| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Original Article
Volume 6, Number 6, December 2014, pages 422-428
Transfusion of Red Blood Cells Is Associated With Improved Central Venous Oxygen Saturation But Not Mortality in Septic Shock Patients
Farid Sadakaa, b, Steven Trottiera, David Tannehilla, Paige L Donnellya, Mia T Griffina, Zerihun Bunayea, Jacklyn O’Briena, Matthew Korobeya, Rekha Lakshmanana
aMercy Hospital St Louis; St Louis University, St. Louis, MO, USA
bCorresponding Author: Farid Sadaka, St Louis University, 621 S. New Ballas Rd, suite 4006B St. Louis, MO 63141, USA
Manuscript accepted for publication May 05, 2014
Short title: Transfusion of Red Blood Cells in Septic Shock
doi: https://doi.org/10.14740/jocmr1843w
| Abstract | ▴Top |
Background: Although the optimum hemoglobin (H) concentration for patients with septic shock (SS) has not been specifically investigated, current guidelines suggest that H of 7 - 9 g/dL, compared with 10 - 12 g/dL, was not associated with increased mortality in critically ill adults. This contrasts with early goal-directed resuscitation protocols that use a target hematocrit of 30% in patients with low central venous oxygen saturation (ScvO2) during the first 6 hours of resuscitation of SS.
Methods: Data elements were prospectively collected on all patients with SS patients (lactic acid (LA) > 4 mmol/L, or hypotension). Out of a total of 396 SS patients, 46 patients received red blood cell (RBC) transfusion for ScvO2 < 70% (RBC group). We then matched 71 SS patients that did not receive RBC transfusion (NRBC group) on the following goals (G): LA obtained within 6 hours (G1), antibiotics given within 3 hours (G2), 20 mL/kg fluid bolus followed by vasopressors (VP) if needed to keep mean arterial pressure > 65 mm Hg (G3), central venous pressure > 8 mm Hg within 6 hours (G4) and ScvO2 > 70% within 6 hours (G5).
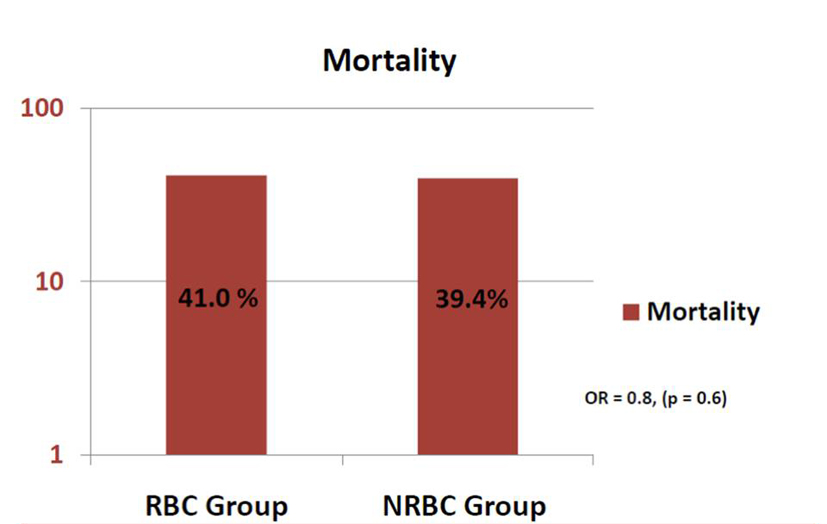
Results: In the RBC group, after one unit of RBC transfusion, ScvO2 improved from average of 63% (± 12%) to 68% (± 10%) (P = 0.02). Sixteen patients required another unit of RBC, and this resulted in increase of ScvO2 to 78% (± 11%) (P < 0.01). The RBC and NRBC groups were matched on sequential organ failure assessment (SOFA) scores and all five goals. There was no difference in mortality between the two groups: 41% vs. 39.4% (OR: 0.8, 95% CI: 0.4 - 1.7, P = 0.6).
Conclusions: In our study, transfusion of RBC was not associated with decreased mortality in SS patients.
