| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Original Article
Volume 6, Number 4, August 2014, pages 287-294
Teneligliptin As an Initial Therapy for Newly Diagnosed, Drug Naive Subjects With Type 2 Diabetes
Eiji Kutoha, b, c, Mitsuru Hiratea, Yu Ikenoa
aBiomedical Center, Tokyo, Japan
bDivision of Diabetes and Endocrinology, Department of Internal Medicine, Gyoda General Hospital, Saitama, Japan
cCorresponding author: Eiji Kutoh, Biomedical Center, 1-5-8-613 Komatsugawa, Edogawa-ku 132-0034, Tokyo, Japan
Manuscript accepted for publication April 23, 2014
Short title: Teneligliptin for T2DM
doi: https://doi.org/10.14740/jocmr1841e
| Abstract | ▴Top |
Background: Teneligliptin is a novel, highly selective dipeptidyl peptidase-4 (DPP-4) inhibitor. The aim of this study is to explore the glycemic and non-glycemic efficacies of teneligliptin as an initial therapy.
Methods: Newly diagnosed, drug naive Japanese subjects with type 2 diabetes (T2DM) were assigned to 20 mg/day teneligliptin monotherapy (n = 31). At 3 months, levels of glycemic and other parameters were compared with those at baseline.
Results: Significant reductions of HbA1c (from 10.34 ± 2.06 to 8.38 ± 2.23%) and fasting blood glucose (FGB, from 211.3 ± 68.4 to 167.3 ± 70.2 mg/dL) levels were observed without any clinically significant adverse events. However, significant increases of uric acids (UA) levels were observed and two subjects reported mild hypoglycemic events. Homeostasis model assessment-B (HOMA-B) levels significantly increased, while high HOMA-R levels significantly decreased. Significant correlations were observed between the changes (Δ) of HbA1c and those of HOMA-B, and between ΔFBG and ΔHOMA-R. No changes in lipid and body weight were noted.
Conclusions: Teneligliptin might be effectively and safely used as an initial therapy for newly diagnosed T2DM. Glycemic efficacy of teneligliptin is obtained through activating beta-cell function as well as decreasing insulin resistance.
Keywords: Teneligliptin; DPP-4 inhibitor; Insulin resistance; Beta-cell function
| Introduction | ▴Top |
With an increasing number of newly diagnosed patients with T2DM worldwide, it is important to establish therapeutic strategies for those patients. Currently metformin together with life style modifications (healthy eating, body weight control, increased physical activity) is regarded as the initial drug to start [1], although other drugs could be potential candidates as well. For example, in patients with renal or heart failure where metformin is contraindicated, and/or in elderly individuals or those with corticosteroid-induced diabetes, the use of other drugs as the first-line therapy may be justifiable and reasonable [2, 3]. Dipeptidyl peptidase-4 (DPP-4) inhibitors have recently emerged as a new class of oral hypoglycemic agent and show favorable results in improving glycemic control (in particular postprandial hyperglycemic control) with low risk of hypoglycemia and weight gain, and overall good tolerability profile [4-6]. DPP-4 inhibitors are associated with enhanced beta-cell function, making them a good therapeutic option early in the disease when the patients still maintain sufficient levels of beta-cell function [7-9].
Teneligliptin, a novel chemotype prolylthiazolidine-based DPP-4 inhibitor, shows a unique chemical structure which is characterized by five consecutive rings (J-shaped), thereby potentially producing unique characteristics including its glucose lowering efficacy and half-time [10-12]. It is administered with 20 - 40 mg once daily. Since the metabolites of this drug are excreted through hepatic (approximately 35%) and renal (about 65%) route, no dose adjustment is necessary in patients with renal impairment [13, 14]. The efficacy and safety profiles of teneligliptin are similar to those of other DPP-4 inhibitors [15, 16]. Particularly because of its long half-life (approximately 24 h [10, 14]), this drug was shown to stabilize the glucose fluctuations throughout the day [15, 17].
