| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Original Article
Volume 15, Number 6, June 2023, pages 300-309
Preoperative Risk Factors in Patients With Pancreatic Cancer
Naomi Kusamaa, Yuta Mitobeb, f, Natsuko Hyodoa, Tetsuya Miyashitac, Yasuko Babac, Takuya Hashimotod, Yoshimi Inagakie
aMaster’s Program, International University of Health and Welfare, Tokyo, Japan
bGraduate School of Health and Welfare Science, International University of Health and Welfare, Tokyo, Japan
cDepartment of Anesthesiology, International University of Health and Welfare, Mita Hospital, Tokyo, Japan
dDepartment of Hepatobiliary Surgery, Japanese Red Cross Medical Center, Tokyo, Japan
eDepartment of Anesthesiology, International University of Health and Welfare, Narita Hospital, Chiba, Japan
fCorresponding Author: Yuta Mitobe, Graduate School of Health and Welfare Science, International University of Health and Welfare, Tokyo, Japan
Manuscript submitted March 7, 2023, accepted June 17, 2023, published online June 29, 2023
Short title: Preoperative Factors in Pancreatic Cancer
doi: https://doi.org/10.14740/jocmr4906
| Abstract | ▴Top |
Background: Pancreatic cancer is gastrointestinal cancer with a poor prognosis. Although surgical techniques and chemotherapy have improved treatment outcomes, the 5-year survival rate for pancreatic cancer is less than 10%. In addition, resection of pancreatic cancer is highly invasive and is associated with high rates of postoperative complications and hospital mortality. The Japanese Pancreatic Association states that preoperative body composition assessment may predict postoperative complications. However, although impaired physical function is also a risk factor, few studies have examined it in combination with body composition. We examined preoperative nutritional status and physical function as risk factors for postoperative complications in pancreatic cancer patients.
Methods: Fifty-nine patients with pancreatic cancer who underwent surgical treatment and were discharged alive from January 1, 2018, to March 31, 2021, at the Japanese Red Cross Medical Center. This retrospective study was conducted using electronic medical records and a database of departments. Body composition and physical function were evaluated before and after surgery, and the risk factors between patients with and without complications were compared.
Results: Fifty-nine patients were analyzed: 14 and 45 patients in the uncomplicated and complicated groups, respectively. The major complications were pancreatic fistulas (33%) and infections (22%). There were significant differences in: age, 74.0 (44 - 88) (P = 0.02); walking speed, 0.93 m/s (0.3 - 2.2) (P = 0.01); and fat mass, 16.50 kg (4.7 - 46.2) (P = 0.02), in the patients with complications. On Multivariable logistic regression analysis, age (odds ratio: 2.28; confidence interval (CI): 1.3400 - 569.00; P = 0.03), preoperative fat mass (odds ratio: 2.28; CI: 1.4900 - 168.00; P = 0.02), and walking speed (odds ratio: 0.119; CI: 0.0134 - 1.07; P = 0.05) were identified as risk factors. Walking speed (odds ratio: 0.119; CI: 0.0134 - 1.07; P = 0.05) was the risk factor that was extracted.
Conclusions: Older age, more preoperative fat mass, and decreased walking speed were possible risk factors for postoperative complications.
Keywords: Pancreatic cancer; Complications; Body composition; Walking speed
| Introduction | ▴Top |
Pancreatic cancer is gastrointestinal cancer with a poor prognosis [1]. Treatment outcomes have improved in recent years with the development of advanced medical care, surgical techniques, and chemotherapy. However, the 5-year survival rate remains the lowest among malignant neoplasms at approximately 10% [2].
Surgical radical resection is an important therapeutic intervention for improving the long-term survival of patients with pancreatic cancer. However, pancreatic cancer resections, such as pancreatoduodenectomy (PD), distal pancreatectomy (DP), and total pancreatectomy (TP), are highly invasive procedures in the field of gastrointestinal surgery [3, 4]. The complication and in-hospital mortality rates for these surgeries are high [3, 4]. The risk factors for in-hospital mortality include weight loss, age, and decreased activity levels [3, 4].
