| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Original Article
Volume 16, Number 6, June 2024, pages 302-309
Clinically Evident Cardiopulmonary Congestion Does Not Significantly Impact the Prognosis of Patients With Septic Acute Kidney Injury
Charlotte Munda, Katharina Asmusa, Wajima Safia, Oliver Rittera, b, Dominique Petrusa, Susann Patschana, Daniel Patschana, b, c
aDepartment of Internal Medicine I - Cardiology, Nephrology and Internal Intensive Medicine, Brandenburg University Hospital, Brandenburg Medical School (Theodor Fontane), Brandenburg an der Havel, Germany
bFaculty of Health Sciences (FGW), Joint Faculty of the University of Potsdam, the Brandenburg Medical School Theodor Fontane and the Brandenburg Technical University Cottbus-Senftenberg, Cottbus, Germany
cCorresponding Author: Daniel Patschan, Cardiology, Nephrology and Internal Intensive Medicine, Brandenburg University Hospital, Brandenburg Medical School (Theodor Fontane), Brandenburg an der Havel, Germany
Manuscript submitted April 18, 2024, accepted May 20, 2024, published online June 30, 2024
Short title: Cardiac Involvement in Septic AKI
doi: https://doi.org/10.14740/jocmr5190
| Abstract | ▴Top |
Background: Acute kidney injury (AKI) is a common issue among in-hospital patients, with high mortality rates. Sepsis is a primary cause of AKI, particularly in the intensive care unit. Patients with septic AKI often experience cardiovascular congestion, leading to the formal classification of cardiorenal syndrome type 5. The study aimed to evaluate the prognosis of septic AKI patients with and without clinical evidence of cardiovascular congestion.
Methods: This was a retrospective observational study. AKI patients were identified using the in-hospital AKI alert system. Sepsis was diagnosed based on laboratory, clinical, and hemodynamic characteristics, with additional consideration of the quickSOFA score. Cardiovascular congestion was diagnosed by assessing clinical (edema), radiographic (pulmonary congestion), echocardiographic (e.g., wall motion abnormalities), and laboratory variables (e.g., N-terminal pro-B-type natriuretic peptide). Endpoints included in-hospital survival, the need for kidney replacement therapy (KRT), and recovery of kidney function (ROKF).
Results: In total, 102 patients were included, and cardiopulmonary congestion was diagnosed in 78.4%. Individuals with congestion did not differ from patients without congestion in any of the pre-defined endpoints.
Conclusions: It is justified not to consider clinically apparent cardiovascular congestion in septic AKI patients as a risk factor for death per se. Rather, especially in the case of sepsis, clinically apparent positive fluid balance does not seem to be a disadvantage in terms of survival, KRT, and ROKF.
Keywords: Sepsis; AKI; Cardiac involvement; Mortality; KRT; Recovery of kidney function; Cardiorenal syndrome type 5
| Introduction | ▴Top |
Acute kidney injury (AKI) is a highly relevant issue in inpatient medicine worldwide. The incidence varies between 15% and 30% [1], with 50% or more of all patients treated in intensive care conditions [2] experiencing an acute deterioration in renal excretory function. On average, 50% of all AKI patients receiving intensive care do not survive the course of treatment, and any initiated kidney replacement therapy (KRT) only has limited impact [3]. AKI is considered an independent predictor of mortality [2, 4, 5], with the manifestation of the syndrome typically reflecting the severity of the underlying condition that ultimately leads to death.
In the intensive care setting, AKI often manifests as a complication of sepsis. The latter affects an average of 30% of the individuals treated in the intensive care unit (ICU), at least when considering Central Europe [6]. The mechanisms underlying acute kidney function decline in sepsis are intricate. They involve systemic perfusion imbalances, leading to oxygen and nutrient depletion in the kidneys and other organs, as well as toxic effects of bacterial antigens on cells. Additionally, the systemic inflammatory response exacerbates vascular dysfunction and cellular damage, ultimately resulting in stimulated tubular cell apoptosis [7]. Finally, diagnostic and therapeutic procedures used for identifying, staging, and managing sepsis (e.g., radiocontrast media, nephrotoxic drugs) may exacerbate AKI even further.
