. 2012;8(5):569-573.
doi - Wake up America: A national sleep alert: report of the national commission on sleep disorders research. Washington, D.C.? The Commission; 1993.
- Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 1997;20(9):705-706.
doi pubmed - McNicholas WT. Diagnosis of obstructive sleep apnea in adults. Proc Am Thorac Soc. 2008;5(2):154-160.
doi pubmed - Mulgrew AT, Fox N, Ayas NT, Ryan CF. Diagnosis and initial management of obstructive sleep apnea without polysomnography: a randomized validation study. Ann Intern Med. 2007;146(3):157-166.
doi pubmed - Rosen CL, Auckley D, Benca R, Foldvary-Schaefer N, Iber C, Kapur V, Rueschman M, et al. A multisite randomized trial of portable sleep studies and positive airway pressure autotitration versus laboratory-based polysomnography for the diagnosis and treatment of obstructive sleep apnea: the HomePAP study. Sleep. 2012;35(6):757-767.
doi pubmed - Farney RJ, Walker BS, Farney RM, Snow GL, Walker JM. The STOP-Bang equivalent model and prediction of severity of obstructive sleep apnea: relation to polysomnographic measurements of the apnea/hypopnea index. J Clin Sleep Med. 2011;7(5):459-465B.
doi - Chung F, Subramanyam R, Liao P, Sasaki E, Shapiro C, Sun Y. High STOP-Bang score indicates a high probability of obstructive sleep apnoea. Br J Anaesth. 2012;108(5):768-775.
doi pubmed - Vasu TS, Doghramji K, Cavallazzi R, Grewal R, Hirani A, Leiby B, Markov D, et al. Obstructive sleep apnea syndrome and postoperative complications: clinical use of the STOP-BANG questionnaire. Arch Otolaryngol Head Neck Surg. 2010;136(10):1020-1024.
doi pubmed - Chung F, Abdullah HR, Liao P. STOP-Bang Questionnaire: A Practical Approach to Screen for Obstructive Sleep Apnea. Chest. 2016;149(3):631-638.
doi pubmed - U. S. Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, Curry SJ, Davidson KW, Epling JW, Jr., Garcia FA, et al. Screening for Obstructive Sleep Apnea in Adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2017;317(4):407-414.
doi pubmed - AASM response to "Screening for obstructive sleep apnea in adults: evidence report and systematic review for the US Preventive Services Task Force" - American Academy of Sleep Medicine (AASM). AASM.
- January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Jr., Conti JB, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130(23):2071-2104.
doi pubmed - Epstein LJ, Kristo D, Strollo PJ, Jr., Friedman N, Malhotra A, Patil SP, Ramar K, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263-276.
pubmed - Mooe T, Gullsby S, Rabben T, Eriksson P. Sleep-disordered breathing: a novel predictor of atrial fibrillation after coronary artery bypass surgery. Coron Artery Dis. 1996;7(6):475-478.
doi pubmed - Kapur V, Blough DK, Sandblom RE, Hert R, de Maine JB, Sullivan SD, Psaty BM. The medical cost of undiagnosed sleep apnea. Sleep. 1999;22(6):749-755.
doi pubmed - Mold JW, Quattlebaum C, Schinnerer E, Boeckman L, Orr W, Hollabaugh K. Identification by primary care clinicians of patients with obstructive sleep apnea: a practice-based research network (PBRN) study. J Am Board Fam Med. 2011;24(2):138-145.
doi pubmed - Catcheside PG. Predictors of continuous positive airway pressure adherence. F1000 Med Rep. 2010;2:70.
doi - Levendowski D, Cunnington D, Swieca J, Westbrook P. User compliance and behavioral adaptation associated with supine avoidance therapy. Behav Sleep Med. 2018;16(1):27-37.
doi pubmed - Colrain IM, Black J, Siegel LC, Bogan RK, Becker PM, Farid-Moayer M, Goldberg R, et al. A multicenter evaluation of oral pressure therapy for the treatment of obstructive sleep apnea. Sleep Med. 2013;14(9):830-837.
doi pubmed - Berry RB, Kryger MH, Massie CA. A novel nasal expiratory positive airway pressure (EPAP) device for the treatment of obstructive sleep apnea: a randomized controlled trial. Sleep. 2011;34(4):479-485.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


