| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Original Article
Volume 10, Number 6, June 2018, pages 466-477
Impact of Dapagliflozin Therapy on Renal Protection and Kidney Morphology in Patients With Uncontrolled Type 2 Diabetes Mellitus
Seigo Sugiyamaa, b, e, f, Hideaki Jinnouchia, b, c, e, Noboru Kurinamia, Kunio Hieshimaa, Akira Yoshidaa, Katsunori Jinnouchia, Motoko Tanakad, Hiroyuki Nishimuraa, Tomoko Suzukia, Fumio Miyamotoa, Keizo Kajiwaraa, b, Tomio Jinnouchia, b
aDiabetes Care Center, Jinnouchi Hospital, Kumamoto, Japan
bCardiovascular Division, Diabetes Care Center, Jinnouchi Hospital, Kumamoto, Japan
cDivision of Preventive Cardiology, Department of Cardiovascular Medicine, Kumamoto University Hospital, Kumamoto, Japan
dDepartment of Nephrology, Akebono Clinic, Kumamoto, Japan
eThese authors contributed equally to this study.
fCorresponding Author: Seigo Sugiyama, Cardiovascular Division, Diabetes Care Center, Jinnouchi Hospital, 6-2-3 Kuhonji, Chuo-ku, Kumamoto City 862-0976, Japan
Manuscript submitted March 25, 2018, accepted April 3, 2018
Short title: Renoprotective effect of dapagliflozin
doi: https://doi.org/10.14740/jocmr3419w
| Abstract | ▴Top |
Background: We examined whether the sodium-glucose cotransporter-2 inhibitor (SGLT2i) dapagliflozin can improve urine albumin-to-creatinine ratio (UACR) associated with a reduction in body weight or body fat in patients with type 2 diabetes mellitus (T2DM).
Methods: We prospectively recruited T2DM patients having inadequate glycemic control (hemoglobin A1c (HbA1c) > 7.0%) not on SGLT2i therapy. We treated the patients with add-on dapagliflozin treatment or intensification of non-SGLT2 inhibitor therapies for 6 months. We measured UACR, urine N-acetyl-β-glucosaminidase (uNAG), and body composition including total body fat mass (TBFM) as assessed by bioelectrical impedance analysis. We also investigated changes in length and radiation attenuation properties of the kidneys and abdominal fat area using computed tomography.
Results: We enrolled 62 patients with a mean HbA1c of 8.0%. The HbA1c and fasting blood glucose were significantly decreased in both the dapagliflozin-group and non-SGLT2i-group, with no significant difference between the two groups. Dapagliflozin treatment, but not non-SGLT2i treatment, significantly decreased UACR and uNAG. The changes in UACR and uNAG were significantly greater in the dapagliflozin group compared with the non-SGLT2i group. Dapagliflozin treatment, but not non-SGLT2i treatment, significantly decreased the body weight, TBFM, and abdominal fat area and significantly increased kidney length and radiation attenuation. The percentage change in UACR was significantly correlated with changes in TBFM, but not with body weight. By multivariate logistic regression analysis, dapagliflozin treatment was significantly associated with the improvement of UACR.
Conclusions: Add-on treatment with dapagliflozin exhibited significant renoprotective effects, with improvement of UACR and uNAG and increased kidney length and radiation attenuation in patients with uncontrolled T2DM.
Keywords: Sodium-glucose co-transporter 2 inhibitor; Dapagliflozin; Kidney; Urine albumin-to-creatinine ratio; N-acetyl-β-glucosaminidase; Type 2 diabetes; Kidney length
| Introduction | ▴Top |
The number of patients with type 2 diabetes mellitus (T2DM) is rapidly increasing globally [1]. Diabetic nephropathy/diabetic kidney disease (DN/DKD) is considered a serious and unsolved complication of T2DM because it is the leading cause of both end stage renal disease and requirement for renal replacement therapy, resulting in important issues not only in clinical practice but also in public health [1, 2]. Increased attention has been focused on investigating and developing practical strategies for preventing and improving DN/DKD clinically [3]. Glucose-lowering therapies that exhibit renoprotective effects are considered to have great additional clinical impact on the comprehensive management of T2DM [4]. Regarding practical approaches to treating DN/DKD, identification and assessment of early renal damage by measuring the urine albumin-to-creatinine ratio (UACR) and estimated glomerular filtration rate (eGFR) is recommended to permit early intervention with renoprotective therapies [3, 4]. Recently, renal tubular damage as assessed by urinary markers such as urine N-acetyl-β-glucosaminidase (uNAG) has also been recognized as an important pathogenic indicator of DN/DKD [5, 6].
Obesity and excess daily calorie intake are critically involved not only in the pathogenesis of T2DM but also of DN/DKD in the current era of satiation [7]. The harmful consequences of obesity and fat overload include ectopic fat accumulation in the kidney resulting in the obesity-related kidney disease “fatty kidney” which promotes DN/DKD progression [8]. Obesity and weight gain are correlated with increasing albuminuria and worsening renal function [8]. Thus, reducing body weight and body fat mass is recognized as an important and effective clinical strategy for practical management of T2DM and DN/DKD [9]. These observations suggest the possible clinical approach of anti-diabetic therapies accompanied by reducing body weight to achieve renoprotection with improvement of albuminuria in DN/DKD.
Sodium-glucose co-transporter 2 (SGLT2) inhibitors exert their glucose-lowering effects via the inhibition of SGLT2 protein in the renal proximal tubule, thus blocking reabsorption of urinary glucose and sodium. Several beneficial pleiotropic effects of SGLT2 inhibitors (SGLT2i) have been reported including improving cardiovascular outcomes, lowering blood pressure, decreasing serum uric acid, and body weight loss, mainly through a reduction in fat mass [10]. Regarding hemodynamics in the kidney, SGLT2i restores homeostasis of the tubuloglomerular feedback mechanism [11] and protects the glomerulus. It has been suggested that SGLT2i may protect kidneys against being overloaded with reabsorbed glucose and sodium and improve energy and oxygen balance in renal proximal tubular epithelial cells [12-14], leading to recovery of tubular cells and the tubulointerstitial area [15]. Taken together, SGLT2i could provide renoprotective benefits through reducing renal stress in tubular cells and glomeruli, resulting in the improvement of albuminuria accompanied by decreased body fat mass.
