| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Original Article
Volume 15, Number 8-9, September 2023, pages 415-422
Opioid Free Total Intravenous Anesthesia With Dexmedetomidine-Esketamine-Lidocaine for Patients Undergoing Lumpectomy
Xia Li Qiana, b, Ping Lia, b, Ya Jie Chena, Shi Qin Xua, Xian Wanga, c, Shan Wu Fenga, c
aDepartment of Anesthesiology, Women’s Hospital of Nanjing Medical University, Nanjing Maternity and Child Health Care Hospital, Nanjing, Jiangsu Province, China
bThese two authors contributed equally to this work.
cCorresponding Author: Xian Wang and Shan Wu Feng, Department of Anesthesiology, Women’s Hospital of Nanjing Medical University, Nanjing Maternity and Child Health Care Hospital, Nanjing, Jiangsu Province, Chinaand
Manuscript submitted July 21, 2023, accepted August 18, 2023, published online September 30, 2023
Short title: OFA for Patients Undergoing Lumpectomy
doi: https://doi.org/10.14740/jocmr5000
| Abstract | ▴Top |
Background: The aim of the study was to evaluate the feasibility of the opioid-free anesthesia (OFA) technique with dexmedetomidine, esketamine, and lidocaine among patients diagnosed with benign breast mass and scheduled for lumpectomy.
Methods: We enrolled 80 female patients who were aged from 18 to 60 years, graded with American Society of Anesthesiologists physical status I or II, diagnosed with benign breast mass, and scheduled for lumpectomy. These patients were randomly treated with OFA or opioid-based anesthesia (OBA). Dexmedetomidine-esketamine-lidocaine and sufentanil-remifentanil were administered in OFA and OBA group, respectively. We mainly compared the analgesic efficacy of OFA and OBA technique, as well as intraoperative hemodynamics, the quality of recovery, and satisfaction score of patients.
Results: There was no significant difference between the two groups with regard to visual analogue scale (VAS) score at 2, 12, and 24 h after extubation. However, the time to first rescue analgesic was prolonged in OFA group than that in OFB group (6.18 ± 1.00 min vs. 7.40 ± 0.92 min, P = 0.000). Further, mean arterial pressure and heart rate at T0 (entering operating room), T1 (before anesthesia induction), T2 (immediately after intubation), T3, T4, and T5 (1, 5, and 10 min after surgical incision, respectively) were significantly higher in OFA group than that in OBA group. Incidence of hypotension and bradycardia was lower in OFA group. Consistently, fewer patients in OFA group consumed atropine (8% vs. 32%, P = 0.019) and ephedrine (5% vs. 38%, P = 0.001) compared to OBA group. Furthermore, patients in OFA group had a longer awakening time (7.14 ± 2.63 min vs. 4.54 ± 1.14 min, P = 0.000) and recovery time of orientation (11.76 ± 3.15 min vs. 6.92 ± 1.19 min, P = 0.000). Fewer patients in the OFA group experienced postoperative nausea and vomiting (PONV) (11% vs. 51%, P = 0.000) and consumed ondansetron (5% vs. 35%, P = 0.003) compared to OBA group. And patients in OFA group had a higher satisfaction score than those in OBA group (9 (8 - 9) vs. 7 (7 - 8), P = 0.000).
Conclusion: For patients undergoing lumpectomy, OFA technique with dexmedetomidine-esketamine-lidocaine showed a better postoperative analgesic efficacy, a more stable hemodynamics, and a lower incidence of PONV. However, such advantage of OFA technique should be weighed against a longer awakening time and recovery time of orientation in clinical practice.
Keywords: Opioid-free anesthesia; Dexmedetomidine; Esketamine; Lidocaine; Lumpectomy
| Introduction | ▴Top |
Opioids are usually associated with various adverse events such as respiratory depression, muscle rigidity, pruritus, chills, urinary retention, nausea, vomiting, drug tolerance, addiction, and hyperalgesia [1, 2]. Opioid tolerance and hyperalgesia can lead to increased postoperative opioid use and related side effects [3]. The traditional opioid-based anesthesia (OBA) technique is increasingly being challenged due to opioid-related adverse events [4] and the global opioid crisis. Accordingly, diverse opioid-sparing or opioid-free anesthesia techniques have been explored.
