| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Case Report
Volume 16, Number 4, April 2024, pages 189-195
Successful Implementation of Combined Treatment Methods for a Rare Recurrence of Waldenstrom Macroglobulinemia With Extramedullary Lesions
Irina Yakovlevna Sokolovaa , Sabina Maximovna Sokolovaa
, Olga Valentinovna Bochkarnikovaa
, Ayshat Eldarovna Rasulovab
, Timur Raisovich Izmailovc
, Pavel Vladimirovich Polushkind
, Yulia Yurevna Kirichenkob, e
, Yuriy Nikitich Belenkovb
, Irina Sergeevna Ilgisonisb
aDepartment of Hematology, University Clinical Hospital No.1, Sechenov University, Moscow 119048, Russia
bDepartment of Hospital Therapy No.1, Sechenov University, Moscow 119048, Russia
cDepartment of Oncology, Federal State Budgetary Institution National Medical and Surgical Center Named After N.I. Pirogov of the Ministry of Health of Russia, Moscow 105203, Russia
dFederal State Budgetary Institution “Russian Scientific Center for Roentgen Radiology” of the Ministry of Health of the Russia, Moscow 117997, Russia
eCorresponding Author: Yulia Yurevna Kirichenko, Department of Hospital Therapy No.1, Sechenov University, Moscow 119048, Russia
Manuscript submitted January 23, 2024, accepted March 9, 2024, published online April 30, 2024
Short title: Treatment for a Rare Case of WM
doi: https://doi.org/10.14740/jocmr5115
| Abstract | ▴Top |
A 67-year-old woman was admitted to the Hematology Department in 2014 with complaints of weakness and a low-grade fever. After conducting various tests, it was confirmed that she had Waldenstrom macroglobulinemia. She underwent several rounds of chemotherapy and maintenance therapy with rituximab, which resulted in a good clinical response. However, in 2019, an abnormal growth in the soft tissues of patient’s frontal region was discovered, which was diagnosed as lymphoplasmacytic lymphoma. This later progressed to an intracranial lesion. The patient underwent radiation therapy for both the extramedullary and intracranial growths, which had a positive effect. A year later, she developed a lesion in her lymph nodes and soft tissues of her right leg, which was confirmed to be a recurrence of Waldenstrom disease. She underwent further treatment and is currently in complete remission. This case highlights the rare occurrence of relapse in Waldenstrom disease and the challenges in diagnosing extramedullary lesions. It also demonstrates the success of modern treatment approaches using a combination of therapies.
Keywords: Waldenstrom macroglobulinemia; Extramedullary lesions; Long-wave radiation therapy; Meninges lesions
| Introduction | ▴Top |
Waldenstrom macroglobulinemia (WM) is a B-cell lymphoplasmacytic lymphoma characterized by immunoglobulin (Ig)M secretion and chronic indolent course [1, 2]. In the structure of all hemoblastosis, the morbidity of Waldenstrom disease is no more than 2%. The annual incidence in the world is two to five cases per 1 million populations. At the same time, the average age of patients is 63 years, and men get sick more often (the incidence rate is about 70%) [2]. However, there is currently no documented statistical information on WM incidence in the Russian Federation.
The etiology of WM continues to be unknown. One of the possible reasons affecting the further development of the disease is considered as genetic predisposition (mutation of the MYD88 protein gene) [3, 4]. The MYD88 gene is localized on the short arm of chromosome 3 of macrophages and B-lymphocytes and encodes the MYD88 protein of the same name. The function of this protein is to transmit a signal from membrane toll-like receptor (TLR) to the NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) group of proteins (NF-κB1, NF-κB2, RelA, RelB) [5, 6]. One of the most important functions of the nuclear transcription factor NF-κB is to protect cells from programmed apoptosis. With an increase in NF-κB activity, cells with this mutation acquire the properties of “pathological survival”, which do not respond to blocking signals. Thus, the MYD88 gene mutation promotes uncontrolled proliferation of lymphocytes, which in turn leads to the formation of a tumor substrate for lymphoma. At the moment, a large number of various MYD88 mutations are being identified; one of the most common variants is the L265P substitution, which occurs in 90% of patients with WM.
Typical laboratory features are anemia, thrombocytopenia, elevated erythrocyte sedimentation rate (ESR) (> 30 mm/h), hyperproteinemia of blood serum due to paraprotein secretion. However, it should be noted that at the beginning of the disease, peripheral blood counts may be within the normal range. Often the leukocyte blood formula remains without clinically significant changes. An electropherogram of blood proteins reveals an M-gradient in the fraction of gamma globulins. In most patients, there is an increase in the level of C-reactive protein (CRP), possibly increased β2-microglobulin level [2].
