| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Original Article
Volume 8, Number 7, July 2016, pages 513-518
Evaluation of the Sympathetic Skin Response to the Dry Needling Treatment in Female Myofascial Pain Syndrome Patients
Ali Veysel Ozdena, Hasan Kerem Alptekina, f, Sina Esmaeilzadehb, Cem Cihanc, Semih Akid, Cihan Aksoyb, Julide Oncue
aBahcesehir Health Sciences Faculty, Physiotherapy and Rehabilitation Department, Istanbul, Turkey
bIstanbul University Medical Faculty, Physical Medicine and Rehabilitation Department, Istanbul, Turkey
cSakarya University Education and Research Hospital, Physical Medicine and Rehabilitation Department, Istanbul, Turkey
dJohn Hopkins Anadolu Hospital, Physical Medicine and Rehabilitation Department, Istanbul, Turkey
eHamidiye Sisli Etfal Education and Research Hospital, Physical Medicine and Rehabilitation Department, Istanbul, Turkey
fCorresponding Author: Hasan Kerem Alptekin, Bahcesehir University Health Sciences Faculty, Vice Dean, Sahrayi Cedid Mah, Batman Sokak, No: 66-68, Yenisahra/Kadikoy, Istanbul, Turkey
Manuscript accepted for publication May 10, 2016
Short title: Myofascial Pain Syndrome
doi: http://dx.doi.org/10.14740/jocmr2589w
| Abstract | ▴Top |
Background: The aim of this study was to evaluate sympathetic nervous system (SNS) activity following dry needling (DN) treatment, by using the sympathetic skin response (SSR) method in female patients diagnosed with myofascial pain syndrome (MPS).
Methods: Twenty-nine MPS patients with trapezius muscle pain and 31 healthy subjects were included in this study. During a single treatment session, DN treatment was applied into trigger points, for a duration of 10 minutes. Healthy patients were subjected to SSR in weeks 1 and 4; whereas the patient group was subjected to SSR 1 week prior to their treatment and in the first, second, third and fourth weeks following the completion of their treatment.
Results: We found diminished latency on both sides. A significantly high algometer measurement (P < 0.05) was observed in the control group. DN treatment was effective in diminishing the visual analog scale (VAS) (P < 0.001), pressure pain threshold (PPT) (P < 0.01), and SSR (P < 0.001). No SSR change was detected in the healthy group after the follow-up period (P > 0.05).
Conclusion: DN is an effective treatment in MPS and trigger point (TP). This original study is the first to deal with the SSR in MPS and weekly SSR trailing, requiring further investigation to solidy findings.
Keywords: Myofascial pain syndrome; Trigger point; Sympathetic skin response; Dry needling
| Introduction | ▴Top |
Myofascial pain syndrome (MPS) can be defined as both a sensory-motor and an autonomic symptom complex [1]. Myofascial trigger points (MTrPs) are a common component of MPS in 30% of individuals, characterized by palpable taut bands within skeletal muscle fibers [2]: local hypersensitive points. Although this is known to be common in individuals with musculoskeletal dysfunction, they often go undiagnosed which may lead to chronic conditions [3].
The etiology of trigger point (TP) formation in muscle and its mechanism of producing somatic symptoms is not fully understood. However, peripheral mechanisms, such as biochemical changes in neuromuscular junctions due to overuse injuries, stand as a common explanation for this formation [2, 4]. Conversely, it has become clear that a majority of chronic musculoskeletal pain cases are characterized by alterations in central nervous system processes and autonomic nervous system activation [2, 5].
Patients with MTrPs may present autonomic symptoms, such as sweating, pilomotor activity, changes in skin temperature, lacrimation and salivation [6]. Furthermore, sympathetic nervous system activity increases motor activity, leading to muscle pain in MTrPs [7-9].