Keywords: RBC; Transfusion; Septic shock; EGDT; Early goal-directed therapy; Mortality; ScvO2; Central venous oxygen saturation
| Introduction | ▴Top |
In the United States, approximately 750,000 cases of sepsis occur each year, of which at least 225,000 are fatal. If it also causes organ dysfunction, the diagnosis is severe sepsis. If severe sepsis is accompanied with tissue hypoperfusion, the diagnosis is septic shock (SS). Organ failure occurs in about one-third of patients with sepsis, and severe sepsis is associated with an estimated mortality rate of 30-50%. There is wide variation in the incidence of sepsis and severe sepsis in the intensive care unit (ICU) setting, with reported rates ranging from 20% to 80%, and reported mortality of 20% to 50%. SS, defined as a state of acute circulatory failure characterized by persistent hypotension unexplained by other causes, despite adequate fluid resuscitation, affects between 10% and 30% of patients managed in the ICU, and its incidence is increasing. Mortality from SS in the ICU is estimated to range between 45% and 63% in observational studies [1-7].
Guidelines published as part of the surviving sepsis campaign (SSC) [8] have endorsed use of red blood cells (RBCs) in the treatment of patients with severe sepsis and SS who show evidence of hypoperfusion. This recommendation is primarily based on studies that evaluated a bundle approach to patients in severe sepsis [9]. RBC transfusion to obtain a hematocrit of 30% is included in this bundle for patients with a central venous oxygen saturation (ScvO2) < 70%. Patients achieving this goal had better outcomes than patients who did not reach the goal. The specific effect of transfusion was not evaluated in this study, however, as the investigation was designed to assess the overall bundle rather than its component parts. On the other hand, several studies, summarized in literature [10] point to several problems documented with RBC transfusions, such as infection, pulmonary complications such as transfusion-related acute lung injury, fluid overload, transfusion-related immunomodulation, multiorgan failure and increased mortality. As a result, outside this window during the first 6 h of early-goal directed therapy (EGDT) for severe sepsis and SS, a “restrictive” strategy of RBC transfusion (transfuse when Hb < 7 g/dL) is recommended.
The primary objective of this study is to evaluate the effect of RBC transfusion on ScvO2 and mortality when used during EGDT for patients with SS.
| Methods | ▴Top |
As discussed above, following SSC guidelines with rapid recognition and aggressive intervention of SS dramatically improves outcome. As a result, a sepsis team has been created in our institution in order to increase compliance with SSC guidelines. As soon as an SS patient is identified anywhere in the hospital, the sepsis team is called to go to the bedside and initiate EGDT. Data are prospectively collected on these patients in order to examine the effectiveness of this effort and its impact on improving compliance with the EGDT bundle and outcomes. As a result, data elements were prospectively collected on all patients. This study is a retrospective review of this database.
SS patients (defined as lactic acid (LA) > 4 mmol/L, or hypotension persistent after initial fluid challenge) admitted between June 2011 and March 2013 were included. We identified a total of 396 SS patients. The protocol was as follows: As soon as a patient was diagnosed with sepsis and sepsis team is notified, an LA was measured. Once patient is diagnosed with SS, a 20 mL/kg bolus of crystalloid was given to achieve a central venous pressure of 8 - 12 mm Hg. If the mean arterial pressure was less than 65 mm Hg, vasopressors were given to maintain a mean arterial pressure of at least 65 mm Hg. Once these goals are achieved, if the central venous oxygen saturation was less than 70%, RBCs were transfused to achieve a hematocrit of at least 30%.