Waldenstrom disease is characterized by chronic course with slow progression. According to Olmer et al [6], and Alexanian et al [7], cases of completely asymptomatic course of the disease have also been described. Usually, the main complaints are nonspecific and characterize the intoxication syndrome.
One of the manifestations may be peripheral polyneuropathy (impairment of tactile, pain sensitivity as “gloves” and “socks” symptom, paresthesia), the substrate of which is lymphoid-plasmacytic infiltration of nerve fibers, secondary amyloidosis, accumulation of pathological IgM in the endo- and perineurium (Table 1).
 Click to view | Table 1. Frequency of the Main Clinical and Laboratory Manifestations of Waldenstrom Disease |
Clinical manifestations of WM are due to lymphoid elements’ proliferation in the bone marrow, lymph nodes, spleen, liver, lungs, skin, gastrointestinal tract, as well as the secretion of IgM in the blood serum as a component of the protein pathology syndrome. In cytological samples, large lymphocytes with a rim of plasmatized cytoplasm are predominantly found. To a lesser extent, a large number of monocytoid cells, small lymphocytes, and mast cells are found. Histological examination of the bone marrow in most patients reveals diffuse lymphoid-plasmacytic infiltration. Pronounced fibrosis of the bone marrow stroma is a significant differential sign from multiple myeloma [8].
When establishing the diagnosis of WM, the following criteria must be present: monoclonal IgM (regardless of paraprotein level); small lymphocyte, plasmacytoid-like and plasma cell infiltration of the bone marrow with a characteristic immunohistochemical (IHC) pattern [8]. Clonal WM lymphocytes are characterized by constant expression of pan-B markers (CD19, CD20, CD22, CD24). CD10 antigen is not expressed in WM patients, less than one-fifth of patients are mostly positive for CD5 and CD23, while CD27, CD45RA and B-cell lymphoma 2 (BCL-2) are present in most malignant cells. Pathognomonic mutation of MYD88 L265P in a cytogenetic study is identified [5]. The differential diagnosis should include mainly monoclonal gammopathy of uncertain significance, Ig-secreting lymphomas, and multiple myeloma.
Particular attention should be paid to the diversity and complexity of diagnosing possible extramedullary lesions. In domestic literature for the moment there are no cases describing such lesions. However, when analyzing foreign experience, there are descriptions of cases with lesions in the lungs, terminal parts of the colon, stomach, hard palate, and humerus diaphysis [3, 6, 9-14]. Particularly noteworthy is the damage to the central nervous system, the meninges, and the involvement of the cerebrospinal fluid in the pathological process, which forms the pathogenetic basis of the Bing-Neеl syndrome and is classified as a complication of WM [15].
The team of authors presents a clinical observation of a patient, who was observed for a long time in the Hematology Department of the Sechenov’s University Clinical Hospital No.1, with a diagnosis of WM. This description is of particular clinical and practical interest due to the appearance of rare extramedullary lesions, uncharacteristic for this hemoblastosis, in the relapse of the disease (formations of the frontal region soft tissues, intracranial formations involving the pia mater, formation of soft tissues of the anterior surface of the right leg). The histological substrate of all lesions was Waldenstrom’s lymphoplasmacytic lymphoma. Modern combined methods of treatment (chemotherapy (CMT), conformal external beam radiation therapy) made it possible to achieve complete remission of the disease and improve the long-term prognosis of the patient.
| Case Report | ▴Top |
Patient G., a 67-year-old woman, was first in-charged to the Hematology Department of the Sechenov’s University Clinical Hospital No.1, in September 2014 with complaints of general weakness, fatigue, sweating, and episodes of low-grade fever without an objective reason. The complete blood count (CBC) revealed normochromic anemia (minimum hemoglobin (Hb) level 75 g/L), the level of platelets, leukocytes, leukocyte count was normal, ESR elevated to 65 mm/h. In the biochemical blood analysis, attention was first paid to hyperproteinemia up to 87 g/L (normal range: 65 - 82 g/L) with an albumin level of 35 g/L (normal range: 32 - 46 g/L), dysproteinemia in the spectrum of gamma globulins: α1-globulin 5.1% (normal range: 2.9-4.9%), α2-globulin 10.4% (normal range: 7.1-11.8%), β2-globulin 8.9% (normal range: 7.9-13.7%), γ-globulin 9.3% (normal range: 11.1-19.8%), M-protein 23.7%. At the same time, biochemically, the level of total calcium was 2.1 mmol/L (normal range: 2.15 - 2.58 mmol/L), lactate dehydrogenase (LDH) 235 U/L (normal range: 240 - 480 U/L), creatinine 48 µmol/L (normal range: 44 - 80 µmol/L), calculated glomerular filtration rate (GFR) 97.6 mL/min/1.73 m2. IHC examination of blood serum and urine by immunofixation method revealed paraprotein IgM kappa-type 19.2 g/L (normal range: 0); at the same time, the level of normal Igs was not reduced, secretion of free light chains, Bence-Jones proteinuria was not detected. There was an increase in the level of CRP up to 20 g/L (normal range: 0 - 5 g/L), β2-microglobulin up to 4.5 mg/L (normal range: 0.9 - 2.0 mg/L). Physical and instrumental examination (computed tomography (CT) of the chest, abdomen, pelvic area with intravenous (IV) contrast) did not reveal peripheral and intracavitary lymphadenopathy or splenomegaly. Organic pathology of the gastrointestinal tract (gastro- and colonoscopy) was not detected.