Some studies suggested a multimodal administration of nonopioid agents including α2-receptor agonists such as clonidine and dexmedetomidine, N-methyl-D-aspartate (NMDA) antagonists such as ketamine and esketamine, non-steroidal anti-inflammatory drugs, as well as local anesthetics such as lidocaine can provide stable intraoperative hemodynamics without compromising perioperative analgesia. For example, Hublet et al [5] reported that a combination of continuous intravenous (IV) infusion of dexmedetomidine, esketamine, and lidocaine reduced postoperative pain and opioid requirement in patients undergoing pancreatic surgery. Similarly, Toleska et al reported in patients undergoing laparoscopic cholecystectomy, opioid-free anesthesia (OFA) with lidocaine, ketamine, and magnesium sulphate showed a lower pain score at rest and coughing, as well as a reduced postoperative opioid consumption [6]. However, in Beloeil et al’ s study [7], compared with remifentanil, OFA with dexmedetomidine resulted in a higher incidence of serious adverse events, especially hypoxemia and bradycardia during major or intermediate noncardiac surgery. Collectively, current literature is inconsistent with regard to the definition of OFA and its advantage compared to traditional OBA technique.
Lumpectomy is largely day surgery for breast disease and a key limiting factor for success is the development of postoperative pain, nausea, or vomiting. As OFA is reported to have an opioid sparing effect, the feasibility of OFA technique in patients scheduled for lumpectomy remains largly unknown. Thus, this study was designed to compare the analgesic effect, hemodynamic fluctuations, quality of recovery, and postoperative adverse events in patients who scheduled for lumpectomy under OFA versus OBA.
| Materials and Methods | ▴Top |
Trial design and participants
This parallel trial was approved by the Institutional Ethics Committee of Nanjing Maternity and Child Health Care Hospital (2020KY071) and was registered at the Chinese Clinical Trial Registry (ChiCTR2100044230). The study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
Non-smoking patients aged 18 to 60 years, graded with American Society of Anesthesiologists (ASA) physical status classes I or II, diagnosed with benign breast mass, and planned to undergo lumpectomy were invited to participate in the study the day before surgery. Patients having a history of chronic pain, motion sickness, postoperative nausea or vomiting (PONV), or patients with allergies to any study medication were excluded. If preoperative heart rate (HR) was lower than 50 beats/min, or oxygen saturation detected by pulse oximetry (SpO2) was less than 95%, the patient was excluded as well. Once enrolled, a written informed consent was obtained. And this study was conducted between March 2021 and March 2022.
Randomization and blinding
Eligible participants were assigned to receive either OFA or OBA technique through a computer-generated randomization allocation protocol. Numbers with regard to the study group assignment were sealed. Once the patient entering the operating theater, the group assignment number was allocated to determine which anesthesia regimen would be given. Except for the anesthesiologist in charge of the patient, the other study members and the patients were blinded to the group assignment.
Characteristics of patients
The following general information were collected in this study: age, height, and weight of the patient, anesthesia time, surgery time, and blood loss.
Intraoperative anesthetic management
Routine monitoring was applied for patients in the operating theater including HR, electrocardiogram (ECG), noninvasive blood pressure (NIBP), respiratory rate (RR), SpO2, partial pressure of end-tidal carbon dioxide (PetCO2). Before anesthesia induction, patients were preoxygenated for 3 - 5 min, IV injected with dexamethasone 5 mg and penehyclidine hydrochloride 0.01 mg/kg.