According to the 2019 Japanese Pancreatic Cancer Treatment Guidelines, nutritional assessment and body composition evaluation combined with preoperative biochemical findings in patients with pancreatic cancer may contribute to the prediction of long-term prognosis and postoperative complications in patients undergoing pancreatic cancer surgery. Perioperative malnutrition has a negative impact on short-term outcomes, including postoperative complications. Moreover, patients at high risk of malnutrition have been reported to have an increased length of hospital stay and a significantly higher complication rate than those at low risk, thereby affecting survival [5, 6].
Previous studies on nutritional assessment using body mass index (BMI), suggested BMI alone did not accurately assess nutritional status, and that the assessment of body composition, such as muscle mass and fat mass, also contributed to evaluate postoperative complication rates and prognosis [6].
A decrease in activity and impaired physical function are also risk factors for the development of postoperative complications [3, 4]. However, few studies have examined the factors that contribute to the development of postoperative complications in combination (or along) with body composition [7].
Therefore, this study aimed to elucidate whether preoperative nutritional status and physical function influence the development of postoperative complications in patients with pancreatic cancer.
| Materials and Methods | ▴Top |
Study design, setting, population, and study approval
This study was approved by the Ethics Review Committee of the International University of Health and Welfare, Akasaka Campus (Approval No. 21-Ig-76, September 10, 2021), and the Ethics Review Committee of the Japanese Red Cross Medical Center (Organizing No. 1362, October 29, 2021). This study was conducted in accordance with the Declaration of Helsinki. In addition, the “Information Disclosure Document on Clinical Research”, which clearly states ethical considerations so that population can withdraw from participation in the research at any time, was posted on the website of the Japanese Red Cross Medical Center.
This was a retrospective study using electronic medical records and databases of department.
Study population
The population of this study were patients who underwent surgical treatment for pancreatic cancer at the Japanese Red Cross Medical Center Hospital from January 1, 2018, to March 31, 2021, and were discharged alive. Body composition (Tanita MC-780MA-N), walking speed, and grip strength were measured preoperatively in 59 patients. Patients who had been on enteral nutrition before surgery and those who had undergone inoperative surgery, such as ileus removal, even if they had pancreatic cancer, were excluded from the study (Fig. 1).
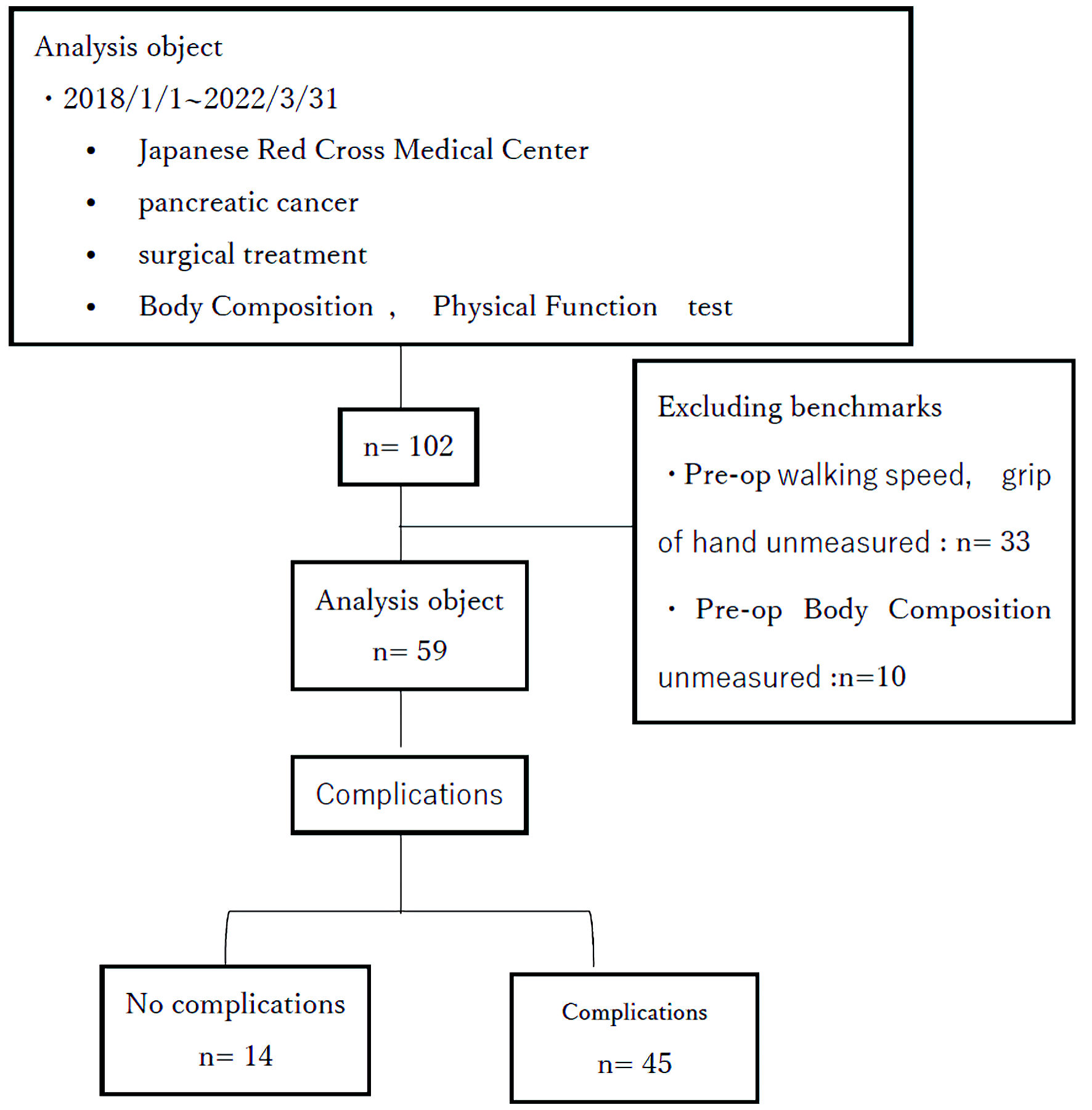 Click for large image | Figure 1. Flowchart of analysis study population. Pre-op: preoperative. |
Data collection