In regards to the utilization of different mechanisms, a variety of treatment methods are available: modified proprioceptive neuromuscular facilitation stretching [10], manual compression [11, 12], tricyclic antidepressants [13, 14], topical and injectable form of thiocolchicoside [15, 16], tizanidine [17], botulinum toxin injection of lidocaine [9, 16, 18], TENS [18], US with therapeutic intensity [4], cold spray and stretch [1, 19], ischemic compression [11], and superficial dry needling (DN) [16, 19-21]. DN stands as a popular, yet, invasive method employed for the treatment of MTrPs [2, 16]. Following thorough investigation, it is evident that only a single study focusing on the effects of DN on neuromuscular junction response (NMRJ) and autonomic responses in a population with MTrPs exists; however, no results were presented in this study. Thus, our study aimed to investigate whether deep DN treatment would alter sympathetic nervous system activity; evaluating sympathetic skin responses. This study stands as the first to attempt interpreting and presenting results in this area of study.
| Patients and Methods | ▴Top |
A prospective controlled study was conducted in a single center, Istanbul University, Medical Faculty, Department of Physical Medicine and Rehabilitation, between June 2011 and September 2011. Informed consent form samples for patient evaluation and follow-up were presented to the Istanbul Faculty of Medicine Ethics Committee, Istanbul University.
Thirty-two female patients who met all inclusion criteria were recruited from Istanbul University, Istanbul Faculty of Medicine, Physical Medicine and Rehabilitation Department Outpatient Clinic. The control group consisted of 33 healthy women in the same age group. Inclusion and exclusion criteria for this study are presented in Table 1.
 Click to view | Table 1. Inclusion and Exclusion Criteria |
In accordance with the standard set by the Ethics Committee of Istanbul University, demographical information of patients was recorded: age, height, occupation, marital status and level of education. Medical history was attained from all participants prior to recruitment. Musculoskeletal and neurological examination were performed by the clinician.
Following alcohol sterilization of the skin area covering the trapezius muscle, TPs were subjected to deep DN. No more than six needles (three right and three left) were applied in a single treatment session. Depending on the thickness of skin and subcutaneous tissue, acupuncture needles sized 0.25 × 40 mm or 0.25 × 25 mm were selected. Needles were winded at 10 min to re-create the stimulus and were then removed after 20 min. Treatments were scheduled once a week, equating to a total of three sessions for the entire treatment. No concommitant medical or physical therapies were allowed.
The outcome measures included sympathetic skin responses (SSR), pain intensity (visual analog scale (VAS)) and pressure pain threshold (PPT) obtained via an algometer, which were taken and recorded before and immediately after DN treatment (fourth week).
Pain intensity was self rated by the participants on a 0 - 10 numerical rating scale: with 0 showing no pain and 10 representing severe pain.
The physiatrist used a pressure algometer (Wagner Pain Test, Model FPK/FPN Mechanical Algometer; USA) to measure the PPT. Participants were debriefed before the treatment.
In order to measure PPT, the most painful area in the upper trapezius MTrP was identified while the patients were situated in a comfortable sitting position. The algometer’s metal rod was then pressed perpendicular to the skin over the identified TPs in the upper trapezius muscle. The applied pressure was increased at a rate of 1 kg/cm2. Participants in the control group were asked to notify the examiner by stating “yes” once pain or discomfort was felt. Whereas, participants in the treatment group (MTrPs group) were required to report pain intensity or discomfort as soon as it was experienced. This procedure was repeated three times in 40 s intervals. The average of the three values was determined as the PPT.
An electromyography instrument (2003 Nihon Kohden The Neuropack Map-9200/9300 series EP/EMG Measure System) with surface electrodes was utilized to assess SSR (impulse time 0.2 ms; severity of impulse 15 mA with interval of more than 30 s). During the assessment of SSR, a maximum of 10 impulses were applied unilaterally. Patients that did not respond to a consecutive of 10 impulses were recorded as “no sympathetic response”. The shortest latency and highest amplitudes were recorded to allow sympathetic response calculations. The measurements were carried out in a silent, dark room with a temparature of 25 °C, and patients were in a supine position with their eyes closed.
SSR was recorded following a single square-wave electric stimulus over the median nerve at the wrist. The recording and reference electrodes were placed on the palm and on the back of the hand, respectively.