We hypothesized that add-on dapagliflozin treatment could improve albuminuria by reducing the body weight or body fat mass in patients with uncontrolled T2DM. We further examined the possible correlation between the change in UACR and changes in body weight, fat mass, and other clinical variables. At the same time, we measured changes in uNAG as a marker of renal tubular damage. The morphological changes in kidneys (kidney length and radiation attenuation) were also assessed by plain abdominal computed tomography (CT).
| Materials and Methods | ▴Top |
Study population and study protocol
The present study was prespecified as the kidney part of the organ-specific arm of a clinical study which examined the changes in body muscle mass after dapagliflozin therapy [16]. We prospectively recruited Japanese patients with stable but uncontrolled T2DM (HbA1c > 7.0%) without severe obesity (body mass index (BMI) < 35 kg/m2) who were not currently treated with SGLT2 inhibitors from the Diabetes Care Center at Jinnouchi Hospital between 2014 and 2017. The exclusion criteria were as follows: type 1 DM, a history of ketoacidosis, age > 80 years, unstable cardiovascular disease, active inflammation, severe liver disease, dementia, chronic kidney disease (estimated glomerular filtration rate < 40 mL/min/1.73 m2), nephrotic syndrome, urinary tract infection, cancer, and those who could not remain standing to have a body composition examination. Patients with newly diagnosed DM not on any treatment and with ketosis were also excluded.
The attending physicians at Jinnouchi Hospital non-randomly separated the enrolled patients into two groups: the dapagliflozin group and the non-SGLT2i group. Inclusion in the treatment arm was left to the physicians’ discretion. Patients in the dapagliflozin group received their standard ongoing treatments plus additional dapagliflozin (5 mg/day) for 6 months. Patients in the non-SGLT2i group received their standard ongoing treatments along with intensification of glucose-lowering medications (increased doses and addition of other types of medications) except SGLT2i to achieve an HbA1c level < 7.0% by the end of the study period.
Before starting each treatment, fasting blood and urine samples were collected in the morning. Body composition was measured by bioelectrical impedance analysis using the InBody770® (Biospace, Seoul, Korea) and kidney morphology was assessed by plain abdominal CT (Aquilion CXL®; Toshiba, Tokyo, Japan). After 6 months of treatment, additional fasting blood and urine samples were collected, and a second body composition analysis using the InBody770® and plain abdominal CT examination were performed.
The primary outcome was the dapagliflozin-induced changes in UACR investigated as a prospective, parallel-arm, and open-label study. This study was conducted in accordance with the Declaration of Helsinki. The study protocol was approved by the Human Ethics Review Committee of Jinnouchi Hospital (2014-5-No.4), and a signed informed consent form was obtained from each patient. This study was registered under the UMIN protocol registration system (ID UMIN0000333354).
Measurement of UACR, uNAG, and blood biochemical parameters
Fasting blood samples were collected from the antecubital vein in the morning. At the same time, spot urine samples were collected. Blood and urine analyses were conducted in the hospital laboratory to measure the levels of blood glucose, HbA1c, cholesterol, triglycerides, C-peptide immunoreactivity (CPR), creatinine, sodium (Na), potassium (K), chloride (Cl), blood urea nitrogen (BUN), uric acid (UA), and B-type natriuretic peptide (BNP). The levels of non-esterified fatty acid (NEFA), uNAG, magnesium (Mg), and glucagon were measured by SRL Corp. (Tokyo, Japan).
Calculation of the estimated glomerular filtration rate (eGFR) and estimated daily salt intake
The eGFR (mL/min/1.73m2) was calculated using the formula of the Japanese Society of Nephrology [17]. To estimate the patients’ daily salt intake, we measured the urinary sodium and creatinine concentrations in spot urine samples. The estimated daily salt intake (g/day) was calculated using a formula created for estimating the 24-h urinary salt excretion [18].
Measurement of the abdominal visceral fat area (VFA), subcutaneous fat area (SFA), and kidney length and radiation attenuation using abdominal CT
We performed plain abdominal CT using a 64-slice multi-detector CT and measured VFA, SFA, and total fat area (TFA) as previously reported [19]. On CT cross-sectional slices that were obtained at the umbilical level, the adipose tissue area located in the peritoneal cavity was defined as VFA (cm2), and the adipose tissue area underlying the skin layer was defined as SFA (cm2). The VFA and SFA in the supine position were calculated by using a commercial software program based on the Japanese guidelines for obesity treatment (Japan Society for the Study of Obesity, in Japanese) [19]. The TFA (cm2) was the sum of the SFA and VFA.
The treatment-induced changes in kidney morphology, kidney length and kidney radiation attenuation were then investigated. The kidney length was measured in the coronal sections of the right and left kidneys on CT images and we selected the maximum values of each kidney length (mm) and calculated the mean values [20]. Values of kidney radiation attenuation (average Hounsfield units; HU values) were measured at the middle level of kidney in the cross sectional images of each kidney using manual tracing [21]. In the present study, the CT HU value was used to assess the tissue lipid content as the degree of “fatty kidney” [8], and a lower CT HU value reflected increased lipid deposition in the kidney [22]. We calculated the mean values of radiation attenuation in both the right and left kidneys.
Measurement of body composition using a bioelectrical impedance analyzer (InBody770®)
Anthropometric measurements were obtained in the standing position. Body composition was measured as previously reported [16]. Elementary body composition, including total body fat mass (TBFM), body fat percentage, and total water mass was measured using a direct segmental multi-frequency bioelectrical impedance analyzer (InBody770®) [23]. This analyzer processes 30 impedance measurements using six different frequencies (1, 5, 50, 250, 500, and 1000 kHz) at each of five segments of the body (right arm, left arm, trunk, right leg, and left leg). In addition, measurements are processed by 15 reactance measurements using tetrapolar 8-point tactile electrodes at three different frequencies (5, 50, and 250 kHz) at each of five segments of the body (right arm, left arm, trunk, right leg, and left leg) [24].