Perioperative information was collected from the patients’ medical records, which provided basic information about the background of the patients, their nutritional status, and physical function.
Medical record review
Basic patient data
Basic patient data included age, sex, weight, height, BMI, and American Society of Anesthesiologists Physical Status (ASA).
Intraoperative items
We extracted information on the type of operation (DP, PD, TP), anesthesia method (general anesthesia, general anesthesia with epidural anesthesia), anesthesia duration, operation duration, intraoperative fluid volume, intraoperative blood loss, intraoperative output, and intraoperative transfusion volume of blood products.
Postoperative period
On postoperative day (POD) 1, the patient got up from bed and walked for the first time (defined as the date when the patient walked for the first time). The date of the first oral intake (defined as the date when the patient drank water), and the duration of hospital stay after surgery were extracted.
Nutritional evaluation method
Nutritional status can be assessed using the controlling nutrition status (CONUT) score, a simple hematological assessment method that reflects protein metabolism, lipid metabolism, and immunity [8]. Using the values of albumin, total cholesterol, and lymphocyte count, the three scores can be summed to obtain the CONUT score.
Normal or mild malnutrition (CONUT score of ≤ 4) was defined as no malnutrition, and moderate or severe malnutrition (CONUT score of ≥ 5) was defined as malnutrition [9]. The prognostic nutritional index (PNI) was calculated using the Onodera index PNI = 10 × serum albumin (g/dL) + 0.005 × lymphocyte count (/µL), to evaluate nutritional status and immunocompetence [9]. In gastrointestinal cancer surgery, a PNI < 47 is an independent predictor of postoperative complications. Caution is required when the PNI is < 40 or < 45; and resection and anastomosis are contraindicated when the PNI is < 40. Therefore, a PNI < 40 was considered poor in this study [9, 10].
Assessment of body composition
The assessment of body composition was performed under the guidance of a dietitian. Body composition included body fat percentage, fat mass, lean body mass, muscle mass, body water mass, and body water content. for example, using an Tanita MC-780MA-N®.
Assessment of physical function
Physical function was assessed by measuring the walking speed and grip strength [11-13]. Physical function was observed by one dietitian. Grip strength was measured twice, each on the left and right sides, and the maximum value was adopted. The measurement device was an Aswan Digital Grip Strength Tester Jammer Model MG-4800. Walking speed was observed by one dietitian. Normal walking speed, excluding acceleration and deceleration, should be evaluated by walking 4 m or more. The time required to walk 4 m from the 0 m point to the 6 m point and from the 1 m point to the 5 m point was measured. As a rule, the measurements are taken once. The dietitian used a stopwatch to measure the time. The time period was from 9:00 to 16:00.