In our study, SPSS version 17.0 was utilized for statistical analysis of the data (Statistical Package for the Social Sciences, Chicago, IL, USA). The average value for evaluation parameters, standard deviation, minimum and maximum values were calculated. Initial evaluation of homogenity between the two groups was accomplished via independent samples t and Chi-square tests.
Wilcoxon signed ranks tests were used to compare changes between groups; whereas, Mann-Whitney and Chi-square tests were employed for inter-group comparisons. Correlation between measurements was assessed using Spearman’s Rho test, considering variable character and distribution.
The results were evaluated in a 95% confidence interval: P < 0.05 was considered statistically significant.
| Results | ▴Top |
Initially, 32 patients who passed the inclusion criteria and 33 control participants were enrolled in the study. Three participants from the patient group and two from the control group were excluded from the study, due to a lack of attendance. The study was completed with a total of 29 patients and 31 controls.
The mean age was 28.31 ± 5.13 (mean ± SD) years in the patient group and 27.39 ± 4.93 (mean ± SD) in the control group. Both of the groups were similar in terms of age distribution (P > 0.05). The mean BMI was measured at 22.78 ± 4.19 kg/m2 (mean ± SD) in patients and 20.85 ± 2.70 kg/m2 (mean ± SD) in the control group. There was a significant difference in BMI between the two groups (P < 0.05). No significant difference between the two groups in terms of dominant hand, drug use, disease, smoking and alcohol addiction, trauma, and exercise status was observed (P > 0.05).
Comparison of algometric mean values for the right trapezius muscle TPs, between patients and participants in the control group, was carried out. Results revealed an algometric value of 5.16 ± 1.73 kg/cm2 (mean ± SD) for the control group and 4.32 ± 1.35 kg/cm2 (mean ± SD) for the patient group: a value of P < 0.05 illustrated statistical significance for this comparison.
Additionally, the same analysis was carried out for the left trapezius muscle TPs. Participants in the control group showed a mean algometric value of 5.29 ± 1.65 kg/cm2 (mean ± SD), whereas those in the patient group revealed a value of 4.36 ± 1.21 kg/cm2 (mean ± SD), with significant difference between the groups (P < 0.05).
In regards to pre- and post-treatment algometric values in the patient group, a statistically significant (P < 0.05) improvement in the right side was evident; yet, no change was identified in the left trapezius muscle post-treatment. However, pre-treatment algometric average values for the right trapezius muscle significantly increased from 4.32 ± 1.35 kg/cm2 to 4.67 ± 1.19 kg/cm2 and 4.36 ± 1.21 kg/cm2 to 4.67 ± 1.29 kg/cm2 for the left muscle (Table 2).
 Click to view | Table 2. Evaluation of the Correlation Between Measurements in the Patient Group |
General pain severity assessed by VAS in the pre-treatment (PT) patient group was 6.82 ± 1.462 (mean ± SD) after dry needling treatment (AT): measured in week 4 as 3.58 ± 2.622 (mean ± SD). VAS scores showed a significant improvement in the patient group (P < 0.05). In relation to SSR values, right side amplitudes were significantly higher, yet, latencies were significantly longer for both sides in the patient group, compared to those in the control group (P < 0.05).
Sympathetic responses in the patient group showed lower amplitudes (Fig. 1) and prolonged latencies post-treatment (Fig. 2) when compared to pre-treatment measures done on both sides (P < 0.05). In contrast, no significant changes were observed in SSRs for participants in the control group (P > 0.05).