Primary and secondary outcomes
The primary endpoint was the treatment-induced changes in UACR. We tested the superiority of the dapagliflozin-induced changes in UACR compared with non-SGLT2i therapy following a 6-month course of treatment. The secondary outcome was the relationship between the percentage changes in UACR and changes in body weight, fat mass, and other clinical parameters. We also investigated the changes in uNAG and kidney morphology as assessed by plain abdominal CT.
Statistical analyses
Based on our preliminary examination at our hospital, a power analysis indicated that enrollment of more than 56 patients (28 patients in each group) was required to detect a mean difference in the change in UACR of -12.0 mg/g in the dapagliflozin group and -2.0 mg/g in the non-SGLT2i group, with a standard deviation of 12.0 mg/g, a power of 80%, and a two-sided alpha of 0.05. The results of normally distributed continuous variables (determined by the Shapiro-Wilk test) were expressed as the mean (standard deviation (SD)), while those of continuous variables with a skewed distribution were expressed as median values (interquartile range). Differences in the baseline characteristics of the two groups were analyzed by Student’s t-test, the Mann-Whitney U test, or Fisher’s exact test for categorical data, as appropriate. Either a paired Student’s t-test or Wilcoxon’s test was used to analyze the effect of each treatment.
To determine the relationships between changes in various clinical parameters and percentage changes in UACR, correlations between variables of interest were analyzed using Spearman’s rank correlation coefficient. Patients with a decrease in UACR (changes in UACR < 0 mg/g) were defined as having an improved UACR in the present study. A logistic regression analysis was used to evaluate the association between improvement in UACR and baseline clinical variables, including age, sex, BMI, HbA1c, fasting plasma glucose (FPG), lipid parameters, CT measures, and therapy allocation (dapagliflozin or non-SGLT2i therapy). Associations between groups and all other parameters were analyzed first by a univariate logistic regression analysis. This was followed by a multivariate logistic regression analysis using the forced inclusion model, and the Hosmer-Lemeshow goodness-of-fit statistic was calculated. A P value < 0.05 was considered statistically significant. Statistical analyses were performed using the Statistical Package for Social Sciences software program, version 23 (SPSS Inc., IBM, Tokyo, Japan).
| Results | ▴Top |
Baseline clinical characteristics of the study participants
A total of 62 Japanese patients with uncontrolled T2DM were enrolled and non-randomly assigned to the two groups (dapagliflozin group or non-SGLT2i group). All patients completed the study protocol. The baseline clinical characteristics of the total cohort and those of each group are shown in Table 1. The mean age was 55.9 years, 71.0% were men, the mean BMI was 27.3 kg/m2, the median HbA1c was 7.7%, the mean FPG level was 143.8 mg/dL, and the mean eGFR was 76.1 mL/min/1.73m2. At enrollment, 41.9% of patients were treated with insulin, and the frequency of baseline anti-diabetic medications was not significantly different between the groups. The baseline characteristics of patients in the dapagliflozin group were similar to those of the non-SGLT2i group (Table 1). The chronic kidney disease (CKD) category and albuminuria category were not significantly different between the two groups.
 Click to view | Table 1. Baseline Clinical Characteristics |
Changes in levels of HbA1c, FPG, CPR and glucagon
For glycemic control, 5 mg/day of dapagliflozin was used in the dapagliflozin group. In the non-SGLT2i group, new medications with the exception of SGLT2i were administered to 25.8% of the patients and the doses of previously prescribed medications including insulin were increased in 93.5% of the patients. The total patients in both treatment groups showed a significant improvement in HbA1c (median (interquartile range): pre-treatment to post-treatment, 7.7% (7.3% - 8.4%) to 6.9% (6.5% - 7.5%), P < 0.01) and FPG levels (mean ± SD: 143.8 ± 39.4 to 125.2 ± 27.6 mg/dL, P < 0.01) at 6 months. As shown in Table 2, the HbA1c and FPG levels were significantly decreased in both groups, while the absolute changes in the HbA1c and FPG levels were not significantly different between the groups. Dapagliflozin treatment significantly decreased the fasting blood CPR levels. We did not find any marked changes in the fasting plasma glucagon levels in either group.
 Click to view | Table 2. Changes in Glucose Metabolic Parameters, Body Weight, Body Composition, and Abdominal Fat Area |
Changes in body weight, total body fat mass, body water mass and abdominal fat area
We found a significant decrease in the body weight, BMI, TBFM, and body fat percentage in the dapagliflozin group, but not in the non-SGLT2i group (Table 2). Dapagliflozin treatment, but not non-SGLT2i treatment, also significantly decreased the waist circumference, abdominal VFA, SFA, and TFA compared with the baseline readings. In contrast to body weight and TBFM, the total body water mass was not significantly changed in either group and the absolute change in the body water mass was not significantly different between the two groups.
Changes in blood pressure, pulse rate, and other metabolic parameters
Among the lipid parameters, dapagliflozin treatment significantly increased HDL cholesterol and significantly decreased triglyceride levels, while the non-SGLT2i therapy did not exhibit any significant effects. There was also no significant difference in lipids between the two groups upon inter-group comparison (Table 3). Dapagliflozin treatment, but not non-SGLT2i treatment, significantly decreased blood pressure and pulse rate compared with the baseline readings. The absolute changes in blood pressure and pulse rate were significantly greater in the dapagliflozin group compared with the non-SGLT2i group as shown in Table 3.
 Click to view | Table 3. Changes in Lipids, Blood Pressure, Pulse Rate, and BNP |
Changes in UACR, uNAG, eGFR, serum BUN, UA, Mg and estimated daily salt intake
Renal function related parameters are presented in Table 4. Neither dapagliflozin nor non-SGLT2i treatments significantly affected eGFR, serum creatinine, BUN, or estimated daily salt intake compared with the baseline. Dapagliflozin treatment significantly increased serum levels of Mg and significantly decreased serum levels of UA while non-SGLT2i therapy did not exhibit significant effects on them. The absolute changes in serum Mg and UA were significantly greater in the dapagliflozin group compared with the non-SGLT2i group as shown in Table 4. Serum levels of Na, K, and Cl were not significantly changed in either group (data not shown). In the total patient cohort, UACR and uNAG decreased after the glucose-lowering therapy, although these treatment-induced changes in UACR and uNAG were not statistically significant (UACR: 18.1 (6.7 - 45.5) to 12.0 (6.4 - 44.7) mg/g, P = 0.196; uNAG: 6.65 (4.76 - 12.10) to 5.74 (3.97 - 9.95) U/L, P = 0.112). Upon intra-group comparison, the add-on dapagliflozin-therapy, but not non-SGLT2i therapy, demonstrated a significant improvement in UACR and uNAG. The absolute changes in UACR and uNAG were significantly greater in the dapagliflozin group than in the non-SGLT2i group (Table 4). The percentage changes in UACR levels were significantly greater in the dapagliflozin group than in the non-SGLT2i group (Fig. 1).