During the examination, a specific histological (trepanobiopsy) and cytological examination of the bone marrow was performed. Trepanobiopsy, IHC study of the bone marrow showed bone beams with bone resorption phenomena; in the bone marrow cavities it was showed focal-intestinal infiltrates of small lymphoid cells with round-oval and irregular nuclei, with signs of plasmacytic differentiation, located inter- and paratrabecularly, and mature plasma cells are present; the granulocytic germ is expanded, represented by elements of varying degrees of maturity, and mature generations predominate; the erythroid germ is relatively narrowed; megakaryocytes are located unevenly; their number is reduced, mostly small in size with hyposegmented nuclei; mastocytes are present on Giemsa stain. IHC study revealed lymphoid infiltrate cells that almost monomorphically express CD20, mature plasma cells which express VS38c+, some cells that express pathological IgM; there are signs of kappa-chain restriction (lambda positive), and mature plasma cells are few; reaction with cyclinD1 is positive in histiocytes; lymphoid cells are negative; scattered T cells (CD3+) are present among the tumor infiltrates. The conclusion was that morphological and IHC-results satisfied the criteria for lymphoplasmacytic Waldenstrom disease.
The patient underwent a molecular study by real-time polymerase chain reaction, and the MYD88 L265P mutation was detected on the bone marrow.
Thus, based on the examination data, Waldenstrom disease was diagnosed in accordance with the Clinical Guidelines of the Russian Federation Ministry of Health for the Diagnosis and Treatment of WM 2014 [16].
During the first and subsequent hospitalizations, antitumor therapy was carried out (MP (melphalan + prednisolone) program three courses: Alkeran 14 mg/day for 4 days, prednisone 90 mg/day, followed by withdrawal for 4 days) in November 2014, February 2015, and April 2015. Against the background of the therapy, there was a clinical improvement (decrease in the severity of asthenic syndrome, normalization of body temperature), a laboratory decrease in the level of paraprotein. However, with the abolition of prednisone, weakness, sweating and low-grade fever resumed. In the control blood tests, an increase in the level of paraprotein was again noted. The CMT program was changed to M-2 (melphalan + cyclophosphamide: eight courses from July 2015 to 2017: cyclophosphamide 600 mg IV, Alkeran 14 mg/day, prednisone 0.5 mg orally for 7 days, followed by a gradual decrease to cancellation) with an incomplete effect: there was an increase in the level of Hb to 105 - 110 g/L, normalization of the level of total protein, a decrease in the level of the M-gradient to 8.1 g/L with persistent daily low-grade fever, weakness, fatigue, elevated levels of CRP and fibrinogen. Due to persistent intoxication syndrome, preservation of the secretion of the kappa-type IgM paraprotein, the patient was transferred to RCD regime (rituximab, cyclophosphamide, dexamethasone), with six courses from January 2018 to June 2018. Against the background of this CMT, there was an improvement in general well-being, the absence of asthenic syndrome, stable normothermia, normalization of the Hb level to 126 - 128 g/L, normalization of the total protein level, a decrease in the paraprotein level to 1.6 g/L, and normalization of acute-phase inflammation indicators. Subsequently, two courses of maintenance therapy with rituximab were carried out (September 2018, December 2018).
It is noteworthy, that since April 2019 the patient noted the appearance of a subcutaneous dense, painless formation of the frontal region, which was initially regarded by oncologists as an osteoma. At the same time, the formation gradually increased in size up to 3 cm, and she had a biopsy in August 2020. According to the cytological study of the biopsy specimen, the substrate of the formation was lymphoplasmacytic lymphoma. Taking into account the preservation of normal parameters of clinical and biochemical blood tests, stable minimum level of paraprotein secretion and the patient’s subjective well-being at the described point of time, it was decided to refer the patient to radiation therapy.