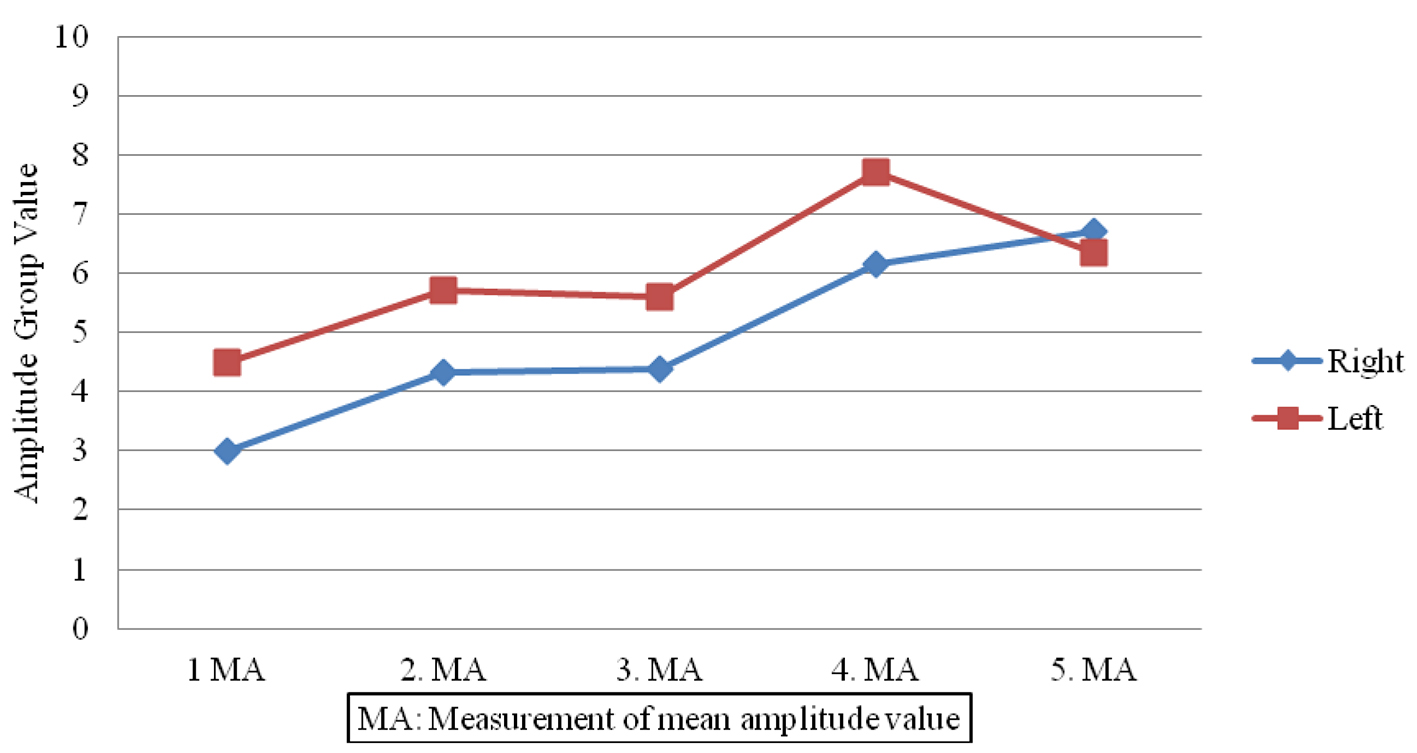 Click for large image | Figure 1. Amplitude value differences in patient group. |
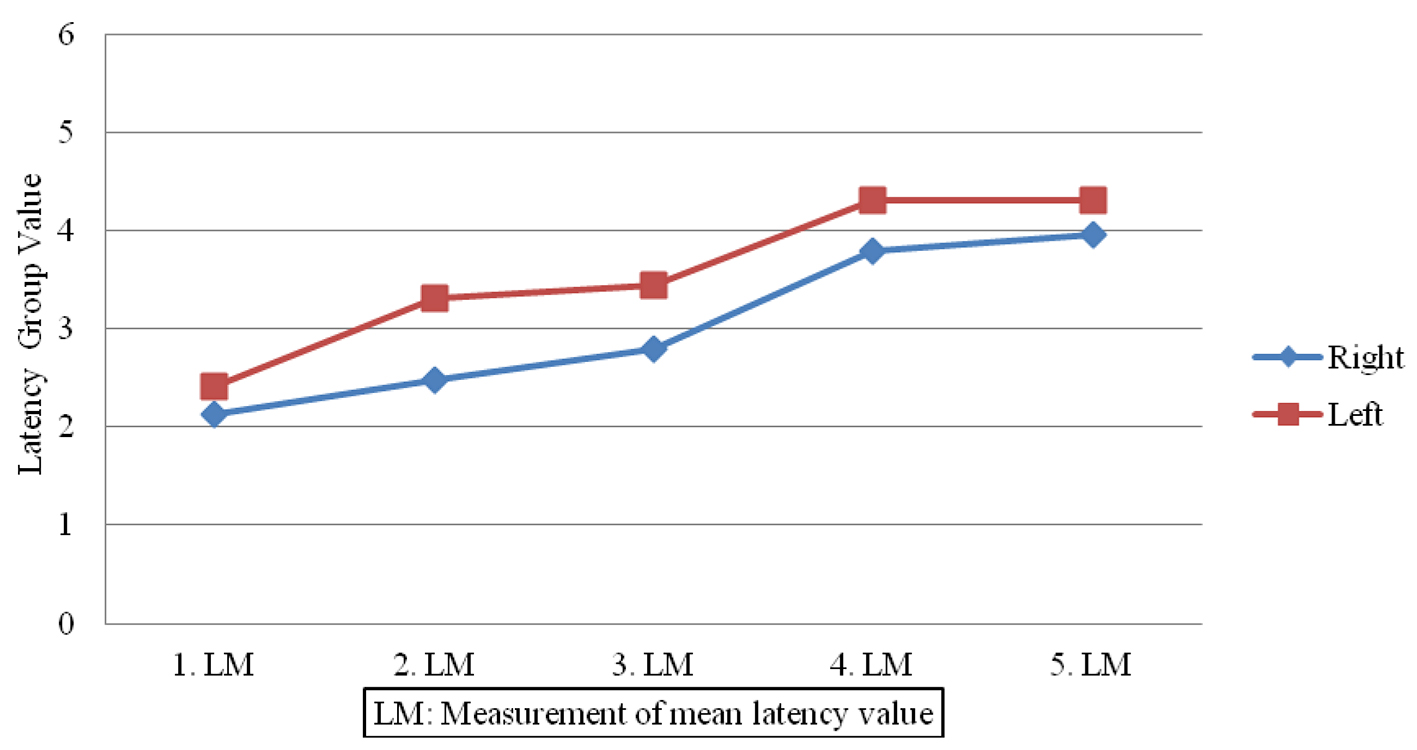 Click for large image | Figure 2. Latency value differences in patient group. |
During treatment with DN and follow-up after 1 week, no adverse effects were observed.
| Discussion | ▴Top |
As SSR is strongly influenced by the autonomic nervous system, it could be used for active TP evaluation and treatment, as well as for peripheral neuropathy treatment accompanying autonomic nervous system disorders. Additionally, SSR may also be utilized in the diagnosis of reflex sympathetic dystrophy [2, 22]. In 50% of multiple sclerosis cases, SSR abnormalities were detected, possibly due to lesions presented in the central sympathetic way. Prolonged latency and amplitude reduction was found in amyotrophic lateral sclerosis, Parkinson’s disease and stroke patients [23].
DN is an effective and reliable method used to treat MPS by reducing VAS and the number of active TPs, with a notible reduction in the conversion of active TPs to latent TPs [24, 25]. As a result, reduced pain by dorsal neuron desensitization, without impeding on existing pathology, is possible [7, 8].
Results indicate that DN treatment for MPS was effective in the reduction of both general pain and the number of TPs. Following DN treatment for 20 min once a week over 3 weeks, the average VAS score was reduced from 6.82 ± 1.46 to 3.58 ± 2.62 after treatment and at the fourth week control (P < 0.001). Similarly, the number of TPs decreased from 5.17 ± 1.19 to 4.38 ± 1.86 (P < 0.01).
Comparisons made between TP sensitivity in the patient group with the control group highlight the average algometric measurement of TP located on the upper fibers of the trapezius muscle (P < 0.05). Although DN treatment increased the pain threshold on both sides, this increase was not statistically significant on the left side.
Studies to date have indicated a rise in the pressure pain threshold with TP treatment. Srbely’s study showed that following an injection to the supraspinatus muscle (C4-C5),the pain threshold levels for patients were elevated at the infraspinatus (C5-C6); yet, no change was evident at the gluteus medius muscles (L4-L5-S1) [26].