 Click to view | Table 4. Changes in Renal Function-Related Factors, and Kidney Length and Radiation Attenuation |
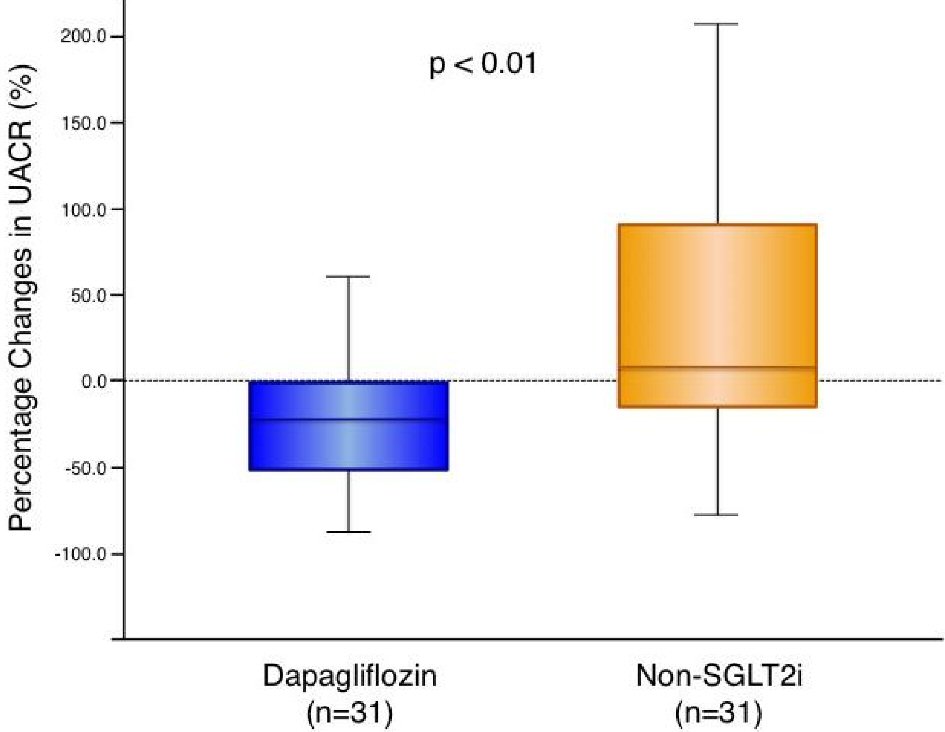 Click for large image | Figure 1. Change in urine albumin-to-creatinine ratio with dapagliflozin therapy and non-SGLT2 inhibitor therapy. Dapagliflozin (n = 31): -21.2 (-52.5 to -1.1) % vs. non-SGLT2i (n = 31): 7.8 (-18.4 to 91.3) %, P = 0.004. Box-and-whisker plots indicate the percentage changes in urine albumin-to-creatinine ratio (UACR) in patients with dapagliflozin therapy (n = 31) or non-sodium-glucose co-transporter 2 (SGLT2) inhibitor therapy (n = 31). In the box-and-whisker plots, lines within boxes represent median values; the top and bottom edges of the boxes represent the 25th and 75th percentiles, respectively; the top and bottom bars outside the boxes represent the 10th and 90th percentiles, respectively. Percentage change in UACR = ((UACR after 6 months of therapy) – (UACR at enrollment)) × 100/(UACR at enrollment). Notably, upon inter-group comparison, the percentage changes in UACR in patients with dapagliflozin therapy were significantly greater than those in the patients with non-SGLT2 inhibitor therapy. |
Logistic regression analysis for an improvement in UACR among the baseline factors
The proportion of patients with an improved UACR (changes in UACR < 0 mg/g) was 77.4% in the dapagliflozin group and 59.7% in the non-SGLT2i group (P = 0.01). Univariate logistic regression analysis showed that the baseline age, waist circumference, abdominal SFA, and dapagliflozin therapy were significantly correlated with an improvement in UACR (Table 5). No lipid parameters were significantly correlated with the UACR improvement (data not shown). A forced inclusion multivariate logistic regression analysis with these significant variables from the univariate analysis showed that the baseline age and dapagliflozin-therapy were significantly and independently correlated with an improvement in UACR. The Hosmer-Lemeshow statistic was appropriate (P = 0.59). The proportion of patients with an improvement in both of UACR and uNAG (changes in UACR < 0 mg/g and uNAG < 0 U/L) was 53.3% in the dapagliflozin group and 11.5% in the non-SGLT2 inhibitor group (P < 0.01, Fig. 2). Univariate logistic regression analysis indicated that dapagliflozin therapy was significantly correlated with an improvement in both UACR and uNAG (odds ratio; 9.52, 95% confidence interval; 2.36 to 38.46, P < 0.01).