For patients receiving OFA technique, a loading dose of dexmedetomidine (0.5 µg/kg IV over 10 min), together with esketamine (0.1 mg/kg) and lidocaine (1.5 mg/kg) was slowly IV injected. Then, anesthesia induction was performed with midazolam (0.03 - 0.04 mg/kg), propofol (1.5 - 2.0 mg/kg), and cisatracurium (0.2 - 0.3 mg/kg). After establishment of laryngeal mask airway, anesthesia was maintained with propofol (4 - 6 mg/kg/h), dexmedetomidine (0.1 - 0.2 µg/kg/h), esketamine (0.1 - 0.2 mg/kg/h) and lidocaine (1 - 1.5 mg/kg/h).
For patients in the OBA group, anesthesia induction was conducted with sufentanil (0.2 - 0.4 µg/kg), together with midazolam (0.03 - 0.04 mg/kg), propofol (1.5 - 2.0 mg/kg), and cisatracurium (0.2 - 0.3 mg/kg). During the operation, anesthesia was maintained with propofol (4 - 6 mg/kg/h) and remifentanil (0.1 - 0.3 µg/kg/min).
During the surgery, additional muscle relaxant was supplied if needed. Mechanical ventilation was performed with a tidal volume set at 6 - 8 mL/kg to maintain PetCO2 between 35 and 45 mm Hg. SpO2 was kept at higher than 95%. Ten minutes before the end of the surgery, IV dezocine 5 mg and ondansetron 4 mg were administered. At the end of the surgery, we terminated all of the medication. Atropine (10 µg/kg) and neostigmine (50 µg/kg) were applied to reverse the residue muscle relaxant. Patients were extubated if conscious recovered and spontaneous aspiration returned, and thereafter transferred to the post-anesthesia care unit (PACU). Only patients with a Steward score higher than 4 were allowed to discharge [8].
Intraoperative and postoperative management
During anesthesia, bradycardia was defined as HR < 45 bpm and treated with bolus atropine 0.5 mg. Besides, intraoperative hypotension and hypertension was defined as mean arterial pressure (MAP) < 60 mm Hg and > 120 mm Hg, and treated with bolus ephedrine 6 mg and urapidil 5 - 10 mg, respectively.
Postoperatively, no regular analgesic was used in our study. The visual analogue scale (VAS) was used for the evaluation of pain. VAS was scored on 0-to-10 with the left end marked “no pain” and the right end “severe intolerable pain”. As a replacement, dezocine 5 mg was used if VAS ≥ 4 for postoperative analgesia. Further, patients were also asked whether they experienced PONV. If PONV reported, IV ondansetron 4 mg was administered. Furthermore, postoperative delirium (POD) was evaluated with the Confusion Assessment Method. If POD identified, supportive treatment and close observation was provided until the symptoms of POD relieved.
Primary outcome
The primary outcome was the time to first rescue analgesic within 24 h postoperatively. The percentage of patients requiring rescue analgesic was recorded as well. To evaluate the analgesia efficacy, VAS score at 2, 12, and 24 h after surgery was examined.
Secondary outcomes
Intraoperative hemodynamics parameters included MAP and HR at the following time points: entering operating room (T0), immediately after anesthesia induction (T1), immediately after intubation (T2), 1 min (T3), 5 min (T4), and 10 min (T5) after surgical incision. Incidence of intraoperative hypotension, hypertension, bradycardia, as well as the percentage of patients receiving vasopressors including atropine, ephedrine, and urapidil were recorded simultaneously. The total amount of propofol consumption, opioids used in OBA group, and dexmedetomidine, esketamine, lidocaine consumption in OFA group were calculated.
Variables indicating recovery quality included awakening time and recovery time of orientation. Postoperative adverse events were collected including PONV, increased oral secretion, POD, and dizziness within the first 24 h after operation. All medications used to treat these side effects were recorded as well. Of note, patient satisfaction on a 0 - 10 scale was obtained at 24 h after operation.
Sample size calculation
In the preliminary trial of 16 patients, the VAS pain scores 2 h after extubation were 0.91 ± 0.55 and 1.26 ± 0.98 in the OBA and OFA group, respectively. Accordingly, we calculated 36 patients would be required in each group for a 90% power to detect a difference of 0.35 in the postoperative pain VAS at 2 h after extubation between the two groups, with a standard deviation (SD) of 0.43 and a significance level of 0.05. Considering a approximately 10% dropout during the study, 40 patients were enrolled in each group.