Since teneligliptin is currently marketed only in Japan, limited data and information are available in actual clinical settings. Furthermore, it is not at all clear whether or not teneligliptin is appropriate for the initial drug for patients with T2DM. Thus it is of therapeutic value to analyze the glycemic and non-glycemic efficacies of teneligliptin under such circumstances. To undertake such studies, it makes sense to perform with drug naive subjects as monotherapy in order to eliminate the influences of other drugs as much as possible. As an initial step towards investigating these issues, teneligliptin 20 mg/day monotherapy was performed with newly diagnosed, drug naive subjects with T2DM and effects on a number of glycemic and non-glycemic parameters were investigated.
| Subjects and Methods | ▴Top |
Subjects
A project of monitoring the effects of oral hypoglycemic drugs in newly diagnosed, drug naive Japanese subjects with 2TDM is ongoing in our group. Inclusion criteria were those who had been recently diagnosed with T2DM according to the criteria of the Japan Diabetes Society [18] and had not received any regularly prescribed drugs in the 3 months prior to the study. The work described in this manuscript is part of this project and its aim is to study the glycemic and non-glycemic efficacies of teneligliptin in newly diagnosed, drug naive Japanese subjects with T2DM. Exclusion criteria were those with clinically significant renal creatinine (CRE) > 1.5 mg/dL, liver glutamic oxalacetic transaminases/glutamic pyruvic transaminases (GOT/GPT) > 70/70 IU/L), hypertensive (blood pressure above 160/100 mm Hg) disorders, type 1 diabetes (T1DM) and pregnancy. These subjects were recruited from the outpatient Division of Diabetes and Endocrinology, in Department of Internal Medicine, Gyoda General Hospital (Saitama, Japan). Most of these patients were identified by the health check screening system usually performed twice a year in Japan. These patients received 20 mg/day teneligliptin monotherapy (n = 31). These subjects were encouraged to follow the exercise suggested by the American Diabetes Association [19]. Informed consents were obtained from the patients, and the protocol for this study was approved by the Ethical Committee. In the case of unacceptable or undesirable therapeutic outcome, the patients were free to leave therapy whenever they wished.
Laboratory measurements
The primary end-point was the change of HbA1c levels from baseline to 3 months. The HbA1c values were assessed by the NGSP standardization [20, 21]. The secondary end-points were the changes of fasting blood glucose (FBG) and other metabolic parameters including total cholesterol (T-C), triglyceride (TG), high density lipoprotein cholesterol (HDL-C), low density lipoprotein (LDL-C), free fatty acid (FFA), uric acid (UA), HOMA-R, HOMA-B and BMI.
Blood was collected in the fasting state before breakfast from the cutaneous vein, followed by the analysis at the central clinical laboratory of the Gyoda General Hospital. Measurements of HbA1c and FBG (measured by the system form Arkray, Shiga, Japan) were performed once a month. Insulin (measured by the kit from Abbott Japan, Tokyo), T-C, TG, HDL-C, LDL-C (measured by the kit from Nittobo, Tokyo, using Hitachi 7180 analyzer) and FFA (Mitsubishi BML, Tokyo, Japan) were measured at the start (baseline) and at 3 months of the study. Anti-glutamic acid decarboxylase (GAD) antibody was measured in some suspected patients in order to exclude those with T1DM (Mitsubishi BML, Tokyo, Japan). HOMA-R and HOMA-B were calculated as described [22] HOMA-R = IRI (µU/mL) × FBG (mg/dL)/405, HOMA-B = IRI (µU/mL) × 360/FBG (mg/dL) - 63. Liver (GOT, GPT, ALP and γ-GTP) and renal (BUN and CRE) functions were also monitored monthly. In the case of any significant increase in these parameters, administration of teneligliptin was planned to discontinue. The drop-out subjects were excluded from data analysis.
Data analyses
Change was calculated as the values at 3 months (post-therapy) minus those at baseline (pre-therapy). Paired Student’s t-test was used to analyze the changes in each parameter. Multiple regression analysis was performed to determine the contributing factors to the changes of HbA1c levels. The following independent variables (baseline levels) including age, HbA1c, FBG, HDL-C, TG, LDL-C, UA, HOMA-R, HOMA-B and BMI were used. Simple regression analysis was performed to analyze the changes between measured parameters. The results were expressed as the mean ± SD. Throughout the statistical analysis, values of P < 0.05 were considered significant.
| Results | ▴Top |
Safety and tolerability
Two out of 31 subjects reported mid hypoglycemic events, which could be easily managed by taking glucose drinks by themselves. One subject reported mild constipation and another complained of skin rashes. These potential adverse events occurred in the first 4 weeks of the initiation of the drug. Significant increases of UA levels were observed (Table 1). Otherwise no subjects had any clinically significant elevations of renal or hepatic enzymes and no gastrointestinal complains were observed. No subject had dropped out because of intolerance or adverse events.