 Click to view | Table 5. Logistic Regression Analysis for the Improvement of Urine Albumin-to-Creatinine Ratio Among Baseline Factors |
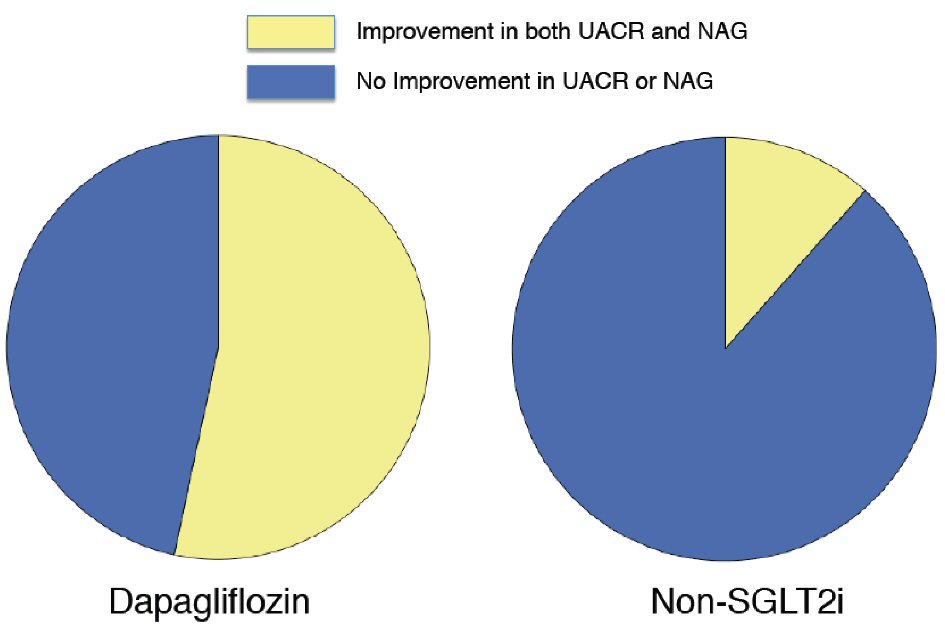 Click for large image | Figure 2. Proportion of patients with improved urine albumin-to-creatinine ratio and urine N-acetyl-β -glucosaminidase who received dapagliflozin therapy and non-SGLT2 inhibitor therapy. The proportion of patients with an improved urine albumin-to-creatinine ratio (UACR) and urine N-acetyl-β-glucosaminidase (uNAG) was 53.3% in the dapagliflozin group and 11.5% in the non-SGLT2 inhibitor group (P = 0.002). SGLT2i: sodium-glucose co-transporter 2 inhibitor. |
Association between changes in UACR and changes in clinical variables
To determine the factors associated with the changes in UACR and the changes in various clinical and laboratory parameters during the treatment period, we undertook Spearman’s rank correlation coefficient analysis between the percentage changes in UACR and the variables. The percentage change in UACR was not significantly correlated with changes in HbA1c, FBG levels, the body weight, BMI, and abdominal fat area (Table 6). No lipid parameters were significantly correlated with the percentage change in UACR (data not shown). However, the percentage change in UACR was significantly and positively correlated with changes in fasting glucagon, TBFM, and body fat percentage.
 Click to view | Table 6. Correlation Between Percent Changes in Urine Albumin-to-Creatinine Ratio and Changes in Clinical Variables |
Changes in kidney length and radiation attenuation as assessed by abdominal plain CT
The treatment-induced changes in kidney morphology, including kidney length and radiation attenuation, are presented in Table 4. Dapagliflozin treatment significantly increased kidney length and radiation attenuation while in contrast non-SGLT2i therapy did not exhibit any significant effects. The absolute change in kidney length was significantly greater in the dapagliflozin group compared with the non-SGLT2i group. The dapagliflozin therapy-induced changes in radiation attenuation were greater than those in non-SGLT2i group, although the difference was not statistically significant (P = 0.14). The change in kidney length was significantly greater in patients with an improvement in both UACR and uNAG compared with those who did not (mean ± SD; 3.92 ± 7.39 mm vs. 0.62 ± 3.38 mm, P = 0.023).
| Discussion | ▴Top |
The add-on treatment of the SGLT2 inhibitor dapagliflozin for 6 months significantly improved UACR and glycemic control associated with a substantial reduction of body fat mass in patients with uncontrolled T2DM. Interestingly, we found that the dapagliflozin therapy produced a significant improvement in the renal proximal tubular injury marker uNAG and significantly increased the radiation attenuation and length of kidney. These findings indicate unique impacts of dapagliflozin on kidney protection and morphology, which could be of great advantage in treating T2DM in clinical practice.
The randomized clinical trials EMPA-REG OUTCOME and CANVAS revealed that SGLT2 inhibition exhibited protective effects on renal events [25, 26]. Similarly, a recent meta-analysis demonstrated that SGLT2i could potentially provide clinical benefits by reducing UACR [27]. Herein, we clearly demonstrated that 6 months dapagliflozin therapy produced significant renoprotective effects such as reducing UACR and uNAG, but without decreasing eGFR, in the daily clinical practice of T2DM treatment. The underlying mechanism reducing UACR might be the improvement of tubuloglomerular feedback, leading to recovery of GFR homeostasis in the glomerulus [11]. Furthermore, the SGLT2i-induced glucose lowering, improvement of insulin resistance, and blood pressure lowering with sodium excretion and reduction of extracellular volume might play a positive role in the mechanisms of improving UACR [27]. Moreover, we demonstrated for the first time that dapagliflozin significantly decreased uNAG, which is a renal tubular damage marker [5]. In patients with T2DM, tubulointerstitial injury is both a key pathogenic feature of DN/DKD and an important predictor of future renal events [6]. Expression of SGLT2 protein [28] and glucose/sodium-reabsorption with ATP consumption are greatly enhanced in renal proximal tubular epithelial cells in patients with T2DM [13, 14]. As SGLT2i blocked the accelerated reabsorption of urinary glucose and sodium, SGLT2i could protect the kidney against stress from the overloaded trafficking of glucose and sodium and improve energy and oxygen balance in renal proximal tubular cells [12-14], leading to recovery of the tubulointerstitial area [15]. Resulting from the dual improving impacts of dapagliflozin on both UACR and uNAG, dapagliflozin may have particularly beneficial effects on not only glomerular injury but also tubulointerstitial damage in DN/DKD.