Edwards and Knowles reported a rise in the algometric value in a patient group via superficial DN and stretching exercises [3]. Pressure pain threshold rises were also observed at the treated TP as well as at satellite TPs located on the pain area [27, 28]. Initially, pressure pain threshold for TPs was found low, but, increased following DN treatment.
Investigation of the SSR measurements for both the control and patient group showed a significant difference between the right amplitude and both side (right and left) latency measurements (P < 0.05). No statistical significance was observed for the left side amplitude measurements, which may have resulted due to habituation, as the right arm was the primary arm measured.
Neither group showed statistical significance between the first and fourth week of treatment (P < 0.001). Insignificance was also evident when SSR measurement differences were compared between the two groups (P ≤ 0.001).
Patient satisfactions in means of treatment adequacy were successfully accomplished, as a prolonged treatment period was employed; stardization was achieved. Another factor that increases the power of this study is that participants had no comorbid diseases.
Blinding was not possible because SSR measurements and treatment controls were conducted by the same clinician. SSR measurements were made twice in the patient group, which may have led to habituation.
Autonomic symptom questions such as increased salivation, changes in skin temperature, sweating, piloerection, impaired proprioception, erythema, etc. were not included in the questionnaires. This may either support or underestimate SSR. Additionally, differences between menstrual cycles could have been standardized.
Following thorough research, only a single study has focused on neurophysiological effects of DN in patients with upper trapezius myofascial TPs, with no results presented [2]. After detailed analysis, our findings confirm that an increase in sympathetic nervous system activity is present in patients with MPS and TP.
Taking habituation and various factors (such as menstrual cycle, patient emotional state, etc.) into consideration, this study suggests that the hyperactivity of the sympathetic nervous system plays an important role in the pathophysiology of MPS and TP. Studies of longer duration, including larger patient numbers, must be performed in order to obtain more robust results regarding CNS function and activity in pathophysiology of MPS and TP.
Conflicts of Interest
None.
| References | ▴Top |
- Lavelle ED, Lavelle W, Smith HS. Myofascial trigger points. Med Clin North Am. 2007;91(2):229-239.
doi pubmed - Abbaszadeh-Amirdehi M, Ansari NN, Naghdi S, Olyaei G, Nourbakhsh MR. The neurophysiological effects of dry needling in patients with upper trapezius myofascial trigger points: study protocol of a controlled clinical trial. BMJ Open. 2013;3(5).
doi pubmed - Edwards J, Knowles N. Superficial dry needling and active stretching in the treatment of myofascial pain - a randomised controlled trial. Acupunct Med. 2003;21(3):80-86.
doi pubmed - Unalan H, Majlesi J, Aydin FY, Palamar D. Comparison of high-power pain threshold ultrasound therapy with local injection in the treatment of active myofascial trigger points of the upper trapezius muscle. Arch Phys Med Rehabil. 2011;92(4):657-662.
doi pubmed - Nijs J, Paul van Wilgen C, Van Oosterwijck J, van Ittersum M, Meeus M. How to explain central sensitization to patients with 'unexplained' chronic musculoskeletal pain: practice guidelines. Man Ther. 2011;16(5):413-418.
doi pubmed - Cummings M, Baldry P. Regional myofascal pain: diagnosis and management. Best Practice & Research Clinical Rheumatology. 2007;21:367-387.
doi pubmed - Majlesi J, Unalan H. Effect of treatment on trigger points. Curr Pain Headache Rep. 2010;14(5):353-360.
doi pubmed - Ceccherelli F, Gioioso L, Casale R, Gagliardi G, Ori C. Neck pain treatment with acupuncture: does the number of needles matter? Clin J Pain. 2010;26(9):807-812.
doi pubmed - Kamanli A, Kaya A, Ardicoglu O, Ozgocmen S, Zengin FO, Bayik Y. Comparison of lidocaine injection, botulinum toxin injection, and dry needling to trigger points in myofascial pain syndrome. Rheumatol Int. 2005;25(8):604-611.