Statistical analysis
Statistical analysis was performed with GraphPad Prism 8.0 software (GraphPad Software, California, USA). Continuous data were tested for normality using the Kolmogorov-Smirnov test. Normally distributed data were presented as mean ± SD, and compared using Student’s t-test. Nonnormally distributed data were shown as median (interquartile range (IQR)) and compared using the Mann-Whitney U test. Categorical data were presented as number (%) and compared using χ2 or Fisher’s exact test. A two-tailed P < 0.05 was considered to be statistically significant.
| Results | ▴Top |
A total of 80 patients underwent lumpectomy in the study period. Six patients were excluded due to surgery cancellation (n = 3), refuse to participate (n = 2), or surgery conversion to a mastectomy (n = 1). Figure 1 shows a detailed flowchart of participants enrollment, allocation, follow-up, and analysis. Finally, there were 37 patients analyzed in each group, respectively. Demographic characteristics were comparable in the two groups (Table 1). As presented, there was no difference with regard to age, height, weight, anesthesia time, surgery time, and blood loss.
 Click for large image | Figure 1. Flowchart of the study. |
 Click to view | Table 1. Characteristics of the Patients |
As the primary outcome, rescue analgesic dezocine was used in all patients in this study. However, the averaged time to first rescue analgesic was significantly prolonged in OFA compared to OBA group (6.18 ± 1.00 min vs. 7.40 ± 0.92 min, P = 0.000). The efficacy of postoperative analgesia was evaluated with VAS score at 2, 12, and 24 h after operation. As shown in Table 2, there was no inter-group difference with regard to postoperative VAS score. Further, the median VAS score was lower than 3 at the three observational time points, suggesting the analgesia regimen was sufficient for all patients.
 Click to view | Table 2. Postoperative Analgesia-Related Variables |
Intraoperative hemodynamics is shown in Figure 2. As shown, preoperative MAP and HR were comparable in both groups. However, at the following observational time points (T1 to T5), both MAP and HR were significant higher in OFA group than those in OBA group. Consistently, the incidence of hypotension and bradycardia was lower in OFA than that in OBA group (Table 3). In addition, more patients in the OFA group used atropine (8% vs. 32%, P = 0.019) and ephedrine (5% vs. 38%, P = 0.001).
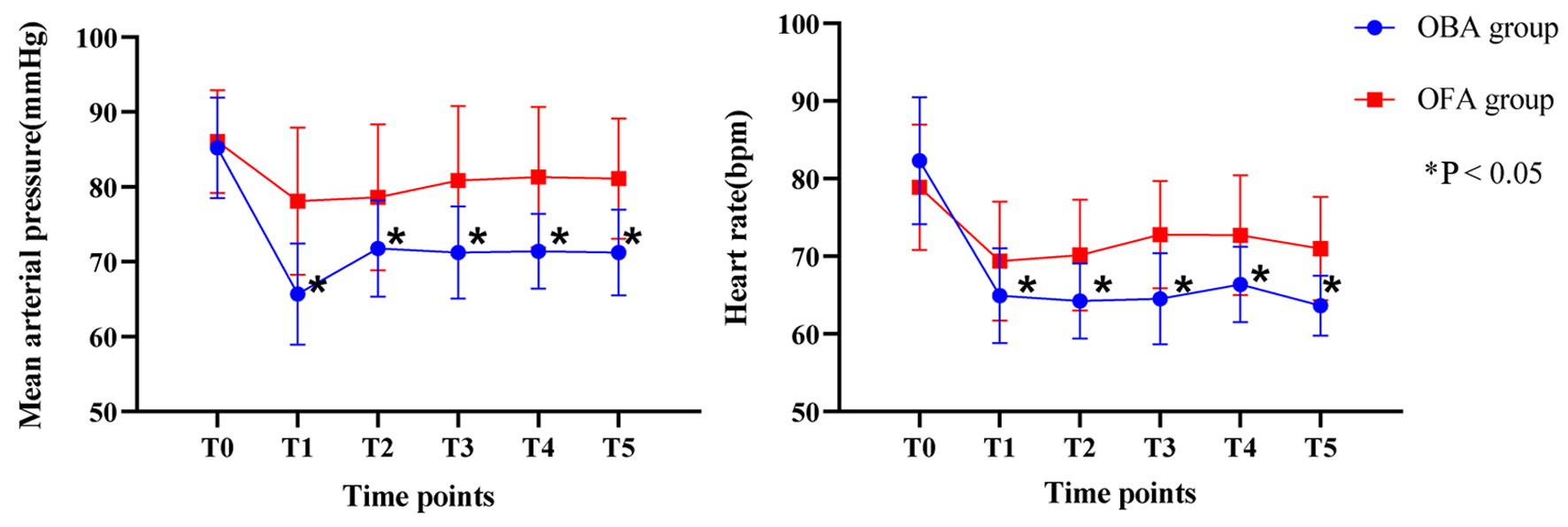 Click for large image | Figure 2. Intraoperative hemodynamics. |
 Click to view | Table 3. Intraoperative Hemodynamics and Drugs Consumption |
The total amount of propofol consumption was comparable (257.2 ± 52.5 mg vs. 278.1 ± 57.6 mg, P = 0.107) in both groups. Besides, sufentanyl and remifentanil consumption in OBA group, as well as dexmedetomidine, esketamine, lidocaine consumption in OFA group were calculated and shown in Table 3.