The Russian Scientific Center for Roentgen Radiology carried out remote beam therapy using a high-energy linear accelerator. At the first stage, pre-radiation preparation was carried out, and an individual fixing device was made. The thermoplastic mask was installed on a carbon deck with the selection of a certain size of the headrest. After that, CT marking with immobilization (headrest, blackboard, mask) was performed. At the second stage, the contouring of the exposure volumes and the allocation of dose-limiting organs, three-dimensional (3D) volumetric dosimetric planning, and verification of the prepared exposure plan were carried out. From October 21, 2020, to November 17, 2020, a course of conformal remote radiation therapy was carried out on a high-tech linear accelerator equipped with a multileaf collimator on the subcutaneous formation of the right forehead area using a single focal dose (SFD) of 2 Gy to a total radiation dose (TRD) of 36 Gy. Radiation sessions were carried out under the control of portal imaging. After that, stabilization of the underlying disease was noted; however, according to brain magnetic resonance imaging (MRI), intracranial formations of the meninges were visualized as asymptomatic finding during a routine examination. The patient was consulted by a neurologist; there were no complaints or evidence of focal neurological symptoms. The patient refused to carry out a biopsy of intracranial lesions.
A study of cerebrospinal fluid was performed with analysis of lymphocytes’ immunophenotyping by flow cytometry. According to the study, no specific lymphoplasmacytic infiltration was obtained; the lymphocytes were polyclonal and predominantly T-cytotoxic lymphocytes and macrophages. According to the results of the tests and the lack of evidence of damage to the central nervous system in Waldenstrom disease, there were no absolute indications for initiating high-dose methotrexate therapy. A multidisciplinary team (hematologist, oncologist, radiotherapist, neurosurgeon, and radiologist) decision was held out: necessity of remote radiation therapy course for intracranial formations of the brain meninges in the right temporal and parietal region, the left temporal-fronto-parietal region.