 Click to view | Table 1. Changes of Glycemic and Non-Glycemic Parameters With Teneligliptin |
Effect of teneligliptin on glycemic control
At 3 months, significant reductions of HbA1c and FBG levels were observed (for each value and statistical significance, Table 1). Ten out of 31 subjects were non-responders whose HbA1c had less than (<) 1% reduction from the baseline. Eleven out of 31 subjects achieved HbA1c < 7%.
In an effort to find any predictive parameters for the response (glycemic efficacy) of teneligliptin, multiple regression analysis was performed between the changes of (Δ)HbA1c levels (as dependable variable) and the baseline levels of metabolic parameters including age, HbA1c, FBG, insulin, HOMA-R, HOMA-B, TG, HDL-C, non-HDL-C, LDL-C, UA and BMI (as independent variables). Among these factors, the baseline HbA1c level was selected as the significant contributing factor for ΔHbA1c (Table 2). This result was confirmed by simple regression analysis showing that significant negative correlations existed between ΔHbA1c and baseline HbA1c levels (Fig. 1).
 Click to view | Table 2. Multiple Regression Analysis With the Factors Associated With the Changes of HbA1c With Teneligliptin |
 Click for large image | Figure 1. Baseline-dependent glucose lowering efficacy of teneligliptin. Simple regression analysis was performed between the changes of (Δ) HbA1c and baseline HbA1c levels. |
Effect of teneligliptin on lipid, body weight and blood pressure
Effects on DPP-4 inhibitors on non-glycemic parameters, for example, lipid profiles, are controversial. As an initial step towards investigating the effects of teneligliptin on lipids, a number of lipid parameters including T-C, TG, HDL, non-HDL-C, LDL-C or FFA were monitored. However, no statistically significant changes of these parameters were observed (Table 1). However, FFA had a tendency to decrease (Table 1). No changes in body weight (as measured by body mass index: BMI) were noted (Table 1). Blood pressure was also monitored. The variations were so large and no conclusions have been made regarding the effect of teneligliptin on blood pressure (results not shown).
| Discussion | ▴Top |
Glycemic effects and safety of teneligliptin
In the present work, teneligliptin 20 mg/day monotherapy in newly diagnosed, drug naive Japanese subjects with T2DM was shown to be rather effective in reducing blood glucose levels (both HbA1c and FBG levels, Table 1) without any clinically significant adverse events on kidney or liver. This glucose-lowering efficacy is comparable to other DPP-4 inhibitors tested in the identical environment (sitagliptin or alogliptin in drug naive subjects as monotherapy [9, 23]). In analogy to other OHAs, the response to teneligliptin is proportional to the baseline HbA1c levels (Fig. 1). However, two out of 31 of the subjects reported mild hypoglycemic events. No gastrointestinal complains or body weight changes were noted (Table 1). However, significant elevations of UA levels, although still within normal range, were observed (Table 1). Currently it is unclear whether the elevated levels of UA have any impact on the increased risk for gout or cardiovascular disorders. Although the numbers of the subjects in this study are small and the study duration is short, these results implicate that teneligliptin could be effectively and safely used as one of the first-line drugs for T2DM.