Of note, there was a significant difference between groups with regard to recovery quality and postoperative adverse events. Patients in OFA group experienced a longer awakening time (7.14 ± 2.63 min vs. 4.54 ± 1.14 min, P = 0.000) and recovery time of orientation (11.76 ± 3.15 min vs. 6.92 ± 1.19 min, P = 0.000) compared to those in OBA group (Table 4). However, patients in OFA group reported fewer cases of PONV (11% vs. 51%, P = 0.000) (Table 4). Consistently, application of rescue ondansetron was statistically fewer in OFA group than those in OBA group (5% vs. 35%, P = 0.003) (Table 4). In addition, no case was reported to have POD, and there was no statistical difference in the incidence of oral secretions and dizziness between the OFA and OBA groups (14% vs. 8%, P = 0.711; 27% vs. 16%, P = 0.398). It is worth mentioning that patients in OFA group had a higher satisfaction score than those in OBA group (9 (8 - 9) vs. 7 (7 - 8), P = 0.000).
 Click to view | Table 4. Recovery Quality, Postoperative Adverse Events, and Satisfaction Score |
| Discussion | ▴Top |
In order to control the opioid crisis and avoid adverse opioid reactions, OFA has gradually emerged. Anesthesologists around the world have developed this technology [9], which has been used in orthopedic surgery, gastrointestinal surgery and other operations [1, 10, 11]. In this randomized controlled trial, the OFA group achieved more stable intraoperative hemodynamic, lower incidence of PONV, and prolonged time to first rescue analgesia with the same analgesic effect. In these patients who underwent lumpectomy, the OFA group had higher postoperative satisfaction scores than the OBA group using opioids. These findings are particularly important in day surgery because patients want rapid discharge, lower costs and rapid return to daily activities. Meanwhile, these results fill the gap in the clinical reports of OFA application in this type of surgery.
Firstly, in terms of analgesic effect, previous studies had led to conflicting results regarding the effect of OFA on postoperative analgesia [6, 12]. These discrepancies could be due to the different methods of compound anesthesia and the differences in postoperative multimodal analgesia [13, 14]. A meta-analysis including 1,304 patients showed that OFA provided similar analgesia in the postoperative period [15]. Our study found that the OFA delayed request for the first analgesic, and, there was no difference in pain scores between the OFA group and the OBA group at each time point after surgery, suggesting that both anesthesia methods can provide effective analgesia in lumpectomy. Esketamine is a right-handed isomer of ketamine, and its affinity for NMDA receptors is 2 - 4 times that of ketamine, allowing it to achieve the same analgesic effect with fewer side effects at lower doses [16, 17].