Excess calorie intake, obesity, and ectopic fat accumulation are important pathogenic factors of T2DM [7]. Recently, the concept of “fatty kidney” as an obesity-related kidney disease has been proposed [8]. Ectopic fat deposition in the kidney can cause glomerular and tubulointerstitial injury by accelerating inflammation and oxidative stress in DN/DKD [7]. Obesity and weight gain are correlated with increasing albuminuria and worsening renal function [8], and reducing body weight and body fat mass has been recognized as important and effective clinical strategies for practical management of T2DM and DN/DKD [9]. We demonstrated that dapagliflozin therapy significantly improved UACR and reduced TBFM and the percent decrease in UACR was significantly correlated with reduction of TBFM, but not body weight, in the present study, indicating the anti-diabetic therapy accompanied by decreasing body fat mass can exhibit clinical benefits on renal protection in T2DM. Furthermore, we report for the first time that dapagliflozin produced significant increases in kidney radiation attenuation by abdominal CT, indicating the reduction of kidney fat deposition [22]. In animal experiments, dapagliflozin significantly decreased ectopic fat accumulation, inflammation, and oxidative stress in the kidney of mice fed a Western diet [29]. The baseline values of kidney radiation attenuation were lower than those previously reported for healthy subjects [21], suggesting increased kidney ectopic fat accumulation in our patients with uncontrolled T2DM. The current study did not show a significant correlation between the treatment-induced improvement in UACR and changes in the kidney radiation attenuation. As the clinical significance of the treatment-induced changes in kidney radiation attenuation is uncertain at present, future studies are needed to clarify the implication of the increasing kidney radiation attenuation.
Interestingly, we demonstrated for the first time that dapagliflozin significantly increased kidney length compared with the non-SGLT2i group after 6 months of treatment. In patients with CKD, the kidney size is positively correlated with eGFR as the kidney function and the impairment of renal function is associated with kidney atrophy and size reduction [30]. There are no previous clinical reports presenting anti-diabetic treatment-induced changes in kidney size in T2DM. In patients with DM, hypertrophy of kidneys was observed in the early disease phase with hyper-filtration [31]. The participants of the present study had uncontrolled glucose levels and T2DM, with an average decreased eGFR of 76 mL/min/1.73m2 and median diabetes duration of more than 10 years. Thus, they already had moderately impaired GFR rather than hyper-filtration, indicating that their kidneys might transition to the atrophic stage from the early hypertrophic stage. In the kidneys of patients with DN/DKD, glomerular lesion development, tubular damage, tubulointerstitial atrophy, fibrosis, and inflammation were histologically observed [32, 33]. Tubulointerstitium makes up approximately 90% of kidney volume [34]. The progression of tubulointerstitium abnormalities could lead to tubular atrophy, apoptosis, and fibrosis, possibly leading to the kidney size reduction. Because SGLT2i might reduce the glucose/sodium reabsorption-dependent metabolic stress in tubulointerstitium [6, 14], it could be speculated that dapagliflozin improves renal tubulointerstitial atrophy associated with the progression of CKD stage in T2DM. Notably, SGLT2i both protected against and improved these pathogenic changes in animal models [28, 29]. Regarding the changes in kidney size in animal experiments, knockout of SGLT2 did not significantly effect renal growth and kidney size in a mouse model [35], and several studies demonstrated that SGLT2i tended to increase kidney weight (kidney weight/body weight) in diet-induced obese mice [36-38] and sub-totally nephrectomized rats [39]. To determine the possible clinical effects of SGLT2i on renal protection through increased kidney size, further studies are required.
SGLT2i was shown to elevate serum Mg levels in a previous meta-analysis [40], which was confirmed in the present study. In contrast, a previous study showed that the serum Mg levels were significantly decreased in patients with DM [41, 42]. Lower serum Mg levels have a significant clinical correlation with death from cardiovascular events, arrhythmia, heart failure, coronary spasm, and coronary artery disease [43]. We speculate that the elevation of serum Mg levels by dapagliflozin might in part be involved in the stabilization of cardiovascular homeostasis, thereby possibly leading to a reduction in occurrence of the cardiovascular events.
The present results unexpectedly revealed an intriguing connection between the glucose-elevating hormone glucagon and DN/DKD. Specifically, the treatment-induced changes in UACR were significantly and positively correlated with changes in fasting serum levels of glucagon, suggesting the possible usefulness of glucagon-inhibition in renal protection in the future. It has been previously reported that fasting glucagon levels were significantly elevated in patients with DN/DKD as disease severity progressed [44]. Furthermore, chronic glucagon administration exacerbated albuminuria in a T2DM mouse model [45]. Dapagliflozin has been reported to suppress glucagon signaling [46] and a recent clinical study with dapagliflozin demonstrated a significant decrease in fasting glucagon levels after 6 months therapy [47]. Our present results also showed a tendency towards treatment-induced decreases in fasting glucagon levels during the 6 months glucose-lowering therapy. Further studies are required to determine the precise role of glucagon on renal function in T2DM.
There are several limitations to the current study including the small number of participants, relatively short study period, open-label design, non-random allocation, and possible bias in patient selection. This study was the organ-specific arm of our previous study [16] and the majority of participants in the present study had normoalbuminuria (UACR < 30 mg/g). More detailed and longer studies with mainly DN/DKD patients are required to validate the effects of SGLT2i treatment on improving renal function as demonstrated in the present study. The beneficial effects of SGLT2i on renal function and morphology should be confirmed in larger, randomized clinical studies. This study included patients with uncontrolled T2DM (HbA1c > 7.0%) as well as patients without severe obesity (BMI > 35 kg/m2). Further studies evaluating the effects of dapagliflozin on renal function should be considered in patients with T2DM with a normal weight, severe obesity, older age, and well-controlled DM. In addition, the detailed molecular mechanisms underlying the dapagliflozin-induced improvement in the renal function could not be determined in the present clinical study and should be investigated further.
Conclusions
The add-on treatment of a SGLT2 inhibitor dapagliflozin exhibits significant renoprotective effects including reducing UACR and uNAG and increasing kidney size and radiation attenuation associated with a reduction in body fat mass in patients with uncontrolled T2DM.
Acknowledgments
The authors thank Noriko Matsuda, Mayumi Shimizu, and Kazue Furuta for their skillful technical assistance. We thank Simon Teteris, PhD, from the Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Conflict of Interest
Dr. Seigo Sugiyama is on the Speaker’s Bureau of MSD, Inc., and AstraZeneca Pharmaceuticals LP. Dr. Hideaki Jinnouchi has received consultant fees from Sanofi U.S., Novo Nordisk, Inc., and Eli Lilly Japan K.K. Dr. Hideaki Jinnouchi is also on the Speaker’s Bureau of MSD, Inc., Astellas Pharma US, Inc., Sanofi U.S., Novo Nordisk Pharma, Ltd., Taishi Toyama Pharmaceutical, Co., Ltd. Daiichi-Sankyo Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Eli Lilly Japan K.K., Boehringer Ingelheim Pharmaceuticals, Inc., Takeda Pharmaceutical Company Limited, and AstraZeneca Pharmaceuticals LP. All other authors declare that they have no conflicts of interest.