The first description of cardiorenal syndrome (CRS) was already published in 1912 [8]. However, the concept of CRS was “officially” introduced in 2008 by Ronco and colleagues [9]. The term CRS refers to concomitant disorders of the heart and kidneys, which can have either an acute or chronic onset. The type of CRS is determined by the organ that is initially affected [10]. Diagnosing the specific type of CRS from the 5 “classical” types can be challenging clinically [11]. CRS type 5 is nevertheless characterized by the simultaneous presence of heart and kidney disease stemming from conditions outside the heart and kidneys [12]. Possible causes include diabetes mellitus with diabetic nephropathy and cardiomyopathy, autoimmune diseases with heart and kidney involvement (e.g. systemic lupus erythematosus), as well as sepsis [13]. The latter can lead to AKI, as well as potentially causing myocardial dysfunction [14] that may result in cardiac failure and cardiopulmonary congestion.
The aim of the current study was to assess the prognosis of septic AKI patients with and without evident cardiopulmonary congestion. Primary endpoint was the in-hospital mortality, and secondary endpoints were the need for KRT, and the prevalence of recovery of kidney function (ROKF) at discharge.
| Materials and Methods | ▴Top |
Design
It was a retrospective, single-center, observational study. The observational period was January until August 2022. It was conducted at the Department of Internal Medicine I, specifically focusing on Cardiology, Nephrology, and Intensive Care Medicine at Brandenburg University Hospital (Brandenburg Medical School Theodor Fontane). It was not mandatory to require formal approval for the study by the ethics committee of the Medical School, since it was a retrospective investigation. The same applied for written informed consent of included patients. All participants were recruited from the Department of Internal Medicine I, and the data were extracted from the central database of the university hospital (MEDICO® CGM). The study did not require ethical approval due to its retrospective design. The study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
Patient identification, inclusion and exclusion criteria
AKI patients were identified using the in-hospital AKI alert system [15], which is based on an electronic algorithm that detects dynamic changes in serum creatinine according to criteria 1 or 2 of the KDIGO guidelines published in 2012 [16]. Criterion 3, which involves reduced urine output over time, was not included because data on urine production were unavailable for many individuals.
Patients who met either criterion 1 or 2 of the KDIGO criteria and also had a diagnosis of sepsis were included. The diagnosis of sepsis was established based on the following criteria: elevated C-reactive protein (CRP) levels in conjunction with elevated procalcitonin (PCT) levels, along with either positive results in bacterial blood cultures and/or a positive quickSOFA score [17] (respiratory rate > 22/min, Glasgow Coma Scale (GCS) < 15, systolic blood pressure < 100 mm Hg - two criteria must be met) and/or hemodynamic instability necessitating the use of vasopressors in addition to crystalloid solutions.
Patients with known stage 5D chronic kidney disease (CKD) according to KDIGO 2012 [18], terminal malignant disease, and pregnant women were not included in the study.
Diagnosis of cardiovascular congestion
Cardiovascular congestion was diagnosed if at least one of the following criteria was met during a period of 0 to 5 days after the diagnosis of septic AKI:
1) Peripheral edema - the diagnosis could be determined by the attending physicians. Typically, three physicians oversee ICU patients on weekdays, working 8-h shifts. Conversely, on weekends, two physicians are responsible during a 24-h shift. The diagnosis of edema was only confirmed if it was recorded in the central database of the Brandenburg University Hospital.
2) N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels exceeding 800 pg/mL - any NT-proBNP level above 800 pg/mL during the 5-day period - was considered positive.
3) Radiographic signs of pulmonary congestion - radiographic congestion was identified when the official radiological assessment by colleagues from the Department of Radiology documented this finding in writing in the central database of the University Hospital. The spectrum of congestion ranged from mild interstitial fluid accumulation to alveolar pulmonary edema. However, each congestion finding was evaluated equally.
4) Abnormal findings on transthoracic echocardiography - included one or more of the following criteria: a reduction in left ventricular ejection fraction to below 40%, localized or generalized cardiac wall motion abnormalities, and the presence of diastolic dysfunction.
It should be noted that the radiological and echocardiographic assessments were conducted by frequently changing staff. However, all official reports have been validated by specialists in the respective fields. Nevertheless, the influence of subjective factors in the evaluations can never be ruled out in this case.