In this study, MAP and HR of the OBA group were lower than those of the OFA group at all time points, confirming that opioids could effectively inhibit various stress responses, but the incidence of hypotension and bradycardia increased significantly. Although dexmedetomidine with an antisympathetic effect [18, 19] was applied in the OFA group, we observed that the fluctuation range of intraoperative MAP and HR in the OFA group was smaller than that in the OBA group, and only individual cases showed hypotension and bradycardia. In response to this finding, we believe that the dose of dexmedetomidine (0.1 - 0.2 µg/kg/h) in our study could be considered low, and we could hypothesize that the low incidence of bradycardia in the OFA group was a result of this low dose. Additionally, the sympathetic excitatory effect of esketamine used in the OFA group partly counteracts the cardiovascular inhibitory effect of propofol [20].
PONV is the last thing patients want to experience [21], compared to pain and other complications. Of concern, in our study, the incidence of PONV in the OFA group was significantly 43% lower compared to the OBA group, and more than 50% of patients with OBA experienced PONV. The four factors recognized to have the greatest impact on PONV were: female gender, non-smoker, previous history of PONV or motion sickness, and use of opioids postoperatively. Among the gender factors, women are the strongest predictors of postoperative vomiting (odds ratio (OR): 4.89) [22]. Regarding anesthetic drugs, opioids are an independent risk factor for PONV [23, 24]. Ziemann-Gimmel et al found that total IV OFA was associated with a large reduction in relative risk of PONV in patients undergoing bariatric operations [25], which is comparable to our finding.
As for the other postoperative adverse events, no difference was shown between the two groups, and it is worth mentioning that the POD did not occur in the OFA group, probably because midazolam was used to prevent esketamine-induced delirium.
However, in this study, the awakening time and recovery time of orientation were prolonged in the OFA group. This may be due to the use of dexmedetomidine, which can cause delayed recovery [26]. A previous research [7] reports that, patients in OFA group experienced high incidence of delayed awakening and hypoxemia, and, the high dosage (1.2 ± 2 µg/kg/h) of dexmedetomidine might be the main reason. By contrast, the dosage of dexmedetomidine (0.1 - 0.2 µg/kg/h) used in our study was low, which explains the low incidences of adverse events. Furthermore, low doses of dexmedetomidine induced to sleep rather than anesthesia [27], so, it prolonged awakening but would not cause respiratory depression.
We also evaluated the satisfaction of patients and the scores were higher in the OFA group. Satisfaction score is an integral evaluation index, which is very meaningful for the evaluation of anesthesia technology and management.
This study is not without its limitations. Firstly, we did not document the management of PONV and we did not assess the quality of postoperative recovery. Another limitation is the lack of validated nociception monitor. Finally, our definition of OFA (multimodal anesthesia including dexmedetomidine, esketamine and lidocaine) is not definitive, and other ways to administer OFA have to be explored [28].
In conclusion, for patients undergoing lumpectomy, OFA technique with dexmedetomidine-esketamine-lidocaine showed a better postoperative analgesia, a more stable hemodynamics, and a lower incidence of postoperative nausea and vomiting. However, such advantage of OFA technique should be weighed against a longer awakening time and recovery time of orientation in clinical practice. This technique might be suitable for certain patients including those with opioid tolerance, or with a high risk of PONV.
Acknowledgments
We would like to thank all those who participated in this study.
Financial Disclosure
There was no specific funding source to be mentioned.
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
All subjects provided written informed consent.