Grant Support
None.
| References | ▴Top |
- Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, Malanda B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018:pii:S0168-8227(18)30203-1.
doi - Duru OK, Middleton T, Tewari MK, Norris K. The landscape of diabetic kidney disease in the United States. Curr Diab Rep. 2018;18(3):14.
doi pubmed - Doshi SM, Friedman AN. Diagnosis and management of type 2 diabetic kidney disease. Clin J Am Soc Nephrol. 2017;12(8):1366-1373.
doi pubmed - Tsimihodimos V, Filippatos TD, Filippas-Ntekouan S, Elisaf M. Renoprotective effects of SGLT2 inhibitors: beyond glucose reabsorption inhibition. Curr Vasc Pharmacol. 2017;15(2):96-102.
doi pubmed - Fiseha T, Tamir Z. Urinary markers of tubular injury in early diabetic nephropathy. Int J Nephrol. 2016;2016:4647685.
doi pubmed - Zeni L, Norden AGW, Cancarini G, Unwin RJ. A more tubulocentric view of diabetic kidney disease. J Nephrol. 2017;30(6):701-717.
doi pubmed - Mahmoodnia L, Tamadon MR. On the occasion of world kidney day 2017; obesity and its relationship with chronic kidney disease. J Nephropathol. 2017;6(3):105-109.
doi pubmed - de Vries AP, Ruggenenti P, Ruan XZ, Praga M, Cruzado JM, Bajema IM, D’Agati VD, et al. Fatty kidney: emerging role of ectopic lipid in obesity-related renal disease. Lancet Diabetes Endocrinol. 2014;2(5):417-426.
doi - Docherty NG, Canney AL, le Roux CW. Weight loss interventions and progression of diabetic kidney disease. Curr Diab Rep. 2015;15(8):55.
doi pubmed - Vallon V, Thomson SC. Targeting renal glucose reabsorption to treat hyperglycaemia: the pleiotropic effects of SGLT2 inhibition. Diabetologia. 2017;60(2):215-225.
doi pubmed - Thomson SC, Rieg T, Miracle C, Mansoury H, Whaley J, Vallon V, Singh P. Acute and chronic effects of SGLT2 blockade on glomerular and tubular function in the early diabetic rat. Am J Physiol Regul Integr Comp Physiol. 2012;302(1):R75-83.
doi pubmed - O’Neill J, Fasching A, Pihl L, Patinha D, Franzen S, Palm F. Acute SGLT inhibition normalizes O2 tension in the renal cortex but causes hypoxia in the renal medulla in anaesthetized control and diabetic rats. Am J Physiol Renal Physiol. 2015;309(3):F227-234.
doi pubmed - Korner A, Eklof AC, Celsi G, Aperia A. Increased renal metabolism in diabetes. Mechanism and functional implications. Diabetes. 1994;43(5):629-633.
doi pubmed - Satirapoj B. Sodium-Glucose Cotransporter 2 Inhibitors with Renoprotective Effects. Kidney Dis (Basel). 2017;3(1):24-32.
doi pubmed - Sano M, Takei M, Shiraishi Y, Suzuki Y. Increased hematocrit during sodium-glucose cotransporter 2 inhibitor therapy indicates recovery of tubulointerstitial function in diabetic kidneys. J Clin Med Res. 2016;8(12):844-847.
doi pubmed - Sugiyama S, Jinnouchi H, Kurinami N, Hieshima K, Yoshida A, Jinnouchi K, Nishimura H, et al. Dapagliflozin Reduces Fat Mass without Affecting Muscle Mass in Type 2 Diabetes. J Atheroscler Thromb. 2017. DOI:10.5551/jat.40873.
pubmed - Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53(6):982-992.
doi pubmed - Tanaka T, Okamura T, Miura K, Kadowaki T, Ueshima H, Nakagawa H, Hashimoto T. A simple method to estimate populational 24-h urinary sodium and potassium excretion using a casual urine specimen. J Hum Hypertens. 2002;16(2):97-103.
doi pubmed - Yoshizumi T, Nakamura T, Yamane M, Islam AH, Menju M, Yamasaki K, Arai T, et al. Abdominal fat: standardized technique for measurement at CT. Radiology. 1999;211(1):283-286.
doi pubmed - Kang KY, Lee YJ, Park SC, Yang CW, Kim YS, Moon IS, Koh YB, et al. A comparative study of methods of estimating kidney length in kidney transplantation donors. Nephrol Dial Transplant. 2007;22(8):2322-2327.
doi pubmed - Georgiades CS, Moore CJ, Smith DP. Differences of renal parenchymal attenuation for acutely obstructed and unobstructed kidneys on unenhanced helical CT: a useful secondary sign? AJR Am J Roentgenol. 2001;176(4):965-968.
doi pubmed - Ferguson CM, Eirin A, Michalak GJ, Hedayat AF, Abumoawad A, Saad A, Zhu X, et al. Intrarenal fat deposition does not interfere with the measurement of single-kidney perfusion in obese swine using multi-detector computed tomography. J Cardiovasc Comput Tomogr. 2018;12(2):149-152.