Effect of teneligliptin on beta-cell function and insulin sensitivity
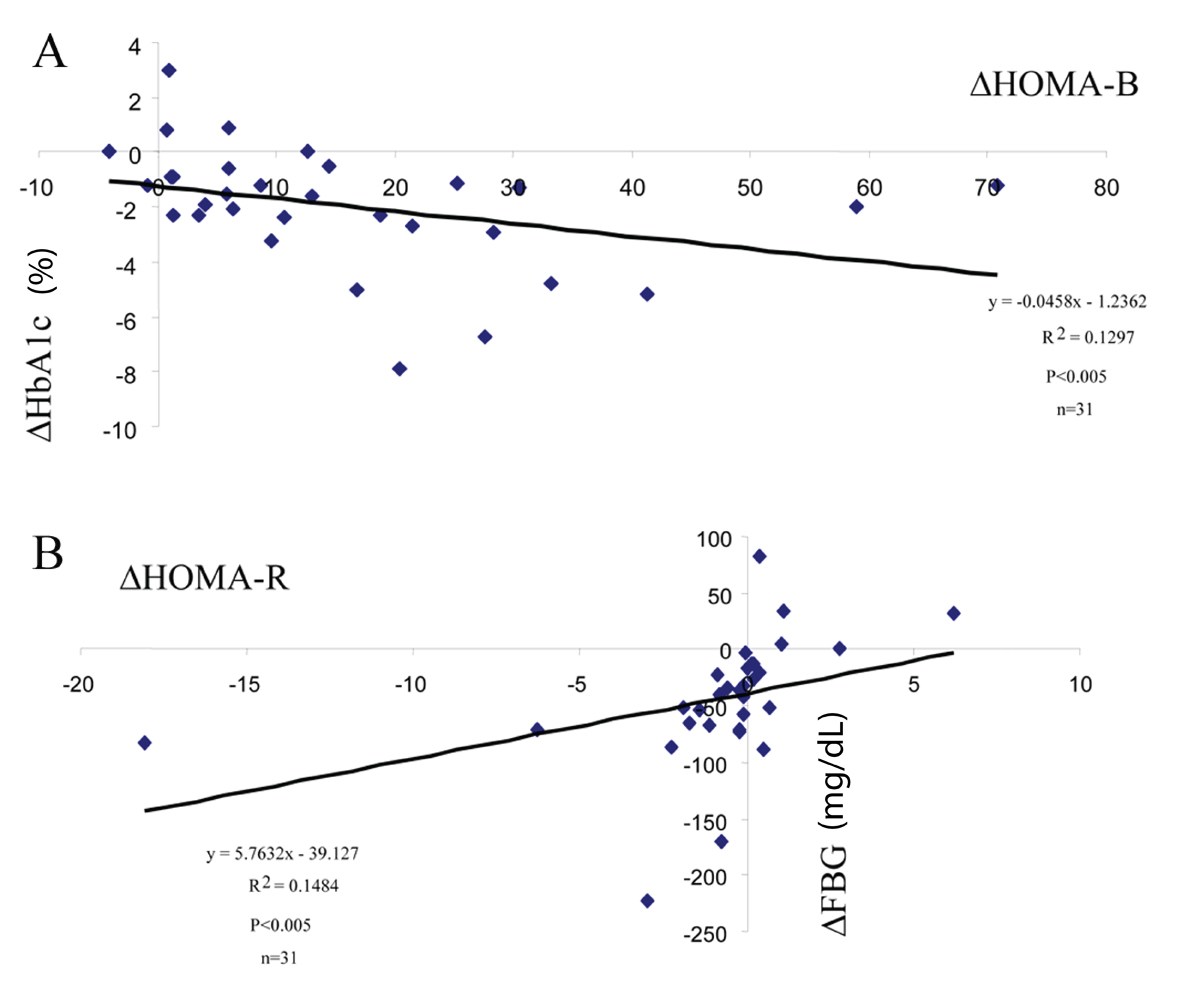
What are the underlying potential mechanisms for the good glycemic efficacy of teneligliptin?DPP-4 inhibitors are a class of incretin-based drugs that stimulate insulin secretion. It was shown that teneligliptin up-regulated beta-cell function (as estimated by HOMA-B, Table 1) and the changes of (Δ) HOMA-B were negatively correlated with those of HbA1c (Fig. 3A) but not with those of FBG (data not shown).
In comparison to GLP-1 receptor agonists, the effect of DPP-4 inhibitors on beta-cell function is less well characterized. DPP-4 inhibitors appear to improve beta-cell function when measured by HOMA-B and proinsulin/insulin ratio [24-26]. The assessments of DPP-4 inhibitors on beta-cell function require further investigation beyond these parameters. Specifically, it is of significance to study whether DPP-4 inhibitors possess beneficial effects on beta-cell mass, and consequently they can eventually delay or convert the progression of the disease process.
Effects of DPP-4 inhibitors on insulin sensitivity (resistance) could be an interesting research area but are scant at present. In the present study, it was shown that teneligliptin decreased HOMA-R levels in the subjects with high baseline HOMA-R levels (Table 1 and Fig. 2A) and changes of (Δ) HOMA-R levels were correlated with ΔFBG (Fig. 3B), but not with ΔHbA1c (result not shown).