Laboratory results
The blood test items included serum albumin, total protein, prealbumin, transferrin, total lymphocyte count, and total cholesterol. Blood tests were performed on admission according to the clinical pathway.
Compare values measured at admission and discharge
Body composition, walking speed, and grip strength were compared to values measured at admission and discharge.
Statistical analysis
Patients were divided into two groups according to the presence or absence of postoperative complications and statistically compared. Non-normally distributed continuous variables were expressed as medians (ranges), and comparisons were made with the Mann-Whitney U test. Categorical variables were compared using either the Chi-squared test or Fisher’s exact test, as appropriate. Cut-off values were calculated from ROC curves for the four items of fat mass, age, walking speed, and fat mass change that differed significantly, and continuous variables were made binary. Finally, we performed a multivariable logistic regression analysis in which all four variables with P values less than 0.05 in the univariate analysis were entered. The significance level was set at < 5%. The statistical analysis software EZR ver. 1.60 was used [14].
| Results | ▴Top |
The patient profiles were shown in Table 1. The median age of the 59 patients was 72 years old, and 32% of the patients were older than 75 years (eight males and 11 females). The median preoperative BMI was 22 kg/m2. By surgical technique, 29 (49%) patients underwent DP, 28 (47%) underwent PD, and two (3%) underwent TP. General anesthesia combined with epidural anesthesia was administered to 55 patients (93%). Postoperatively, 49 (88%) patients were weaned POD1. The first oral intake occurred on the first postoperative day in 26 patients (48%). Twenty-four patients (44%) took their first oral intake on the third postoperative day because the gastric tube was removed on the third postoperative day. All patients were discharged from the hospital after surgery, and no patient died while in the hospital. Forty-five patients developed postoperative complications including pancreatic fistula (33%), infection (22%), and delayed gastric emptying (11%) (Fig. 2).
 Click to view | Table 1. Patient Characteristics in the Two Study Groups |
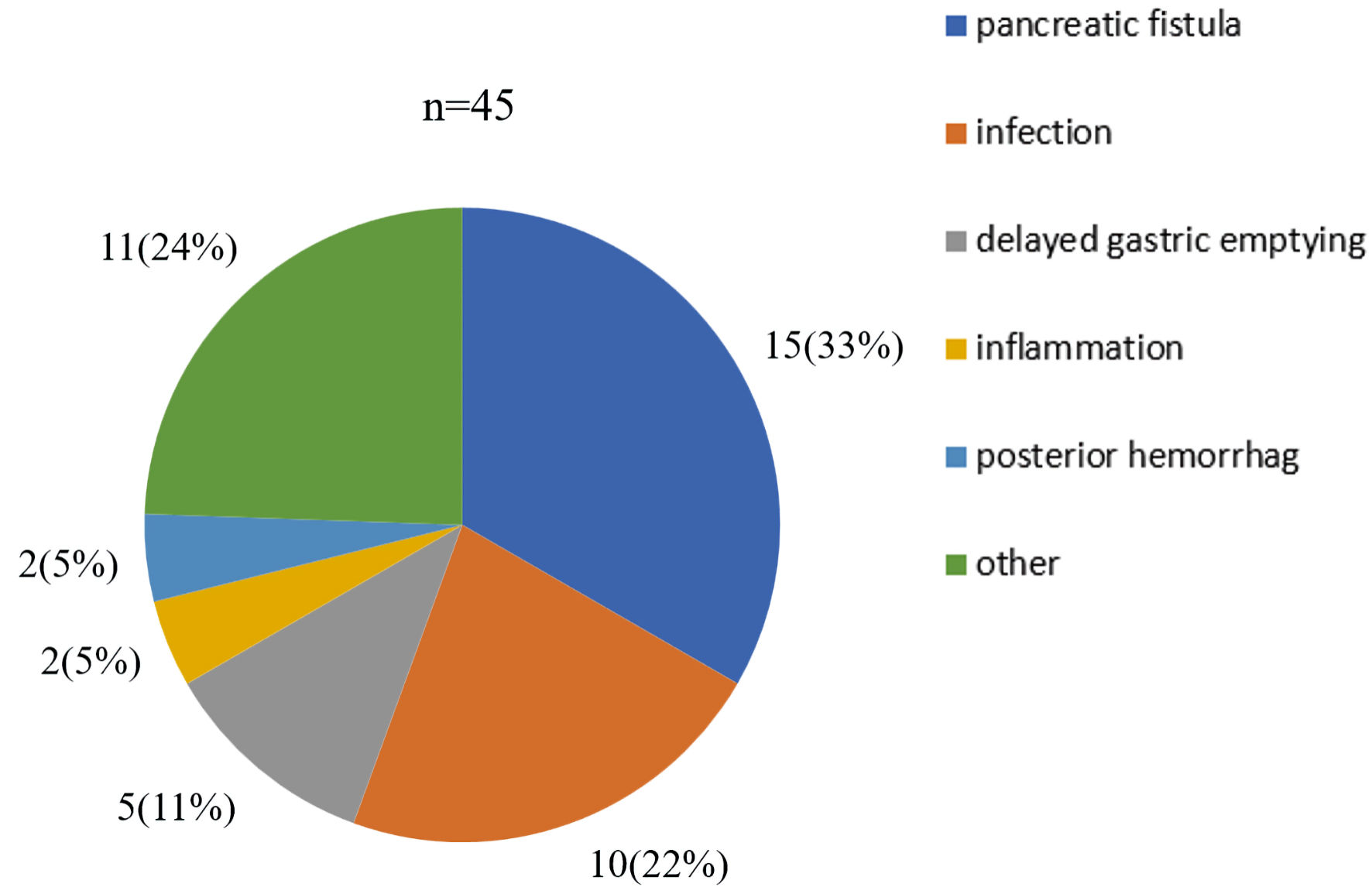 Click for large image | Figure 2. Postoperative complications contents. The number of complications (%) of all pancreatic cancer patients included in the study is indicated. |
Preoperative patient profiles