Before the start of radiation therapy, pre-radiation preparation of the patient was again carried out, which included the manufacture of an individual fixing thermoplastic mask, CT topometry, contouring of radiation volumes taking into account the MRI image and isolation of dose-limiting organs and tissues, volumetric dosimetric planning of radiation therapy and verification of the radiation plan. From January 18, 2021, to October 2, 2021, a course of remote conformal radiation therapy was carried out according to a radical program on a high-energy medical linear accelerator using the RapidArc method. The scope of radiation included the area of intracranial formations of the meninges of the brain in the right temporal and parietal regions, the left temporal-frontal-parietal regions with the capture of subclinical distribution zones. The radiation regime was applied five times a week, using SFD 2 Gy with summing up TRD 36 Gy (totally 18 days). The course of radiation therapy was carried out under the control of portal imaging. The patient tolerated radiation therapy satisfactorily, and no concomitant therapy was used (Figs. 1, 2).
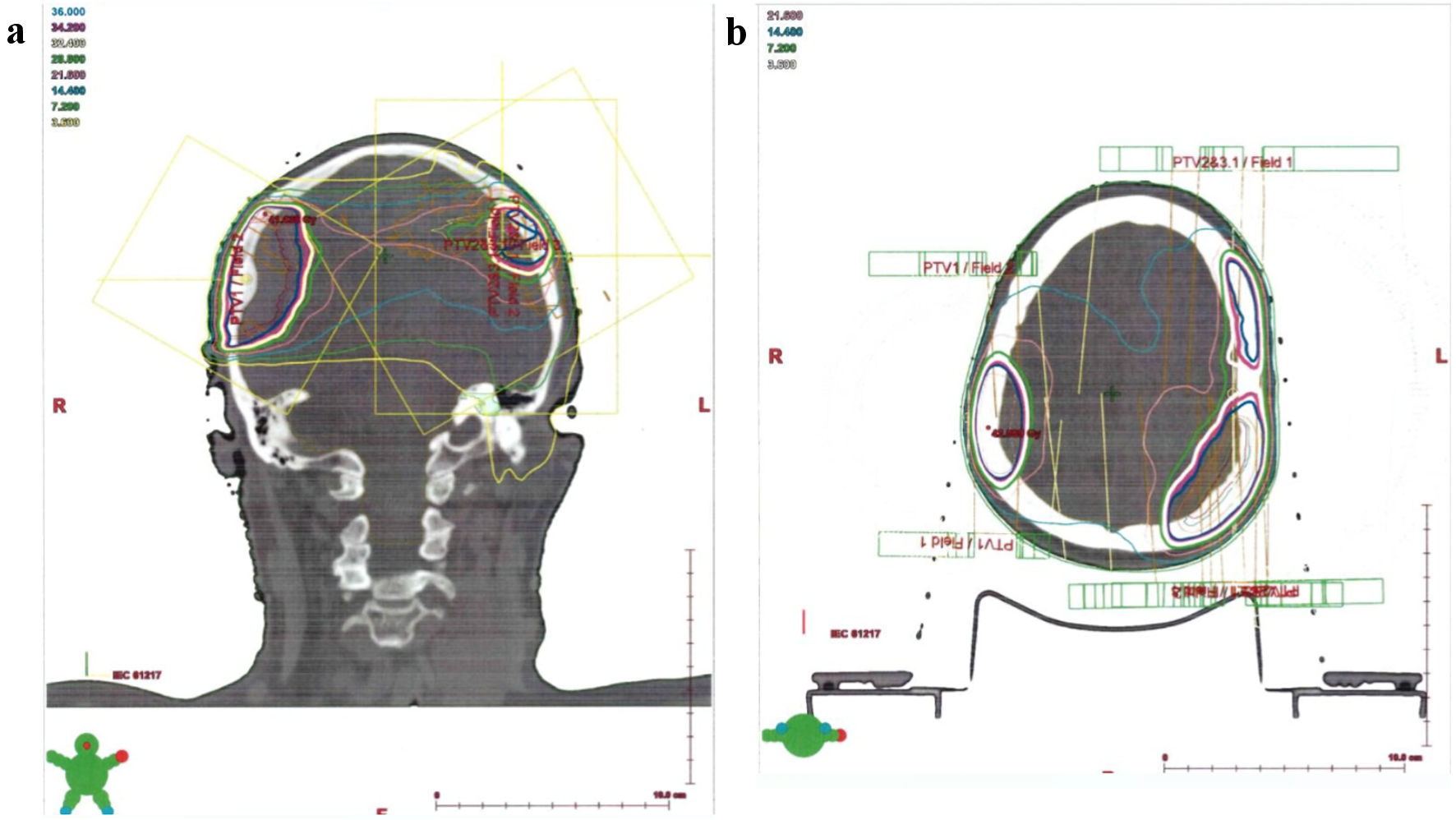 Click for large image | Figure 1. (a, b) Isodose distribution of radiation volume of patient G., who was 67 years old, with the diagnosis of Waldenstrom macroglobulinemia with extramedullary lesion (frontal and axial sections). |
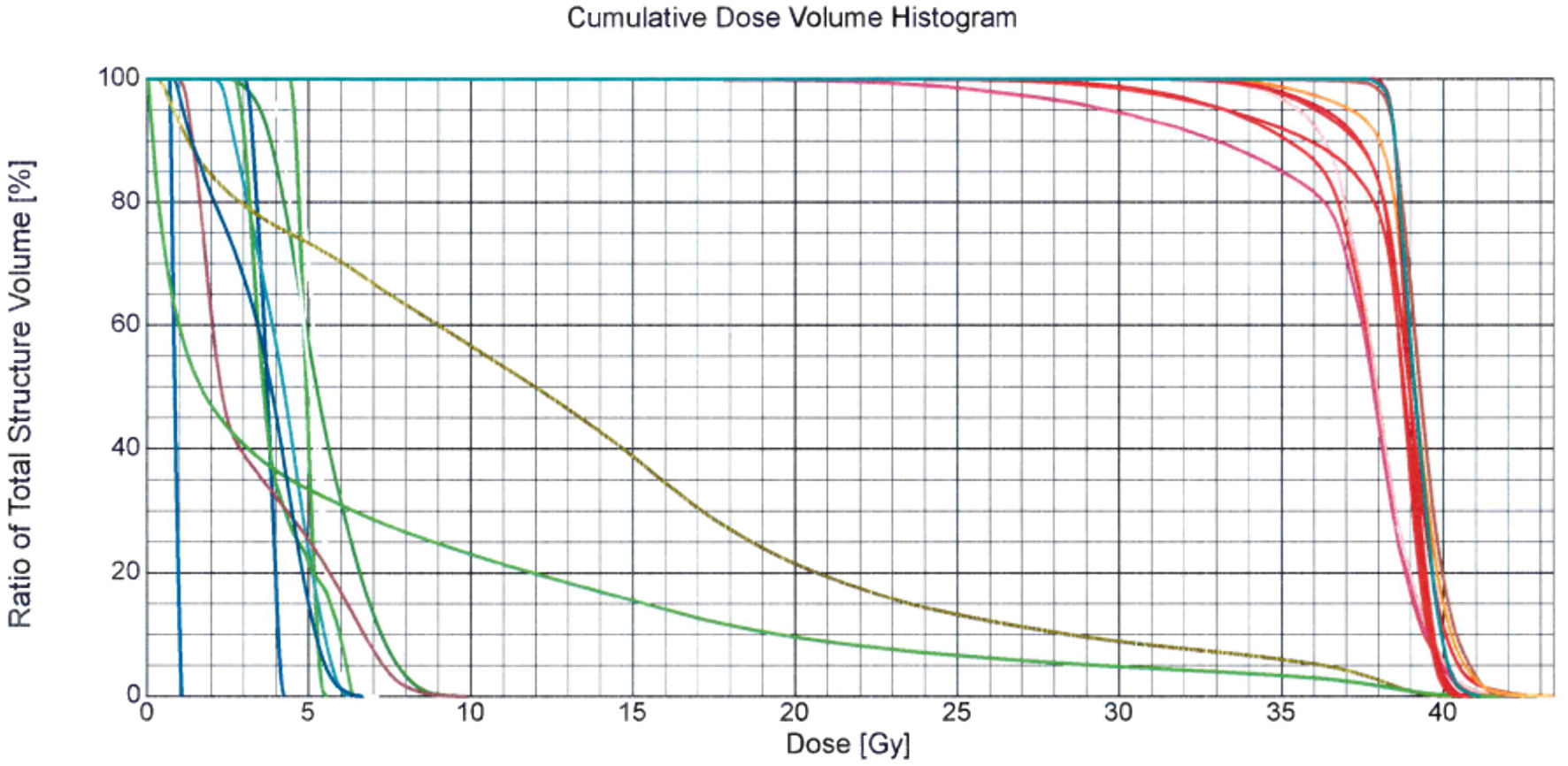 Click for large image | Figure 2. Dose-volume histogram of the radiation therapy plan of patient G., who was 67 years old, with a diagnosis of Waldenstrom macroglobulinemia with extramedullary lesion. According to the control brain MRI with contrasting, a positive trend was noted in the form of a decrease in intracranial formations of the meninges of the brain in the right temporal and parietal region, and in the left temporal-fronto-parietal region. MRI: magnetic resonance imaging. |
In June 2021, the patient suffered from a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, CT-stage 4, complicated by acute respiratory failure and sepsis (received biological therapy with levilimab, corticosteroid and antibiotic therapy), and she was on in-patient treatment for 2 months until August 2021. After recovery, for the first time she noted the appearance of a dense rounded formation on the lateral surface of the lower third of the right leg, hyperemia