Clinical and laboratory variables
The following variables were collected: age (years), gender (female, male), duration of in-hospital therapy (DOIT, days), AKI stage according to KDIGO 2012 [16], pre-existing CKD (diagnosis according to the 2012 published KDIGO criteria [18]), sepsis focus (lungs, urogenital tract, abdominal, other), bacteria identified (no, yes), serum creatinine, blood urea nitrogen (BUN), serum sodium and potassium, CRP (all at baseline, peak, and discharge), use of nephrotoxic drugs (nonsteroidal anti-inflammatory drugs (NSAIDs), systemic vancomycin, systemic aminoglycosides), use of vasopressors (no, yes), respiratory support (no, yes - respiratory support was defined as any type of inspiratory/expiratory pressure support), prevalences of several comorbidities (always no, yes): arterial hypertension, coronary artery disease (CAD), chronic heart failure (CHF), chronic obstructive pulmonary disease (COPD), obesity, diabetes mellitus, history of neoplasia, and smoking.
Endpoints
Primary endpoint was in-hospital survival, with secondary endpoints including the need for KRT and ROKF until discharge. The need for KRT was defined as the requirement for at least one session of individual KRT treatment, whether intermittent or continuous. ROKF was defined as the discontinuation of KRT, accompanied by a decrease in serum levels to the baseline value, with a difference not exceeding 10%.
Statistical analysis
All statistical analyses were conducted using WIZARD for MacOS (version 2.0.16, developed by Evan Miller). Numerical data were initially assessed for normality using the Kolmogorov-Smirnov test. Normally distributed data were compared using either a t-test (for two groups) or analysis of variance (ANOVA) (for more than two groups). Non-normally distributed data were compared using the Mann-Whitney test (for two groups) or the Kruskal-Wallis test (for more than two groups). Categorical data were compared using the Chi-square test. Multiple linear regression analysis was performed with the Application WIZARD® for MacOS (developed by Evan Miller, version 2.0.16). A P-value below 0.05 was considered statistically significant. Results were reported as either mean ± standard error of mean (SEM) or median ± interquartile range (IQR).
| Results | ▴Top |
Patients
In total, 102 patients were included in the study, with 42.2% (n = 43) being females and 57.8% (n = 59) males. The mean age of all individuals was 75.4 ± 11.6 years, and the in-hospital treatment time was 18 ± 16 days. The most common septic focus was urinary tract infections (29.4%), followed by pulmonary (27.5%) and abdominal infections (24.5%). Bacteria were identified in 74.5% of cases, with the sources being blood (60%), bronchial secretions (14.7%), urine (14.7%), bile (4%), and other (6.7%). Cardiopulmonary congestion was diagnosed in 78.4% of patients. In-hospital death occurred in 52.9% (n = 54), with 17.6% (n = 18) requiring at least one KRT session. ROKF, according to the pre-defined criteria, was diagnosed in 53.9% (n = 55) of cases. Table 1 summarizes all clinical and laboratory data.
 Click to view | Table 1. Characteristics of All Included Patients |
Survival
In-hospital mortality rates were not significantly different between septic AKI patients with and without symptoms or findings of cardiopulmonary congestion (53.8% with congestion, 50% without congestion; P = 0.75) (Table 2 and Fig. 1).