Forty-six patients received RBC transfusion for ScvO2 < 70% (RBC group). Of the remaining patients, we then matched 71 SS patients that did not receive RBC transfusion (NRBC group) on the following goals (G) based on SSC 2008 criteria [11]: LA obtained within 6 h (G1), antibiotics given within 3 h (G2), 20 mL/kg fluid bolus followed by vasopressors (VP) if needed to keep mean arterial pressure (MAP) > 65 mm Hg (G3), central venous pressure (CVP) ≥ 8 mm Hg within 6 h (G4) and ScvO2 ≥ 70% within 6 h (G5). We calculated average age, average sequential organ failure assessment (SOFA) scores and fluid balance at the end of 6 h for both groups. In the RBC group, we collected ScvO2 levels before and after each unit of RBC transfused. Outcome was hospital mortality. Matching is used to evaluate the effect of a treatment by comparing the treated and the non-treated patients in an observational study (especially when the treatment is not randomly assigned). The goal of matching is for every treated patient, to find one non-treated patient (in this case transfusion versus no transfusion) with similar observable characteristics against whom the effect of treatment can be assessed. By matching treated patients to similar non-treated patients, matching enables a comparison of outcomes in order to estimate the effect of the treatment without reduced bias due to confounding. In this case, all of the goals (G1-G5) could confound outcome and thus were all matched. The RBC and NRBC groups were compared by Pearson Chi-squared and Fisher’s exact tests to analyze statistical significance. Mean, standard deviation and P value were reported for each comparison. Statistical significance was defined as P ≤ 0.05. This study was approved by Mercy Hospital Institutional Review Board.
| Results | ▴Top |
In the RBC group, after one unit of RBC transfusion, ScvO2 improved from average of 63% (± 12%) to 68% (± 10%) (P = 0.02). Sixteen patients required another unit of RBC, and this resulted in increase of ScvO2 to 78% (± 11%) (P < 0.01) (Fig. 1). All these transfusions were for ScvO2 < 70% as part of EGDT. None of the transfusions were for other indications, such as bleeding, hemolysis, or bone marrow dyscrasias. Although all patients in the RBC group got transfused, not all of them achieved this goal (ScvO2 ≥ 70%) at the 6 h mark, which explains the findings in Table 1.
 Click for large image | Figure 1. ScvO2 values after one and two units in the RBC group. |
| Discussion | ▴Top |
RBC transfusion is one of the most commonly used interventions in the ICU to treat severe anemia, which often occurs in SS. In the United States, more than 14 million units of RBCs are administered annually, many of which are administered in the ICU and in SS patients [12]. Approximately 40-80% of RBC transfusions are not given for bleeding, but rather for low hemoglobin levels, for a decrease in physiological reserve, or for alterations in tissue perfusion [13, 14]. Forty to fifty percent of septic and other critically ill patients require blood transfusion during their ICU stay [15]. By day two in the ICU, nearly 95% of patients are anemic [13].
For years, it was considered that a hemoglobin concentration of 10 g/dL, or a hematocrit of 30%, represented the lowest level acceptable, thereby providing a standard and convenient “transfusion trigger” [16]. This was adopted based on the fact that tissue oxygen delivery (DO2) is the product of tissue blood flow and arterial oxygen content. Tissue blood flow is thus determined by cardiac output and regional vasoregulation, and arterial oxygen content depends on hemoglobin concentration and its percentage saturation. Oxygen flow increases as the hemoglobin falls to a level termed the “optimal hematocrit”, at which point DO2 is highest at the lowest energy cost to the individual. This occurs around a hematocrit of 30% [17]. Below this “optimal” level, the maintenance of tissue oxygen consumption (VO2) and aerobic metabolism at decreasing levels of DO2 is principally provided by increased oxygen extraction. In the critical care environment, there have been several studies examining the interrelationship between hematocrit, DO2 and VO2. Shoemaker et al [18] and Boyd et al [19] initially defined the optimal hematocrit at around 30 % since, below this level, oxygen delivery and consumption were decreased in critically ill patients and mortality was increased. Above this level, there was no change in these variables or outcome. This line of reasoning led to the common practice of maintaining this “10/30 rule” as the transfusion trigger. Subsequently, several problems were documented with RBC transfusions, such as infection, pulmonary complications such as TRALI and transfusion-associated circulatory overload (TACO), transfusion-related immunomodulation (TRIM) and multiorgan failure, and increased mortality [20]. This lead to the need for evaluation of restrictive transfusion strategies.