doi pubmed - Trampas A, Kitsios A, Sykaras E, Symeonidis S, Lazarou L. Clinical massage and modified Proprioceptive Neuromuscular Facilitation stretching in males with latent myofascial trigger points. Phys Ther Sport. 2010;11(3):91-98.
doi pubmed - Bron C, de Gast A, Dommerholt J, Stegenga B, Wensing M, Oostendorp RA. Treatment of myofascial trigger points in patients with chronic shoulder pain: a randomized, controlled trial. BMC Med. 2011;9:8.
doi pubmed - Vernon H, Schneider M. Chiropractic management of myofascial trigger points and myofascial pain syndrome: a systematic review of the literature. J Manipulative Physiol Ther. 2009;32(1):14-24.
doi pubmed - Robinson JP, Arendt-Nielsen L. Muscle Pain Sydromes. Chapter 43. Braddom RL, Buschbacher RM, Chan L, Kowalske KJ, Laskowski ER, Matthews DJ, Ragnarrson KT. Physical Medicine and Rehabilitation. Fourth Edition. 2010; 971-1003.
- Cohen SP, Mullings R, Abdi S. The pharmacologic treatment of muscle pain. Anesthesiology. 2004;101(2):495-526.
doi pubmed - Ketenci A, Basat H, Esmaeilzadeh S. The efficacy of topical thiocolchicoside (Muscoril) in the treatment of acute cervical myofascial pain syndrome: a single-blind, randomized, prospective, phase IV clinical study. Agri. 2009;21(3):95-103.
pubmed - Ay S, Evcik D, Tur BS. Comparison of injection methods in myofascial pain syndrome: a randomized controlled trial. Clin Rheumatol. 2010;29(1):19-23.
doi pubmed - Borg-Stein J. Treatment of fibromyalgia, myofascial pain, and related disorders. Phys Med Rehabil Clin N Am. 2006;17(2):491-510, viii.
doi pubmed - Gul K, Onal SA. [Comparison of non-invasive and invasive techniques in the treatment of patients with myofascial pain syndrome]. Agri. 2009;21(3):104-112.
pubmed - Baldry P. Management of myofascial trigger point pain. Acupunct Med. 2002;20(1):2-10.
doi pubmed - Srbely JZ. New trends in the treatment and management of myofascial pain syndrome. Curr Pain Headache Rep. 2010;14(5):346-352.
doi pubmed - Baldry P. Superficial versus deep dry needling. Acupunct Med. 2002;20(2-3):78-81.
doi pubmed - Vetrugno R, Liguori R, Cortelli P, Montagna P. Sympathetic skin response: basic mechanisms and clinical applications. Clin Auton Res. 2003;13(4):256-270.
doi pubmed - Kucera P, Goldenberg Z, Kurca E. Sympathetic skin response: review of the method and its clinical use. Bratisl Lek Listy. 2004;105(3):108-116.
- Ga H, Choi JH, Park CH, Yoon HJ. Dry needling of trigger points with and without paraspinal needling in myofascial pain syndromes in elderly patients. J Altern Complement Med. 2007;13(6):617-624.
doi pubmed - Yoon SH, Rah UW, Sheen SS, Cho KH. Comparison of 3 needle sizes for trigger point injection in myofascial pain syndrome of upper- and middle-trapezius muscle: a randomized controlled trial. Arch Phys Med Rehabil. 2009;90(8):1332-1339.
doi pubmed - Srbely JZ, Dickey JP, Lee D, Lowerison M. Dry needle stimulation of myofascial trigger points evokes segmental anti-nociceptive effects. J Rehabil Med. 2010;42(5):463-468.
doi pubmed - Hsieh YL, Kao MJ, Kuan TS, Chen SM, Chen JT, Hong CZ. Dry needling to a key myofascial trigger point may reduce the irritability of satellite MTrPs. Am J Phys Med Rehabil. 2007;86(5):397-403.
doi pubmed - Tsai CT, Hsieh LF, Kuan TS, Kao MJ, Chou LW, Hong CZ. Remote effects of dry needling on the irritability of the myofascial trigger point in the upper trapezius muscle. Am J Phys Med Rehabil. 2010;89(2):133-140.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