Author Contributions
Xia Li Qian and Ping Li have designed and performed the study. Xia Li Qian and Ping Li have drafted the manuscript. Xian Wang and Shan Wu Feng have did critical editing. Ya Jie Chen and Shi Qin Xu have assisted and supported in sample collection and subsequent analysis with statistics. Xian Wang and Shan Wu Feng have carefully supervised this manuscript preparation and writing.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
ASA: American Society of Anesthesiologists; ECG: electrocardiogram; HR: heart rate; MAP: mean arterial pressure; NIBP: noninvasive blood pressure; NMDA: N-methyl-D-aspartate; OBA: opioid-based anesthesia; OFA: opioid-free anesthesia; PACU: post-anesthesia care unit; PetCO2: partial pressure of end-tidal carbon dioxide; POD: postoperative delirium; PONV: postoperative nausea or vomiting; RR: respiratory rate; SpO2: pulse oxygen saturation; VAS: visual analogue scale
| References | ▴Top |
- Mulier JP. Is opioid-free general anesthesia for breast and gynecological surgery a viable option? Curr Opin Anaesthesiol. 2019;32(3):257-262.
doi pubmed - Koepke EJ, Manning EL, Miller TE, Ganesh A, Williams DGA, Manning MW. The rising tide of opioid use and abuse: the role of the anesthesiologist. Perioper Med (Lond). 2018;7:16.
doi pubmed pmc - Lavand'homme P, Steyaert A. Opioid-free anesthesia opioid side effects: Tolerance and hyperalgesia. Best Pract Res Clin Anaesthesiol. 2017;31(4):487-498.
doi pubmed - Bugada D, Lorini LF, Lavand'homme P. Opioid free anesthesia: evidence for short and long-term outcome. Minerva Anestesiol. 2021;87(2):230-237.
doi pubmed - Hublet S, Galland M, Navez J, Loi P, Closset J, Forget P, Lafere P. Correction to: Opioid-free versus opioid-based anesthesia in pancreatic surgery. BMC Anesthesiol. 2022;22(1):33.
doi pubmed pmc - Toleska M, Dimitrovski A. Is opioid-free general anesthesia more superior for postoperative pain versus opioid general anesthesia in laparoscopic cholecystectomy? Pril (Makedon Akad Nauk Umet Odd Med Nauki). 2019;40(2):81-87.
doi pubmed - Beloeil H, Garot M, Lebuffe G, Gerbaud A, Bila J, Cuvillon P, Dubout E, et al. Balanced opioid-free anesthesia with dexmedetomidine versus balanced anesthesia with remifentanil for major or intermediate noncardiac surgery. Anesthesiology. 2021;134(4):541-551.
doi pubmed - Steward DJ. A simplified scoring system for the post-operative recovery room. Can Anaesth Soc J. 1975;22(1):111-113.
doi pubmed - Soffin EM, Wetmore DS, Beckman JD, Sheha ED, Vaishnav AS, Albert TJ, Gang CH, et al. Opioid-free anesthesia within an enhanced recovery after surgery pathway for minimally invasive lumbar spine surgery: a retrospective matched cohort study. Neurosurg Focus. 2019;46(4):E8.
doi pubmed - Urvoy B, Aveline C, Belot N, Catier C, Beloeil H. Opioid-free anaesthesia for anterior total hip replacement under general anaesthesia: the Observational Prospective Study of Opiate-free Anesthesia for Anterior Total Hip Replacement trial. Br J Anaesth. 2021;126(4):e136-e139.
doi pubmed - Brandal D, Keller MS, Lee C, Grogan T, Fujimoto Y, Gricourt Y, Yamada T, et al. Impact of enhanced recovery after surgery and opioid-free anesthesia on opioid prescriptions at discharge from the hospital: a historical-prospective study. Anesth Analg. 2017;125(5):1784-1792.
doi pubmed pmc - Bakan M, Umutoglu T, Topuz U, Uysal H, Bayram M, Kadioglu H, Salihoglu Z. Opioid-free total intravenous anesthesia with propofol, dexmedetomidine and lidocaine infusions for laparoscopic cholecystectomy: a prospective, randomized, double-blinded study. Braz J Anesthesiol. 2015;65(3):191-199.