 Click to view | Table 2. Endpoint Analysis |
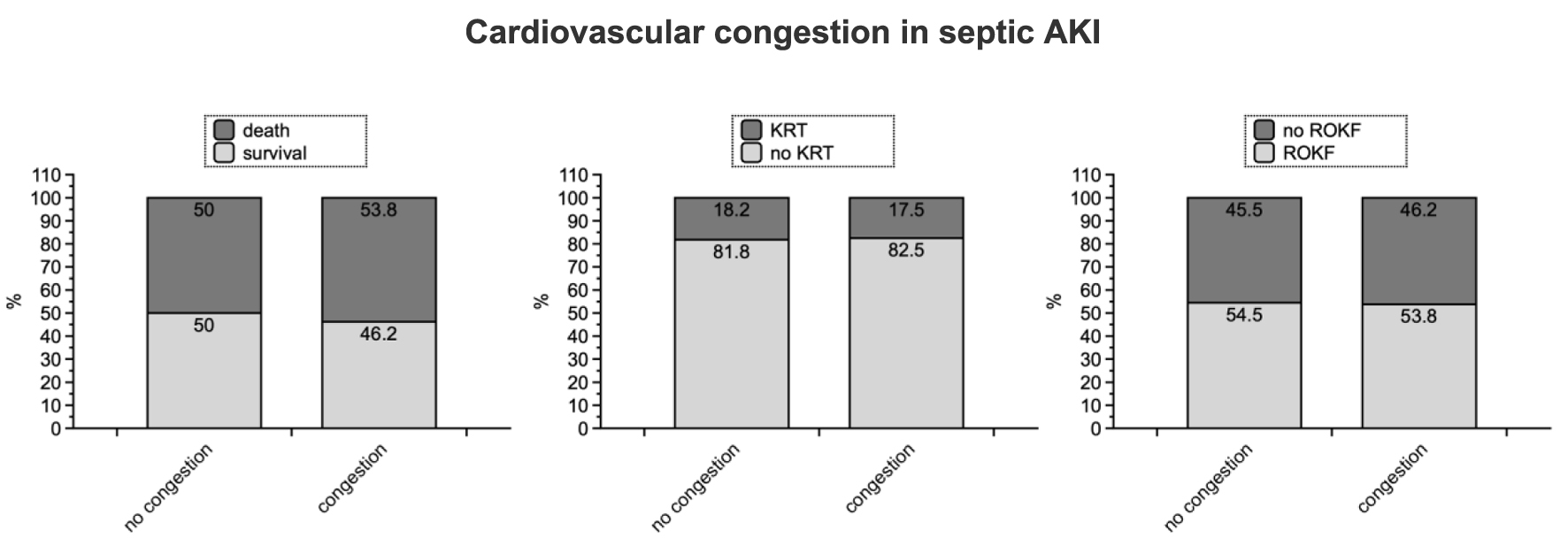 Click for large image | Figure 1. Graphical summary of all three endpoints. The P-values have been listed in Table 2. AKI: acute kidney injury; KRT: kidney replacement therapy; ROKF: recovery of kidney function. |
KRT and ROKF
KRT became not mandatory more often in patients with congestion (17.5% versus 18.2%; P = 0.94). ROKF was diagnosed in 46.2% of all patients with congestion, as opposed to 45.5% without symptoms/findings of fluid overload (P = 0.94) (Table 2 and Fig. 1).
Risk factor analysis
Although no significant differences were found in the primary and secondary endpoints between individuals with and without symptoms of cardiovascular congestion, an analysis of risk factors for reaching the endpoints was conducted. It encompassed the entire study population.
Survival
AKI stage III, as per KDIGO criteria, was more frequently diagnosed in non-survivors; however, they exhibited a lower prevalence of pre-existing CKD. Other distinctions between survivors and non-survivors included (information in the brackets refers to survivors): duration of in-hospital stay (higher), ROKF (more often), serum creatinine at discharge (lower), peak BUN (lower), BUN at discharge (lower), peak serum potassium (lower), serum potassium discharge (lower), minimum serum sodium (lower), CRP at discharge (lower), and use of vasopressors (less often) (Table 3).
 Click to view | Table 3. Risk Factor Analysis |
KRT
Patients requiring KRT were younger and received aminoglykoside therapy more often. A multiple linear regression analysis including independent variables age, gender, arterial hypertension, CHF, diabetes mellitus, and cardiovascular congestion revealed that age was significantly associated with the risk of KRT (P = 0.031). Several laboratory findings differed between individuals with versus without KRT (information in the brackets refers to patients without KRT): baseline and peak serum creatinine, serum creatinine at discharge (each lower), baseline and peak BUN (each lower), and peak serum potassium (lower) (Table 3).