These results indicate that the glycemic effect of teneligliptin is obtained through decreasing insulin resistance as well as activating beta-cell function. However this was not the case with sitagliptin (Fig. 2B). The finding that teneligliptin could ameliorate insulin sensitivity (resistance) may be a unique character of this drug. In order to consolidate the above findings, euglycemic clamp study in humans will be required in order to prove that teneligliptin has indeed beneficial effects on insulin sensitivity.
Effect of teneligliptin on non-glycemic parameters
Effects of DPP-4 inhibitors on non-glycemic parameters such as lipids are controversial [27]. In general, it is regarded as lipid neutral. Recently our group has shown that alogliptin or sitagliptin has favorable effects on lipid profiles (sitagliptin decreases TG and FFA while alogliptin down-regulates atherogenic cholesterols including LDL-C or non-HDL-C [9, 23]).
In the present study, we tested whether teneligliptin has any effects on lipid profiles. However, little effects, if any, on lipid parameters were noted (Table 1). Although all the DPP-4 inhibitors inhibit the same enzyme (DPP-4) and display similar glucose lowering efficacies, they show clearly distinct chemical structures and pharmacological properties such as half-life, bioavailability, protein binding, metabolism, presence of active metabolites or excretion route [28-30]. These differences may have distinct impact on lipids or other non-glycemic profiles.
The limitations and strengthens of the study
The limitations of this study are that the number of the subjects is small and the study duration is short. However one can assume that the observed changes were caused exclusively by teneligliptin based on the design of the study (monotherapy with drug naive patients). Further randomized, double-blind, placebo-controlled longer period study with increased number of subjects will be necessary to strengthen the finding in this study.
Acknowledgments
The author thanks Drs. Jan Wajs, Yoichi Morishita, Takeo Kikkawa, Sachiko Yokoyama, Asuka Wada and Hiroshi Kawashima for discussions and Naoki Takeda for the advice of statistical analysis.
Disclosure
The authors have nothing to disclose.
| References | ▴Top |
- Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL,
et al . Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Diabetologia. 2012;55(6):1577-1596.
doi pubmed - Kutoh E. Differential effects of pioglitazone on metabolic parameters in newly diagnosed, drug-naive Japanese patients with type 2 diabetes with or without metabolic syndrome. Endocr Res. 2010;35(3):118-127.
doi pubmed - Giaccari A, Giorda CB, Riccardi G, De Micheli A, Bruno G, Monge L, Frontoni S. Comment on: Inzucchi et al. Comment on: Inzucchi et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35(10):e71, author reply e72-73.
- Lovshin JA, Drucker DJ. Incretin-based therapies for type 2 diabetes mellitus. Nat Rev Endocrinol. 2009;5(5):262-269.
doi pubmed - Neumiller JJ. Clinical pharmacology of incretin therapies for type 2 diabetes mellitus: implications for treatment. Clin Ther. 2011;33(5):528-576.
doi pubmed - Russell-Jones D, Gough S. Recent advances in incretin-based therapies. Clin Endocrinol (Oxf). 2012;77(4):489-499.
doi pubmed - Nagakura T, Yasuda N, Yamazaki K, Ikuta H, Tanaka I. Enteroinsular axis of db/db mice and efficacy of dipeptidyl peptidase IV inhibition. Metabolism. 2003;52(1):81-86.
doi pubmed - Bosi E. Time for testing incretin therapies in early type 1 diabetes? J Clin Endocrinol Metab. 2010;95(6):2607-2609.
doi pubmed - Kutoh E, Ukai Y. Alogliptin as an initial therapy in patients with newly diagnosed, drug naive type 2 diabetes: a randomized, control trial. Endocrine. 2012;41(3):435-441.
doi pubmed - Yoshida T, Akahoshi F, Sakashita H, Kitajima H, Nakamura M, Sonda S, Takeuchi M,
et al . Discovery and preclinical profile of teneligliptin (3-[(2S,4S)-4-[4-(3-methyl-1-phenyl-1H-pyrazol-5-yl)piperazin-1-yl]pyrrolidin-2-y lcarbonyl]thiazolidine): a highly potent, selective, long-lasting and orally active dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. Bioorg Med Chem. 2012;20(19):5705-5719.
doi pubmed - Nabeno M, Akahoshi F, Kishida H, Miyaguchi I, Tanaka Y, Ishii S, Kadowaki T. A comparative study of the binding modes of recently launched dipeptidyl peptidase IV inhibitors in the active site. Biochem Biophys Res Commun. 2013;434(2):191-196.