The best evidence available regarding the efficacy of RBC transfusion among critically ill patients including SS is from a randomized controlled trial, the transfusion requirements in critical care (TRICC) trial, conducted by the Canadian Critical Care Trials Group [21]. In this study, a liberal transfusion strategy (hemoglobin 10 - 12 g/dL, with a transfusion trigger of 10 g/dL) was compared to a restrictive transfusion strategy (hemoglobin 7 - 9 g/dL, with a transfusion trigger of 7 g/dL) in a general medical and surgical critical care population. Patients who were euvolemic after initial treatment who had a hemoglobin concentration < 9 g/dL within 72 h were enrolled. The TRICC trial documented an overall nonsignificant trend toward decreased 30-day mortality in the restrictive group; however, there was a significant decrease in mortality in the restrictive group among patients who were less acutely ill (APACHE II scores < 20) and among younger patients (< 55 years of age). Patients in the restrictive group received 54% less RBC units than those in the liberal group [21]. The diversity of patients enrolled in the trial and the consistency of the results suggest that the conclusions may be generalized to most critical care patients including SS patients. In a recent analysis by the Cochrane database of 19 trials involving a total of 6,264 patients, restrictive transfusion strategies were associated with a statistically significant reduction in hospital mortality (RR: 0.77, 95% CI: 0.62 - 0.95) but not 30-day mortality (RR: 0.85, 95% CI: 0.70 - 1.03) [22]. The authors concluded that the existing evidence supports the use of restrictive transfusion triggers in most patients including those with pre-existing cardiovascular disease.
Guidelines published as part of the SSC [8] have endorsed use of RBCs in the treatment of patients with severe sepsis and SS who show evidence of hypoperfusion. EGDT thus calls for an increase in hematocrit to at least 30% in the setting of perceived oxygen deficit. The specific effect of transfusion was not evaluated in this study, however, as the investigation was designed to assess the overall bundle rather than its component parts. The pathologic effects of RBC transfusion in sepsis could be numerous. Several studies have demonstrated that RBC rheology is impaired (increased aggregation, decreased deformability, alterations of RBC shape) in recipient RBCs in SS patients [23-26]. RBC can also act as oxygen sensor, which can modulate tissue oxygen flow variables, by the release of the vasodilators, nitric oxide [27, 28] or ATP [29]. This release of vasodilators from RBCs during hypoxia could be impaired during storage and/or sepsis/septic shock. Storage of RBCs decreases levels of 2,3-diphosphoglycerate and adenosine triphosphate (ATP) levels with a resultant increase in oxygen affinity and a decrease in the ability of hemoglobin to offload oxygen. Morphological changes in erythrocytes occur during storage which may result in increased fragility, decreased viability and decreased deformability of RBCs. A release of a number of substances occurs during storage resulting in such adverse systemic responses as fever, cellular injury, alterations in regional and global blood flow, and organ dysfunction. Using near infrared spectroscopy (NIRS) or sidestream dark field (SDF), several investigators have reported that microcirculation is markedly altered in sepsis, that these alterations are more severe in nonsurvivors than in survivors, that persistent microvascular alterations are associated with development of multiple organ failure and death and that microvascular alterations are the most sensitive and specific predictor of outcome in septic patients [30-34]. In severe septic patient requiring leukoreduced RBC transfusion, using sidestream dark field (SDF), Sakr et al showed that the sublingual microcirculation was globally unaltered; however, it improved in patients with altered capillary perfusion at baseline [35]. Using both SDF and NIRS, Sadaka et al looked at patients that got non-leukoreduced RBCs for a hemoglobin < 7.0, or for a hemoglobin between 7.0 and 9.0 with either lactic acidosis or central venous oxygen saturation < 70% [36]. Sadaka et al showed that muscle tissue oxygen consumption, microvascular reactivity and sublingual microcirculation were globally unaltered by RBC transfusion in severe septic and SS patients. However, muscle oxygen consumption improved in patients with low baseline and deteriorated in patients with preserved baseline [36]. In this study, we showed that transfusion of RBC during the EGDT period of resuscitation of SS was not associated with decreased mortality, despite improvement of ScvO2 values. Improvement in oxygen delivery with RBC transfusion could have been offset by the multiple pathophysiologic effects of transfusing RBCs. Given the clinical evidence and the aforementioned pathophysiologic alterations associated with RBCs, our findings are thus not surprising and further question the rationale of transfusion during EGDT for SS patients.
