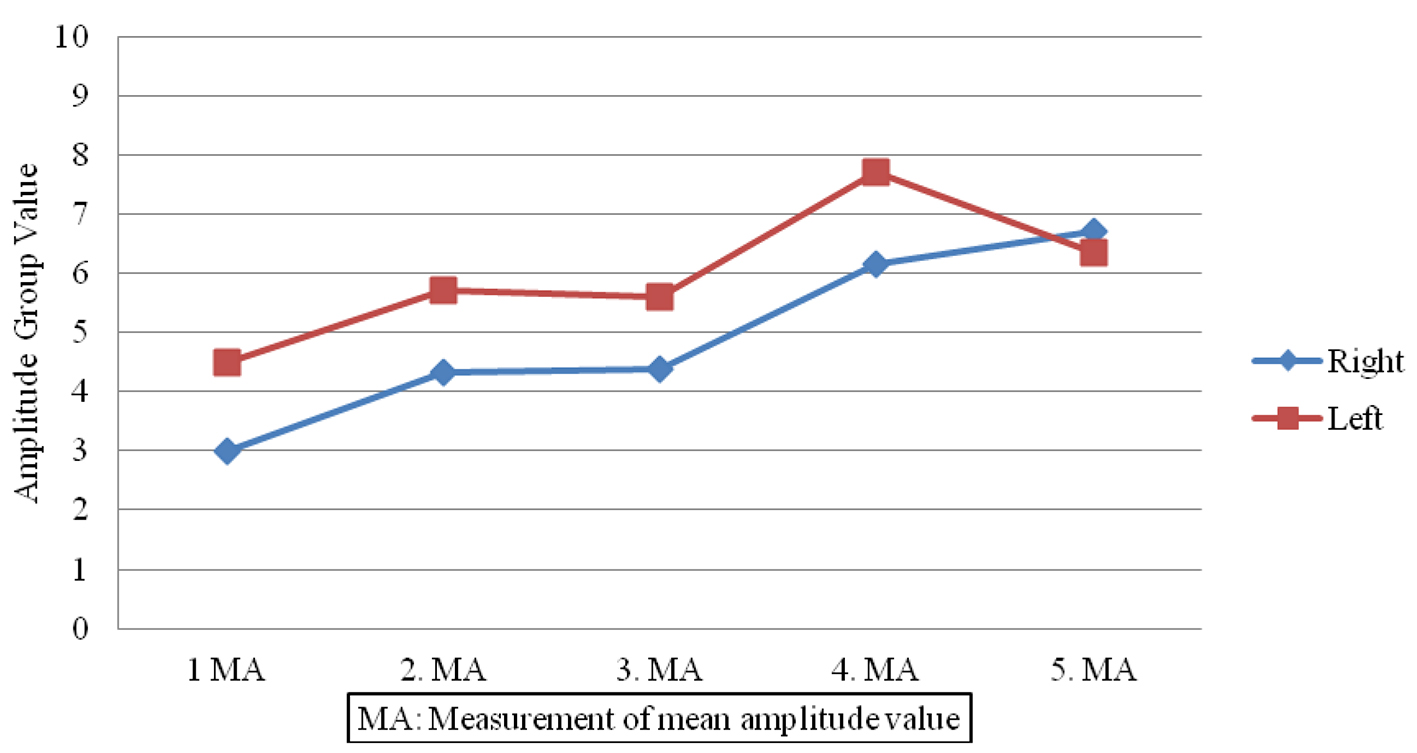
Figure 1. Amplitude value differences in patient group.
| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Original Article
Volume 8, Number 7, July 2016, pages 513-518
Evaluation of the Sympathetic Skin Response to the Dry Needling Treatment in Female Myofascial Pain Syndrome Patients
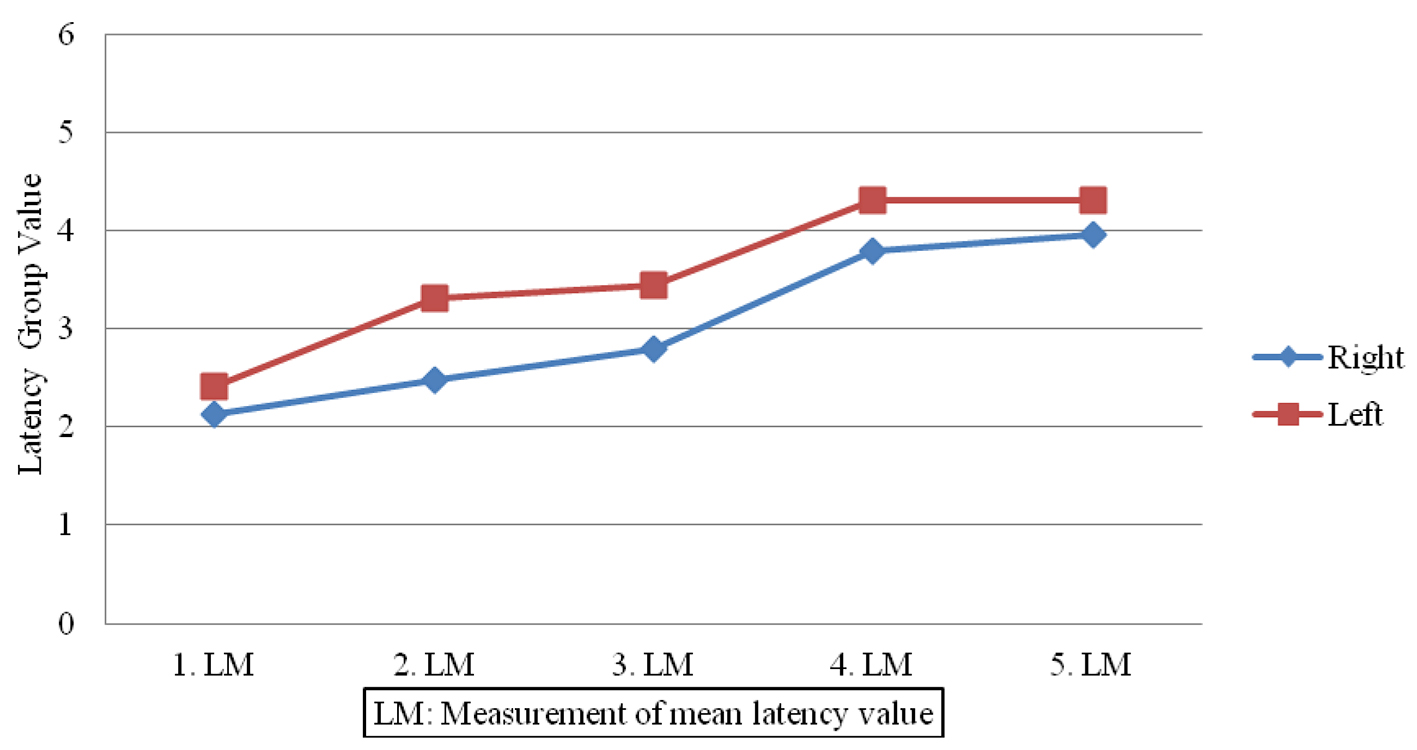
Figures

Tables
| Inclusion criteria |
| 18 - 40 aged female |
| Having regular menstrual cycle |
| Duration of pain longer than 3 months (for patient group) |
| Absence of any pain (for the control group) |
| Exclusion criteria |
| Comorbid conditions that may affect sympathetic skin response answers such as (diabetes mellitus, rheumatoid arthritis, Sjogren’s syndrome, psoriasis and vitiligo, Behcet’s disease, Fabry disease, botulism, primary autoimmune hypothyroidism, reflex sympathetic dystrophy, central nervous system diseases, peripheral nervous system diseases, peripheral vascular disease, entrapment neuropathies) |
| Patients taking antidepressant and anxiolytic drugs |
| Surgical or chemical sympathectomy |
| Major trauma or surgery history of head, neck and upper extremities |
| Scar tissue located in neck, trapezius muscle or in upper extremities that are greater than 2 cm2 and longer than 2 cm |
| Scoliosis greater than 10° which is detected in examination |
| Significantly symptomatic (grade 3 and 4) cervical and dorsal spondylosis |
| Cardiac pace-maker |
| SPerman’s rho | ALIS | ARIS | VASENRF | VASENLF | ALGENRF | ALGENLF | LRIS | LLIS | TNRF | TNLF |
|---|---|---|---|---|---|---|---|---|---|---|