and burning feeling of this area was periodically observed. In December 2021 the patient was again hospitalized to the Hematology Department of Sechenov’s University. Physical examination revealed for the first time extensive isolated right-sided inguinal and femoral lymphadenopathy up to 6 cm; palpation determined the formation of the lower third of the right leg up to 7 - 8 cm in size, protruding 1 - 2 cm above the skin surface, accompanied by moderate soreness, hyperemia and skin swelling in this area. According to the results of positron emission tomography-computed tomography (PET-CT) scan, metabolic activity was determined in the right inguinal, femoral lymph nodes with the maximum standardized uptake value (SUVmax) of 5.6; splenomegaly, lymphadenopathy of other localization was not revealed. However, the soft tissue area of the lower leg was not included in the PET-CT study. The patient underwent a CT scan with IV contrast to clarify the nature of the formation, according to which there was an active accumulation of contrast in the soft tissues of the right leg.
Excisional biopsy of the inguinal lymph node and core biopsy of the right leg soft tissue formation were performed. According to histological and IHC studies, lymphoplasmacytic lymphoma was also a substrate for lymph node biopsy and soft tissue formation. Thus, there was a recurrence of WM [17]. At the same time, CBC parameters remained within the normal range, as well as the level of total protein, secretion of paraprotein M was 2.3 g/L. Then, two courses of CMT (RD program: rituximab, dexamethasone) were conducted. According to control CT scan and ultrasound, there was a decrease in the size of the right inguinal and femoral lymph nodes, but the formation size of right leg soft tissues and monoclonal secretion remained at the same level, as a result of which the program was changed to BRD (bortezomib, rituximab, dexamethasone). By present time, three courses of BRD program have been carried out with positive clinical and instrumental dynamics: paraprotein secretion (immunoelectrophoresis) is not detected, lymphadenopathy, and formation of right leg soft tissues have regressed. In the future, it is planned to conduct a control examination with the determination of prolonged therapeutic tactic and the patient’s monitoring plan.