ROKF
Subjects without ROKF were more likely to reach AKI stage III, but they also had a lower prevalence of pre-existing CKD. Non-recovering patients had shorter in-hospital treatment times and significantly lower survival rates compared to patients who recovered. In the additional multiple linear regression analysis with independent variables including age, gender, duration of in-hospital treatment, survival, arterial hypertension, CHF, diabetes mellitus, and cardiovascular congestion, only survival was found to be independently associated with ROKF (P < 0.001). Additionally, the following laboratory parameters differed between individuals who recovered and those who did not (information in the brackets pertains to patients with ROKF): serum creatinine at discharge (lower), BUN at discharge (lower), serum potassium at discharge (lower), and baseline and minimum serum sodium (lower). Also, CRP at discharge was lower (Table 3).
| Discussion | ▴Top |
The study assesses a crucial aspect in sepsis and septic AKI: cardiovascular congestion. First, the term “cardiovascular congestion”, as used in the current study, needs to be elaborated upon. Essentially, in this study, it refers to a positive fluid balance, meaning an increase in total body water (and sodium) above the norm. Generally, the increase in total body water can occur due to illness, such as a complication of a condition like AKI with reduced diuresis. Alternatively, the positive balance may be of therapeutic origin. The present study did not differentiate between these two possible causes. If the methodology provided evidence of a positive fluid balance, it was defined as cardiovascular congestion. In the context of septic AKI, it may indicate cardiac involvement and support the diagnosis of CRS type 5 [9]. Current recommendations for treating sepsis involve giving large volumes of fluids over relatively short periods of time [19]. Systemic volume overload can have a significant impact on the prognosis of patients with AKI, as reported by Macedo and colleagues in 2010 [20]: firstly, expanding extracellular fluid can lead to dilution of creatinine in the extracellular space, potentially underestimating the severity of AKI. Fluid overload may, in addition, increase the likelihood of ROKF due to improved renal perfusion. However, it also raises the risk of mortality: Samoni and colleagues [21] performed a prospective trial in ICU treated patients and utilized bioelectrical impedance vector analysis for the assessment of hyperhydration. A fluid overload (FO) percentage of more than 10 indicated severe hyperhydration. Finally, the study included 125 individuals and found that severe hyperhydration was associated with ICU mortality. A 2018 published meta-analysis discussed 12 selected studies on bioelectrical impedance analysis and confirmed these findings [22]. Our study did not indicate differences in mortality rates between septic AKI patients with and without signs or symptoms of cardiovascular congestion (mortality rates 53.8% among patients diagnosed with congestion and 50% among those without the diagnosis). This discrepancy could have various reasons. On the one hand, we did not utilize bioelectrical impedance analysis. The hydration status was assessed by considering clinical, laboratory, radiological, and echocardiographic criteria. This approach does not allow for a numerical quantification of extracellular fluid volume. In contrast, bioimpedance analyses provide percentages for hydration, related to the lean body mass, for instance. As previously mentioned [20], a threshold of over 10% was identified as a risk factor for mortality. It is possible that in our cohort, this cutoff was mostly not exceeded. It should also be considered the positive influence of hyperhydration on the course of sepsis itself. According to current recommendations, positive fluid balance is mainly recommended in the early phases following the diagnosis of sepsis [19]. It is conceivable that the overhydration we observed was rather a reflection of successful sepsis management, ultimately resulting in a reduction of the risk of mortality.
The findings regarding KRT are somewhat surprising. There was no difference in the frequency of KRT performance between individuals with and without congestion. Hyperhydration is a crucial criterion in the decision to initiate dialysis. In our study, we assessed the prevalence of KRT throughout the entire ICU stay. It is important to consider that in the early stages of sepsis, hyperhydration may have been tolerated therapeutically to align with current guidelines for sepsis management. However, persistent overhydration following the initial phase worsens the prognosis of sepsis significantly [23]. It is not without reason that achieving a negative fluid balance again is recommended after the initial positive balance [24]. In our cohort, this goal seems to have been achieved without a significantly higher rate of KRT utilization.
It is almost most surprising that no differences in ROKF could be identified between patients with and without cardiovascular congestion. Considering the work of Macedo et al [20], while hyperhydration may potentially increase the risk of mortality, it could also enhance the chances of ROKF. Ensuring adequate renal perfusion is indeed one of the central goals in the management of AKI. While there are few established beneficial interventions for AKI, optimizing renal blood flow is certainly among them, as outlined in the KDIGO guidelines published in 2012 [16]. However, our study did not consider any hemodynamic data of the patients. Also not taken into account was the aspect of intravascular dehydration in conjunction with extravascular overhydration. This phenomenon manifests, for example, in more severe albumin deficiency states (such as liver cirrhosis, malabsorption). Although total body water may be expanded in these cases, there is sustained systemic underperfusion. Therefore, it remains unclear whether the cardiovascular congestion we observed was actually associated with an increase in renal perfusion.