| ALIF: difference between initial and final amplitude measurements on left side; ARIF: difference between initial and final amplitude measurements on right side; VASMDR: the difference between highest VAS scores before and after treatment on the right side; VASMDL: the difference between highest VAS scores before and after treatment on the left side; ALGDR: the difference between lowest algometric values before and after treatment on the right side; ALGDL: the difference between lowest algometric values before and after treatment on the left side; LDR: the difference between first and last latency measurements on right side; LDL: the difference between first and last latency measurements on left side; TPDR: trigger point difference before and after treatment on right side; TPDL: trigger point difference before and after treatment on left side. | ||||||||||
| ALIF | r = -0.372 P = 0.051 | r = 0.427 P = 0.021* | r = -0.244 P = 0.202 | |||||||
| ARIF | r = -0.308 P = 0.104 | r = 0.304 P = 0.109 | r = -0.430 P = 0.020* | |||||||
| VASMDR | r = -0.308 P = 0.104 | r = -0.425 P = 0.022* | r = -0.284 P = 0.136 | r = 0.302 P = 0.111 | ||||||
| VASMDL | r = -0.372 P = 0.051 | r = -0.536 P = 0.003** | r = -0.427 P = 0.024* | r = 0.603 P = 0.001** | ||||||
| ALGDR | r = 0.304 P = 0.109 | r = -0.425 P = 0.022* | r = 0.153 P = 0.429 | r = -0.276 P = 0.147 | ||||||
| ALGDL | r = 0.427 P = 0.021* | r = -0.536 P = 0.003** | r = 0.143 P = 0.459 | r = -0.363 P = 0.053 | ||||||
| LDR | r = -0.284 P = 0.136 | r = 0.153 P = 0.429 | r = -0.286 P = 0.132 | |||||||
| LDL | r = -0.427 P = 0.024* | r = 0.143 P = 0.459 | r = -0.121 P = 0.531 | |||||||
| TPDR | r = -0.430 P = 0.020* | r = 0.302 P = 0.111 | r = -0.276 P = 0.147 | r = -0.286 P = 0.132 | ||||||
| TPDL | r = -0.244 P = 0.202 | r = 0.603 P = 0.001** | r = -0.363 P = 0.053 | r = -0.121 P = 0.531 | ||||||