doi pubmed - Kishimoto M. Teneligliptin: a DPP-4 inhibitor for the treatment of type 2 diabetes. Diabetes Metab Syndr Obes. 2013;6:187-195.
doi pubmed - Otsuki H, Kosaka T, Nakamura K, Shimomura F, Kuwahara Y, Tsukamoto T. Safety and efficacy of teneligliptin: a novel DPP-4 inhibitor for hemodialysis patients with type 2 diabetes. Int Urol Nephrol. 2014;46(2):427-432.
doi pubmed - Nakamaru Y, Hayashi Y, Ikegawa R, Kinoshita S, Perez Madera B, Gunput D, Kawaguchi A,
et al . Metabolism and disposition of the dipeptidyl peptidase IV inhibitor teneligliptin in humans. Xenobiotica. 2014;44(3):242-253.
doi pubmed - Goda M, Kadowaki T. Teneligliptin for the treatment of type 2 diabetes. Drugs Today (Barc). 2013;49(10):615-629.
- Kadowaki T, Kondo K. Efficacy, safety and dose-response relationship of teneligliptin, a dipeptidyl peptidase-4 inhibitor, in Japanese patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2013;15(9):810-818.
doi pubmed - Fukuda-Tsuru S, Anabuki J, Abe Y, Yoshida K, Ishii S. A novel, potent, and long-lasting dipeptidyl peptidase-4 inhibitor, teneligliptin, improves postprandial hyperglycemia and dyslipidemia after single and repeated administrations. Eur J Pharmacol. 2012;696(1-3):194-202.
doi pubmed - Tominaga M. [Diagnostic criteria for diabetes mellitus]. Rinsho Byori. 1999;47(10):901-908.
pubmed - Ma Y, Olendzki BC, Merriam PA, Chiriboga DE, Culver AL, Li W, Hebert JR,
et al . A randomized clinical trial comparing low-glycemic index versus ADA dietary education among individuals with type 2 diabetes. Nutrition. 2008;24(1):45-56.
doi pubmed - Little RR, Rohlfing CL, Wiedmeyer HM, Myers GL, Sacks DB, Goldstein DE. The national glycohemoglobin standardization program: a five-year progress report. Clin Chem. 2001;47(11):1985-1992.
pubmed - Miedema K. Towards worldwide standardisation of HbA1c determination. Diabetologia. 2004;47(7):1143-1148.
doi pubmed - Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412-419.
doi pubmed - Kutoh E, Yamashita H. Differential effects on metabolic parameters between sitagliptin and alogliptin in drug naïve subjects with type 2 diabetes. Journal of Diabetes Research and Clinical Metabolism. 2012;1:17.
doi - Zerilli T, Pyon EY. Sitagliptin phosphate: a DPP-4 inhibitor for the treatment of type 2 diabetes mellitus. Clin Ther. 2007;29(12):2614-2634.
doi pubmed - Karasik A, Aschner P, Katzeff H, Davies MJ, Stein PP. Sitagliptin, a DPP-4 inhibitor for the treatment of patients with type 2 diabetes: a review of recent clinical trials. Curr Med Res Opin. 2008;24(2):489-496.
doi pubmed - Pratley RE, Kipnes MS, Fleck PR, Wilson C, Mekki Q. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor alogliptin in patients with type 2 diabetes inadequately controlled by glyburide monotherapy. Diabetes Obes Metab. 2009;11(2):167-176.
doi pubmed - Monami M, Lamanna C, Desideri CM, Mannucci E. DPP-4 inhibitors and lipids: systematic review and meta-analysis. Adv Ther. 2012;29(1):14-25.
doi pubmed - Neumiller JJ. Differential chemistry (structure), mechanism of action, and pharmacology of GLP-1 receptor agonists and DPP-4 inhibitors. J Am Pharm Assoc (2003). 2009;49(Suppl 1):S16-29.
- Baetta R, Corsini A. Pharmacology of dipeptidyl peptidase-4 inhibitors: similarities and differences. Drugs. 2011;71(11):1441-1467.
doi pubmed - Golightly LK, Drayna CC, McDermott MT. Comparative clinical pharmacokinetics of dipeptidyl peptidase-4 inhibitors. Clin Pharmacokinet. 2012;51(8):501-514.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