Our study has several limitations. The small sample size is an obvious limitation. Although we prospectively collected robust data and accounted for bundle compliance data during EGDT, we still cannot exclude the possibility of unaccounted, unmatched or missing data, which may lead to bias and undetected differences in baseline characteristics. In addition, there may be unaccounted differences in treatment of the patients in the two groups, which can lead to differences in outcomes. Although the two groups were matched on basic characteristics and goals of EGDT, exact matching remains challenging due to the retrospective nature of the study, the small number of patients, and the inability to match on baseline hematocrit and ScvO2 levels which lead to one group (RBC group) getting transfused and not the other group (NRBC group). However, patients are closely matched on severity of illness as apparent by nonsignificant difference in SOFA scores between the two groups. Another limitation is lack of multivariate analysis that was not used due to the small number of patients. By the same token, a power analysis was not undertaken since this is a retrospective review of data and thus the results of this study are considered hypothesis generating and not conclusive. All this makes drawing final conclusions difficult based on findings of this trial alone.
Conclusion
In our study, transfusion of RBC was not associated with decreased mortality in SS patients, despite improvement in ScvO2 values. This and other studies mentioned above raise a concern about liberal transfusion during resuscitation phase of SS patients. This study does suggest that better means of identifying a need for transfusion are needed and that blindly transfusing to an arbitrarily set (and high) Hb may be detrimental. Future research with larger samples is needed to further examine the association between RBC transfusion and outcomes of patients resuscitated early in SS.
Acknowledgments
None.
Sponsorship and Disclosures
This study was not sponsored. None of the authors have anything to disclose.
| References | ▴Top |
- Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546-1554.
doi pubmed - Brun-Buisson C, Doyon F, Carlet J, Dellamonica P, Gouin F, Lepoutre A, Mercier JC,
et al . Incidence, risk factors, and outcome of severe sepsis and septic shock in adults. Incidence, risk factors, and outcome of severe sepsis and septic shock in adults. A multicenter prospective study in intensive care units. JAMA. 1995;274(12):968-974.
doi pubmed - Karlsson S, Ruokonen E, Varpula T, Ala-Kokko TI, Pettila V. Long-term outcome and quality-adjusted life years after severe sepsis. Crit Care Med. 2009;37(4):1268-1274.
doi pubmed - Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303-1310.
doi pubmed - Annane D, Aegerter P, Jars-Guincestre MC, Guidet B. Current epidemiology of septic shock: the CUB-Rea Network. Am J Respir Crit Care Med. 2003;168(2):165-172.
doi pubmed - Pittet D, Rangel-Frausto S, Li N, Tarara D, Costigan M, Rempe L, Jebson P,
et al . Systemic inflammatory response syndrome, sepsis, severe sepsis and septic shock: incidence, morbidities and outcomes in surgical ICU patients. Intensive Care Med. 1995;21(4):302-309.
doi pubmed - Quenot JP, Binquet C, Kara F, Martinet O, Ganster F, Navellou JC, Castelain V,
et al . The epidemiology of septic shock in French intensive care units: the prospective multicenter cohort EPISS study. Crit Care. 2013;17(2):R65.
doi pubmed - Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE,
et al . Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580-637.
doi pubmed - Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E,
et al . Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368-1377.
doi pubmed - Napolitano LM, Kurek S, Luchette FA, Corwin HL, Barie PS, Tisherman SA, Hebert PC,
et al . Clinical practice guideline: red blood cell transfusion in adult trauma and critical care. Crit Care Med. 2009;37(12):3124-3157.
doi pubmed - Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K,
et al . Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36(1):296-327.
doi pubmed - Comprehensive report on blood collection and transfusion in the US in 2001. Available online at: www.nbdrc.org.
- Vincent JL, Baron JF, Reinhart K, Gattinoni L, Thijs L, Webb A, Meier-Hellmann A,
et al . Anemia and blood transfusion in critically ill patients. JAMA. 2002;288(12):1499-1507.
doi pubmed - Corwin HL, Gettinger A, Pearl RG, Fink MP, Levy MM, Abraham E, MacIntyre NR,
et al . The CRIT Study: Anemia and blood transfusion in the critically ill--current clinical practice in the United States. Crit Care Med. 2004;32(1):39-52.
doi pubmed - Brown RB, Klar J, Teres D, Lemeshow S, Sands M. Prospective study of clinical bleeding in intensive care unit patients. Crit Care Med. 1988;16(12):1171-1176.
doi pubmed - Zauder HL. Preoperative hemoglobin requirements. Anesth Clin North Am. 1990;8:471-480.