- Malavolti M, Mussi C, Poli M, Fantuzzi AL, Salvioli G, Battistini N, Bedogni G. Cross-calibration of eight-polar bioelectrical impedance analysis versus dual-energy X-ray absorptiometry for the assessment of total and appendicular body composition in healthy subjects aged 21-82 years. Ann Hum Biol. 2003;30(4):380-391.
doi pubmed - Kurinami N, Sugiyama S, Yoshida A, Hieshima K, Miyamoto F, Kajiwara K, Jinnouchi T, et al. Correlation of body muscle/fat ratio with insulin sensitivity using hyperinsulinemic-euglycemic clamp in treatment-naive type 2 diabetes mellitus. Diabetes Res Clin Pract. 2016;120:65-72.
doi pubmed - Cherney DZI, Zinman B, Inzucchi SE, Koitka-Weber A, Mattheus M, von Eynatten M, Wanner C. Effects of empagliflozin on the urinary albumin-to-creatinine ratio in patients with type 2 diabetes and established cardiovascular disease: an exploratory analysis from the EMPA-REG OUTCOME randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017;5(8):610-621.
doi - Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644-657.
doi pubmed - Xu L, Li Y, Lang J, Xia P, Zhao X, Wang L, Yu Y, et al. Effects of sodium-glucose co-transporter 2 (SGLT2) inhibition on renal function and albuminuria in patients with type 2 diabetes: a systematic review and meta-analysis. PeerJ. 2017;5:e3405.
doi pubmed - Wang XX, Levi J, Luo Y, Myakala K, Herman-Edelstein M, Qiu L, Wang D, et al. SGLT2 protein expression is increased in human diabetic nephropathy: SGLT2 protein inhibition decreases renal lipid accumulation, inflammation, and the development of nephropathy in diabetic mice. J Biol Chem. 2017;292(13):5335-5348.
doi pubmed - Wang D, Luo Y, Wang X, Orlicky DJ, Myakala K, Yang P, Levi M. The sodium-glucose cotransporter 2 inhibitor dapagliflozin prevents renal and liver disease in western diet induced obesity mice. Int J Mol Sci. 2018;19(1):E137.
doi - Gupta S, Singh AH, Shabbir A, Hahn PF, Harris G, Sahani D. Assessing renal parenchymal volume on unenhanced CT as a marker for predicting renal function in patients with chronic kidney disease. Acad Radiol. 2012;19(6):654-660.
doi pubmed - Zerbini G, Bonfanti R, Meschi F, Bognetti E, Paesano PL, Gianolli L, Querques M, et al. Persistent renal hypertrophy and faster decline of glomerular filtration rate precede the development of microalbuminuria in type 1 diabetes. Diabetes. 2006;55(9):2620-2625.
doi pubmed - Fioretto P, Mauer M. Histopathology of diabetic nephropathy. Semin Nephrol. 2007;27(2):195-207.
doi pubmed - Lin YC, Chang YH, Yang SY, Wu KD, Chu TS. Update of pathophysiology and management of diabetic kidney disease. J Formos Med Assoc. 2018. pii:S0929-6646(17)30803-3.
doi pubmed - Gilbert RE, Cooper ME. The tubulointerstitium in progressive diabetic kidney disease: more than an aftermath of glomerular injury? Kidney Int. 1999;56(5):1627-1637.
doi pubmed - Vallon V, Rose M, Gerasimova M, Satriano J, Platt KA, Koepsell H, Cunard R, et al. Knockout of Na-glucose transporter SGLT2 attenuates hyperglycemia and glomerular hyperfiltration but not kidney growth or injury in diabetes mellitus. Am J Physiol Renal Physiol. 2013;304(2):F156-167.
doi pubmed - Xu L, Nagata N, Nagashimada M, Zhuge F, Ni Y, Chen G, Mayoux E, et al. SGLT2 inhibition by empagliflozin promotes fat utilization and browning and attenuates inflammation and insulin resistance by polarizing M2 macrophages in diet-induced obese mice. EBioMedicine. 2017;20:137-149.
doi pubmed - Nagata T, Fukuzawa T, Takeda M, Fukazawa M, Mori T, Nihei T, Honda K, et al. Tofogliflozin, a novel sodium-glucose co-transporter 2 inhibitor, improves renal and pancreatic function in db/db mice. Br J Pharmacol. 2013;170(3):519-531.
doi pubmed - Takagi S, Li J, Takagaki Y, Kitada M, Nitta K, Takasu T, Kanasaki K, et al. Ipragliflozin improves mitochondrial abnormalities in renal tubules induced by a high-fat diet. J Diabetes Investig. 2018. DOI:10.1111/jdi.12802.
- Rajasekeran H, Reich HN, Hladunewich MA, Cattran D, Lovshin JA, Lytvyn Y, Bjornstad P, et al. Dapagliflozin in focal segmental glomerulosclerosis: a combined human-rodent pilot study. Am J Physiol Renal Physiol. 2018;314(3):F412-F422.
doi pubmed - Tang H, Zhang X, Zhang J, Li Y, Del Gobbo LC, Zhai S, Song Y. Elevated serum magnesium associated with SGLT2 inhibitor use in type 2 diabetes patients: a meta-analysis of randomised controlled trials. Diabetologia. 2016;59(12):2546-2551.
doi pubmed - Sales CH, Pedrosa Lde F. Magnesium and diabetes mellitus: their relation. Clin Nutr. 2006;25(4):554-562.
doi pubmed - Ramadass S, Basu S, Srinivasan AR. SERUM magnesium levels as an indicator of status of Diabetes Mellitus type 2. Diabetes Metab Syndr. 2015;9(1):42-45.
doi pubmed - DiNicolantonio JJ, O’Keefe JH, Wilson W. Subclinical magnesium deficiency: a principal driver of cardiovascular disease and a public health crisis. Open Heart. 2018;5(1):e000668.
doi pubmed - Wang X, Yang J, Chang B, Shan C, Xu Y, Zheng M, Wang Y, et al. Glucagon secretion is increased in patients with Type 2 diabetic nephropathy. J Diabetes Complications. 2016;30(3):488-493.
doi pubmed - Li XC, Liao TD, Zhuo JL. Long-term hyperglucagonaemia induces early metabolic and renal phenotypes of Type 2 diabetes in mice. Clin Sci (Lond). 2008;114(9):591-601.
doi pubmed - Wang MY, Yu X, Lee Y, McCorkle SK, Chen S, Li J, Wang ZV, et al. Dapagliflozin suppresses glucagon signaling in rodent models of diabetes. Proc Natl Acad Sci U S A. 2017;114(25):6611-6616.
doi pubmed - Shigiyama F, Kumashiro N, Miyagi M, Ikehara K, Kanda E, Uchino H, Hirose T. Effectiveness of dapagliflozin on vascular endothelial function and glycemic control in patients with early-stage type 2 diabetes mellitus: DEFENCE study. Cardiovasc Diabetol. 2017;16(1):84.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