doi pubmed - Guinot PG, Spitz A, Berthoud V, Ellouze O, Missaoui A, Constandache T, Grosjean S, et al. Effect of opioid-free anaesthesia on post-operative period in cardiac surgery: a retrospective matched case-control study. BMC Anesthesiol. 2019;19(1):136.
doi pubmed pmc - King CA, Perez-Alvarez IM, Bartholomew AJ, Bozzuto L, Griffith K, Sosin M, Thibodeau R, et al. Opioid-free anesthesia for patients undergoing mastectomy: A matched comparison. Breast J. 2020;26(9):1742-1747.
doi pubmed - Frauenknecht J, Kirkham KR, Jacot-Guillarmod A, Albrecht E. Analgesic impact of intra-operative opioids vs. opioid-free anaesthesia: a systematic review and meta-analysis. Anaesthesia. 2019;74(5):651-662.
doi pubmed - Trimmel H, Helbok R, Staudinger T, Jaksch W, Messerer B, Schochl H, Likar R. S(+)-ketamine : Current trends in emergency and intensive care medicine. Wien Klin Wochenschr. 2018;130(9-10):356-366.
doi pubmed pmc - Wang X, Lin C, Lan L, Liu J. Perioperative intravenous S-ketamine for acute postoperative pain in adults: A systematic review and meta-analysis. J Clin Anesth. 2021;68:110071.
doi pubmed - Kleiman AM, Johnson KB. Untapped potential of dexmedetomidine. Anesth Analg. 2019;129(6):1450-1453.
doi pubmed - Kaye AD, Chernobylsky DJ, Thakur P, Siddaiah H, Kaye RJ, Eng LK, Harbell MW, et al. Dexmedetomidine in enhanced recovery after surgery (ERAS) protocols for postoperative pain. Curr Pain Headache Rep. 2020;24(5):21.
doi pubmed pmc - Wang X, Chen Y, Zhou X, Liu F, Zhang T, Zhang C. Effects of propofol and ketamine as combined anesthesia for electroconvulsive therapy in patients with depressive disorder. J ECT. 2012;28(2):128-132.
doi pubmed - Macario A, Weinger M, Carney S, Kim A. Which clinical anesthesia outcomes are important to avoid? The perspective of patients. Anesth Analg. 1999;89(3):652-658.
doi pubmed - Poon YY, Ke TY, Hung KC, Lu HF, Chiang MH, Chin JC, Wu SC. Risk factors of postoperative vomiting in the eye of "Real-World Evidence"-modifiable and clinical setting-dependent risk factors in surgical trauma patients. J Pers Med. 2021;11(5):386.
doi pubmed pmc - Sato J, Tanaka R, Ishikawa H, Suzuki T, Shino M. A preliminary study of the effect of naldemedine tosylate on opioid-induced nausea and vomiting. Support Care Cancer. 2020;28(3):1083-1088.
doi pubmed - Smith HS, Laufer A. Opioid induced nausea and vomiting. Eur J Pharmacol. 2014;722:67-78.
doi pubmed - Ziemann-Gimmel P, Goldfarb AA, Koppman J, Marema RT. Opioid-free total intravenous anaesthesia reduces postoperative nausea and vomiting in bariatric surgery beyond triple prophylaxis. Br J Anaesth. 2014;112(5):906-911.
doi pubmed - Bilotta F, Pugliese F. The evolving clinical use of dexmedetomidine. Lancet. 2020;396(10245):145-147.
doi pubmed - Feng ZX, Dong H, Qu WM, Zhang W. Oral delivered dexmedetomidine promotes and consolidates non-rapid eye movement sleep via sleep-wake regulation systems in mice. Front Pharmacol. 2018;9:1196.
doi pubmed pmc - Fiore JF, Jr., Olleik G, El-Kefraoui C, Verdolin B, Kouyoumdjian A, Alldrit A, Figueiredo AG, et al. Preventing opioid prescription after major surgery: a scoping review of opioid-free analgesia. Br J Anaesth. 2019;123(5):627-636.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