The final risk factor analysis revealed few surprising aspects. Increased mortality, higher prevalence of CKD, and lower likelihood of ROKF were predominantly associated with higher serum concentrations of retention substances and hyperkalemia. Relatively surprising was the lower CKD prevalence in non-survivors. It is possible that not all patients defined as having CKD were actually chronically kidney diseased, at least not according to the KDIGO guideline published in 2012 [16]. To accurately make this diagnosis, one would need estimated glomerular filtration rate values and proteinuria from the 3 months prior to admission to the ICU. In our study, diagnoses from medical records mainly determined the documentation of CKD diagnosis.
Conclusions
It is justified not to consider clinically apparent cardiovascular congestion in septic AKI patients as a risk factor for death per se. Rather, especially in the case of sepsis, clinically apparent positive fluid balance does not seem to be a disadvantage in terms of survival, KRT, and ROKF. On the other hand, congestion does not significantly increase the chance of ROKF also.
Limitations
One limitation is the definition of cardiovascular congestion, which was primarily based on the presence of hypervolemia as indicated by increased NT-proBNP levels, along with clinical or radiographic signs of volume overload/pulmonary congestion. Echocardiographic findings were also considered. As mentioned at the beginning of the discussion, our methodology does not allow for the distinction between therapy-induced and predominantly disease-associated hypervolemia. We have essentially recorded the characteristic of a positive fluid balance. Our study design did also not allow for the grading of congestion, thus limiting the comparison with results from the cited bioimpedance analyses. Additionally, hemodynamic parameters were not documented, which could have provided more precise insights into the renal perfusion status. A minor limitation is the definition of pre-existing CKD, which relied solely on physician documentation lists. The retrospective design is also a limitation.
| ▴Top |
Acknowledgments
None to declare.
Financial Disclosure
No funding was provided for the study.
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
Not applicable.
Author Contributions
Charlotte Mund collected all data. Katharina Asmus helped in data collection and patient identification. Oliver Ritter assisted in writing. Dominique Petrus helped in patient identification. Susann Patschan prepared figures and helped in data analysis. Daniel Patschan designed the study, performed data analysis and wrote the article.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Bienholz A, Wilde B, Kribben A. From the nephrologist's point of view: diversity of causes and clinical features of acute kidney injury. Clin Kidney J. 2015;8(4):405-414.
doi pubmed pmc - Hoste EAJ, Kellum JA, Selby NM, Zarbock A, Palevsky PM, Bagshaw SM, Goldstein SL, et al. Global epidemiology and outcomes of acute kidney injury. Nat Rev Nephrol. 2018;14(10):607-625.
doi pubmed - Ympa YP, Sakr Y, Reinhart K, Vincent JL. Has mortality from acute renal failure decreased? A systematic review of the literature. Am J Med. 2005;118(8):827-832.
doi pubmed - Garcia-Carro C, Bolufer M, Bury R, Castaneda Z, Munoz E, Felip E, Lorente D, et al. Acute kidney injury as a risk factor for mortality in oncological patients receiving checkpoint inhibitors. Nephrol Dial Transplant. 2022;37(5):887-894.
doi pubmed - Chandiraseharan VK, Kalimuthu M, Prakash TV, George T, Rajenesh A, Jayaseelan V, Sudarsanam TD. Acute kidney injury is an independent predictor of in-hospital mortality in a general medical ward: a retrospective study from a tertiary care centre in south India. Indian J Med Res. 2020;152(4):386-392.
doi pubmed pmc - Sakr Y, Jaschinski U, Wittebole X, Szakmany T, Lipman J, Namendys-Silva SA, Martin-Loeches I, et al. Sepsis in intensive care unit patients: worldwide data from the intensive care over nations audit. Open Forum Infect Dis. 2018;5(12):ofy313.