Compliance with ethics guidelines
The study was approved by the Local Ethics Committee of the Federal State Autonomous Educational Institution of Higher Education I.M. Sechenov First Moscow State Medical University of the Ministry of Health of the Russian Federation (Sechenov University), and the study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
| Discussion | ▴Top |
Lymphoplasmacytic lymphoma continues to be a fairly rare form of B-cell lymphoma. At the time of writing this publication, the authors did not find similar cases of extramedullary lesions in WM in domestic sources. However, colleagues from the National Defense Medical Center (Taipei, Taiwan) described a clinical case of a 62-year-old man with WM with rapid development (within 3 months) of soft tissue, painless, non-adhered to the surrounding subcutaneous fat lesions on the right shoulder (7 × 5 × 4 cm), and in the left elbow joint area (4 × 3 × 2 cm) [3]. Clonal lymphoplasmacytic cells were present in all lesions according to the IHC study of the biopsy specimen. According to the results of studies, including IHC study and bone marrow flow cytometry, the presence of expression of clonal IgM (13,000 mg/dL) and CD20 was confirmed, with absence of CD3, CD5 and CD10 expression. There was a history of hemorrhagic syndrome (gingival bleeding at the time of dental intervention during the week before treatment, recurrent nasal bleedings over the past 2 years), weight loss 2 kg monthly for several years, visual impairment of the right eye, progressive general weakness. Laboratory results revealed anemia (Hb 82 g/L), thrombocytopenia 85 ×109; hyperproteinemia 118 g/L, hypoalbuminemia 26 g/L; signs of coagulopathy with prolongation of prothrombin time and activated partial thromboplastin time (APTT); a significant increase in blood serum ratio of free light chains with a predominance of kappa-type light chains, the value of blood serum viscosity was 9.2. Cryoglobulinemia, synthesis of autoantibodies was not detected. The main life-supporting functions of the body were preserved, dialysis-independent (kidney function is normal), no auditory impairment has been identified, the patient was fully capable of self -care. During the period of hospitalization, the patient underwent courses of R-CHOP (rituximab, cyclophosphamide, hydroxydaunorubicin, oncovin, prednisolone) CMT program. Subsequently, serum IgM levels were restored to normal levels, and extramedullary lesions regressed, achieving a complete response to treatment (according to the criteria of the Third International Seminar on Waldenstrom’s macroglobulinemia).
Extramedullary lesions in Waldenstrom disease are extremely rare and are associated with difficulties for correct interpretation. In the presented clinical case, the formation of frontal region soft tissues that first appeared, initially, according to CT scan and consultation with an oncologist, was undoubtedly regarded as an osteoma, and only subsequent morphological verification with a progressive growth of the formation made it possible to diagnose a rare variant of extramedullary lesions. At the same time, the occurrence of extramedullary lesions was not accompanied by intoxication syndrome; normal levels of total protein and minimal secretion of paraprotein were observed. Thus, there was no need to intensify systemic CMT. In clinical practice, extramedullary lesions of soft tissues may be encountered primarily by various specialists: dermatologists, surgeons, oncologists; with extra- and intracranial lesions of the meninges - neurosurgeons, neurologists. The description of such rare lesions in Waldenstrom disease will allow colleagues to suggest, among other things, a specific lymphoplasmacytic lesion that requires morphological verification, especially in the presence of a history of WM. The possibility of using modern combined methods of treatment (complex radiation therapy, CMT) is particularly important, which made it possible to treat this patient with an optimal result and improve her long-term prognosis.
Conclusions
In the described clinical case, the authors draw attention of the scientific medical community to a rare variant of diagnosed WM with extracranial lesions of the frontal region soft tissues, intracranial lesions of the meninges, lesions of the soft tissues of the lower extremity, the complexity of verifying genesis of lesions and their atypical nature for this disease. The treatment of this patient required the use of combined methods (CMT, radiation therapy), which made it possible to achieve a stable positive effect. Currently, the patient’s condition is regarded as a complete remission of the disease, and regular control monitoring of the patient is carried out.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
All authors declare that they have no conflict of interest and financial support.
Informed Consent
Informed consent was obtained from the patient.
Author Contributions
S.I.Y., S.S.M. and R.A.E. performed literature search, data analysis and manuscript writing. B.O.V., I.T.R., P.P.V. and K.Y.Y. participated in data analysis and original draft preparation. I.I.S. and B.Y.N. reviewed and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
3D: three-dimensional; BRD: bortezomib, rituximab, dexamethasone; CBC: complete blood count; CMT: chemotherapy; CRP: C-reactive protein; CT: computed tomography; ESR: erythrocyte sedimentation rate; GFR: glomerular filtration rate; IHC: immunohistochemistry; LDH: lactate dehydrogenase; М-2: melphalan, cyclophosphamide; MP: melphalan, prednisolone; MRI: magnetic resonance imaging; WM: Waldenstrom macroglobulinemia; MYD88: myeloid differentiation primary response 88; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; PET-CT: positron emission tomography-computed tomography; RCD: rituximab, cyclophosphamide, dexamethasone; R-CHOP: rituximab, cyclophosphamide, hydroxydaunorubicin, oncovin, prednisolone; RD: rituximab, dexamethasone; SFD: single focal dose; TRD: total radiation dose
| References | ▴Top |
- Dimopoulos MA, Kastritis E. How I treat Waldenstrom macroglobulinemia. Blood. 2019;134(23):2022-2035.