- Messmer K, Lewis DH, Sunder-Plassmann L, Klovekorn WP, Mendler N, Holper K. Acute normovolemic hemodilution. Acute normovolemic hemodilution. Eur Surg Res. 1972;4(1):55-70.
doi pubmed - Shoemaker WC, Appel PL, Kram HB, Waxman K, Lee TS. Prospective trial of supranormal values of survivors as therapeutic goals in high-risk surgical patients. Chest. 1988;94(6):1176-1186.
doi pubmed - Boyd O, Grounds RM, Bennett ED. A randomized clinical trial of the effect of deliberate perioperative increase of oxygen delivery on mortality in high-risk surgical patients. JAMA. 1993;270(22):2699-2707.
doi pubmed - Sadaka F. Red Blood Cell Transfusion in Sepsis: A Review. J Blood Disord Transfus. 2012;S4:001.
- Hebert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, Tweeddale M,
et al . A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. N Engl J Med. 1999;340(6):409-417.
doi pubmed - Carson JL, Carless PA, Hebert PC. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. 2012;4:CD002042.
pubmed - Powell RJ, Machiedo GW, Rush BF, Jr. Decreased red blood cell deformability and impaired oxygen utilization during human sepsis. Am Surg. 1993;59(1):65-68.
pubmed - Baskurt OK, Gelmont D, Meiselman HJ. Red blood cell deformability in sepsis. Am J Respir Crit Care Med. 1998;157(2):421-427.
doi pubmed - Reggiori G, Occhipinti G, De Gasperi A, Vincent JL, Piagnerelli M. Early alterations of red blood cell rheology in critically ill patients. Crit Care Med. 2009;37(12):3041-3046.
doi pubmed - Hurd TC, Dasmahapatra KS, Rush BF, Jr., Machiedo GW. Red blood cell deformability in human and experimental sepsis. Arch Surg. 1988;123(2):217-220.
doi pubmed - Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK,
et al . Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9(12):1498-1505.
doi pubmed - Jia L, Bonaventura C, Bonaventura J, Stamler JS. S-nitrosohaemoglobin: a dynamic activity of blood involved in vascular control. Nature. 1996;380(6571):221-226.
doi pubmed - Ellsworth ML. The red blood cell as an oxygen sensor: what is the evidence? Acta Physiol Scand. 2000;168(4):551-559.
doi pubmed - De Backer D, Creteur J, Preiser JC, Dubois MJ, Vincent JL. Microvascular blood flow is altered in patients with sepsis. Am J Respir Crit Care Med. 2002;166(1):98-104.
doi pubmed - Trzeciak S, Dellinger RP, Parrillo JE, Guglielmi M, Bajaj J, Abate NL, Arnold RC,
et al . Early microcirculatory perfusion derangements in patients with severe sepsis and septic shock: relationship to hemodynamics, oxygen transport, and survival. Ann Emerg Med. 2007;49(1):88-98, 98 e81-82. - Sakr Y, Dubois MJ, De Backer D, Creteur J, Vincent JL. Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit Care Med. 2004;32(9):1825-1831.
doi pubmed - Pareznik R, Knezevic R, Voga G, Podbregar M. Changes in muscle tissue oxygenation during stagnant ischemia in septic patients. Intensive Care Med. 2006;32(1):87-92.
doi pubmed - Creteur J, Carollo T, Soldati G, Buchele G, De Backer D, Vincent JL. The prognostic value of muscle StO2 in septic patients. Intensive Care Med. 2007;33(9):1549-1556.
doi pubmed - Sakr Y, Chierego M, Piagnerelli M, Verdant C, Dubois MJ, Koch M, Creteur J,
et al . Microvascular response to red blood cell transfusion in patients with severe sepsis. Crit Care Med. 2007;35(7):1639-1644.
doi pubmed - Sadaka F, Aggu-Sher R, Krause K, O'Brien J, Armbrecht ES, Taylor RW. The effect of red blood cell transfusion on tissue oxygenation and microcirculation in severe septic patients. Ann Intensive Care. 2011;1(1):46.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