doi pubmed pmc - Honore PM, Jacobs R, Joannes-Boyau O, De Regt J, Boer W, De Waele E, Collin V, et al. Septic AKI in ICU patients. diagnosis, pathophysiology, and treatment type, dosing, and timing: a comprehensive review of recent and future developments. Ann Intensive Care. 2011;1(1):32.
doi pubmed pmc - Lewis T. A clinical lecture on paroxysmal dyspnoea in cardiorenal patients: with special reference to "cardiac" and "uraemic" asthma: delivered at University College Hospital, London, November 12th, 1913. Br Med J. 1913;2(2761):1417-1420.
doi pubmed pmc - Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol. 2008;52(19):1527-1539.
doi pubmed - Di Lullo L, Bellasi A, Barbera V, Russo D, Russo L, Di Iorio B, Cozzolino M, et al. Pathophysiology of the cardio-renal syndromes types 1-5: an uptodate. Indian Heart J. 2017;69(2):255-265.
doi pubmed pmc - Hatamizadeh P, Fonarow GC, Budoff MJ, Darabian S, Kovesdy CP, Kalantar-Zadeh K. Cardiorenal syndrome: pathophysiology and potential targets for clinical management. Nat Rev Nephrol. 2013;9(2):99-111.
doi pubmed - Mehta RL, Rabb H, Shaw AD, Singbartl K, Ronco C, McCullough PA, Kellum JA. Cardiorenal syndrome type 5: clinical presentation, pathophysiology and management strategies from the eleventh consensus conference of the Acute Dialysis Quality Initiative (ADQI). Contrib Nephrol. 2013;182:174-194.
doi pubmed - Kotecha A, Vallabhajosyula S, Coville HH, Kashani K. Cardiorenal syndrome in sepsis: a narrative review. J Crit Care. 2018;43:122-127.
doi pubmed - Beesley SJ, Weber G, Sarge T, Nikravan S, Grissom CK, Lanspa MJ, Shahul S, et al. Septic cardiomyopathy. Crit Care Med. 2018;46(4):625-634.
doi pubmed - Assem A, Safi W, Ritter O, Patschan D. Electronic acute kidney injury alert at the Brandenburg Medical School: implementation and follow-up. Kidney Blood Press Res. 2023;48(1):701-709.
doi pubmed pmc - Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179-c184.
doi pubmed - Serafim R, Gomes JA, Salluh J, Povoa P. A comparison of the quick-SOFA and systemic inflammatory response syndrome criteria for the diagnosis of sepsis and prediction of mortality: a systematic review and meta-analysis. Chest. 2018;153(3):646-655.
doi pubmed - Inker LA, Astor BC, Fox CH, Isakova T, Lash JP, Peralta CA, Kurella Tamura M, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis. 2014;63(5):713-735.
doi pubmed - Dugar S, Choudhary C, Duggal A. Sepsis and septic shock: guideline-based management. Cleve Clin J Med. 2020;87(1):53-64.
doi pubmed - Macedo E, Bouchard J, Soroko SH, Chertow GM, Himmelfarb J, Ikizler TA, Paganini EP, et al. Fluid accumulation, recognition and staging of acute kidney injury in critically-ill patients. Crit Care. 2010;14(3):R82.
doi pubmed pmc - Samoni S, Vigo V, Resendiz LI, Villa G, De Rosa S, Nalesso F, Ferrari F, et al. Impact of hyperhydration on the mortality risk in critically ill patients admitted in intensive care units: comparison between bioelectrical impedance vector analysis and cumulative fluid balance recording. Crit Care. 2016;20:95.
doi pubmed pmc - da Silva AT, Hauschild DB, de Almeida Oliveira LD, de Fragas Hinnig P, Franco Moreno YM, Wazlawik E. Association of hyperhydration evaluated by bioelectrical impedance analysis and mortality in patients with different medical conditions: systematic review and meta-analyses. Clin Nutr ESPEN. 2018;28:12-20.
doi pubmed - Malbrain ML, Marik PE, Witters I, Cordemans C, Kirkpatrick AW, Roberts DJ, Van Regenmortel N. Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: a systematic review with suggestions for clinical practice. Anaesthesiol Intensive Ther. 2014;46(5):361-380.
doi pubmed - Vincent JL. Fluid management in the critically ill. Kidney Int. 2019;96(1):52-57.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