doi pubmed - Jalali S, Ansell SM. The bone marrow microenvironment in Waldenstrom macroglobulinemia. Hematol Oncol Clin North Am. 2018;32(5):777-786.
doi pubmed - Minzenmayer AN, Miranda RN, Powell PR, Parekh PK. An unusual case of cutaneous Waldenstrom macroglobulinemia with the MYD88 L265P mutation. J Cutan Pathol. 2020;47(9):850-853.
doi pubmed - Treon SP, Xu L, Guerrera ML, Jimenez C, Hunter ZR, Liu X, Demos M, et al. Genomic landscape of Waldenstrom macroglobulinemia and its impact on treatment strategies. J Clin Oncol. 2020;38(11):1198-1208.
doi pubmed pmc - Ngo VN, Young RM, Schmitz R, Jhavar S, Xiao W, Lim KH, Kohlhammer H, et al. Oncogenically active MYD88 mutations in human lymphoma. Nature. 2011;470(7332):115-119.
doi pubmed pmc - Olmer J, Mongin M, Muratore R, Gabriel B. [Long-term asymptomatic development of Waldenstrom's macroglobulinemia]. Nouv Rev Fr Hematol. 1967;7(6):894-896.
pubmed - Alexanian R, Weber D, Delasalle K, Cabanillas F, Dimopoulos M. Asymptomatic Waldenstrom's macroglobulinemia. Semin Oncol. 2003;30(2):206-210.
doi pubmed - Seiter K, Ponce D, Besa E, et al. NCCN Clinical Practice Guidelines in Oncology: Waldenstrom Macroglobulinemia/Lymphoplasmacytic Lymphoma. 2023. Version 1.
- Chang CY, Yeh RH, Chen JH, Wu YY, Huang TC, Chang PY, et al. Case report unusual presentation of waldenstrom macroglobulinemia. J of Cancer Research and Practice. 2018;5(1):35-37.
doi - Abdulfattah O, Rahman EU, Bhattarai B, Dahal S, Alnafoosi Z, Trauber D, Enriquez D, et al. Lung consolidation as a rare presentation of lymphoplasmacytic lymphoma with extramedullary Waldenstrom's macroglobulinemia. J Community Hosp Intern Med Perspect. 2018;8(2):68-72.
doi pubmed pmc - Ravipati P, Bu L, Sachs Z, Nachman PH. Lymphomatous infiltration of the kidney in a patient with Waldenstrom's macroglobulinemia. Clin Nephrol Case Stud. 2022;10:87-90.
doi pubmed pmc - Zelman S, Russell MB, Hojat A, Pilichowska M, Olans LB, Sterling MJ. A rare case of Waldenstrom macroglobulinemia of the rectosigmoid colon. ACG Case Rep J. 2021;8(11):e00689.
doi pubmed pmc - Zhao DF, Ning HY, Cen J, Liu Y, Qian LR, Han ZH, Shen JL. Extensive multifocal and pleomorphic pulmonary lesions in Waldenstrom macroglobulinemia: a case report. World J Clin Cases. 2020;8(11):2305-2311.
doi pubmed pmc - Attallah HS, Moonim M, Fields P, Wrench D, Brady J, Mikhaeel NG. Primary isolated lymphoplasmacytic lymphoma (LPL) of the stomach: a case report. Am J Case Rep. 2020;21:e921840.
doi pubmed pmc - Awad AK, Elbadawy MA, Boury M, Rivera A, Motawea K, Shah J, Parnia S, et al. Simple headache revealed a rare lymphoma: Waldenstrom macroglobulinemia with unique markers: a case report and review of the literature. J Egypt Natl Canc Inst. 2022;34(1):10.
doi pubmed - Mendeleeva L, Votiakova O, Stadnik E, Falaleeva N, Poddubnaya I, et al. Clinical guidelines of macroglobulinemia Waldenstroma of the Ministry of Health of the Russian Federation. 2014. (in Russian).
- Parovichnikova E, Poddubnaya I, Levkovskiy O, Varfolomeeva S, et al. Clinical guidelines of macroglobulinemia Waldenstroma of the Ministry of Health of the Russian Federation. 2022. (in Russian).
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