There were no differences in sex, ASA classification, BMI, CONUT values, or blood test results (Tables 1-3). In the group with complications, there was a significant difference in age, with a mean age of 74.0 years (P = 0.02). There were no significant differences in grip strength or the presence of sarcopenia, and the group with complications had a mean walking speed of 0.93 m/s (P = 0.01), resulting in a slower gait (Table 3). In terms of body composition, there was a difference in fat mass (P = 0.02), but no difference in muscle mass (P = 0.92) (Table 4).
 Click to view | Table 2. Preoperative Blood Tests (or Examinations) in the Two Study Groups |
 Click to view | Table 3. Status of Nutrition and Physical Function in the Two Study Groups |
 Click to view | Table 4. Body Composition and Physical Function in the Two Study Groups in Pre- and Postoperative Periods |
Intraoperative factors
There were no differences in the anesthesia method, anesthesia duration, operative time, operative technique, intraoperative fluid volume, blood loss, urine volume, or blood transfusion (Table 1).
Postoperative factors
There was no difference in the date on which the patients got up from bed and walked for the first time or the date of first drinking water. In terms of body composition, the mean rate of change in fat mass before and after surgery was -0.17% (P = 0.004), indicating a change in fat mass (Table 5). The mean length of hospital stay was 33 days (P = 0.07), and patients with complications had longer stays (Table 1).
 Click to view | Table 5. Comparison of Alterations of Body Composition and Physical Function in Pre- and Postoperative Periods in the Two Study Groups |
Detection of independence factors affecting the incidence of postoperative complications
Univariate and multivariable logistic regression analyses were performed to identify risk factors affecting the occurrence of postoperative complications (Table 6). Cut-off values were calculated from factors including age, preoperative fat mass, fat mass change, and walking speed, and from the ROC curve for the postoperative complication group (Fig. 3). In the univariate analysis, the factors associated with complications were age (P = 0.01), gait speed (P = 0.01), preoperative fat mass (P = 0.01), and pre- and postoperative fat mass changes (P = 0.01). In the multivariable logistic regression analysis, only three factors were identified as risk factors for postoperative complications: age (odds ratio: 2.28; 95% confidence interval (CI): 1.34 - 569.0; P = 0.03), preoperative fat mass (odds ratio: 2.28; 95% CI: 1.49 - 168.0; P = 0.02), and walking speed (odds ratio: 0.119; 95% CI: 0.01 - 1.07; P = 0.05).
 Click to view | Table 6. Logistic Regression Analysis, Univariate and Multivariable Logistic Regression Analysis of Risk Factors Associated With Postoperative Complications |
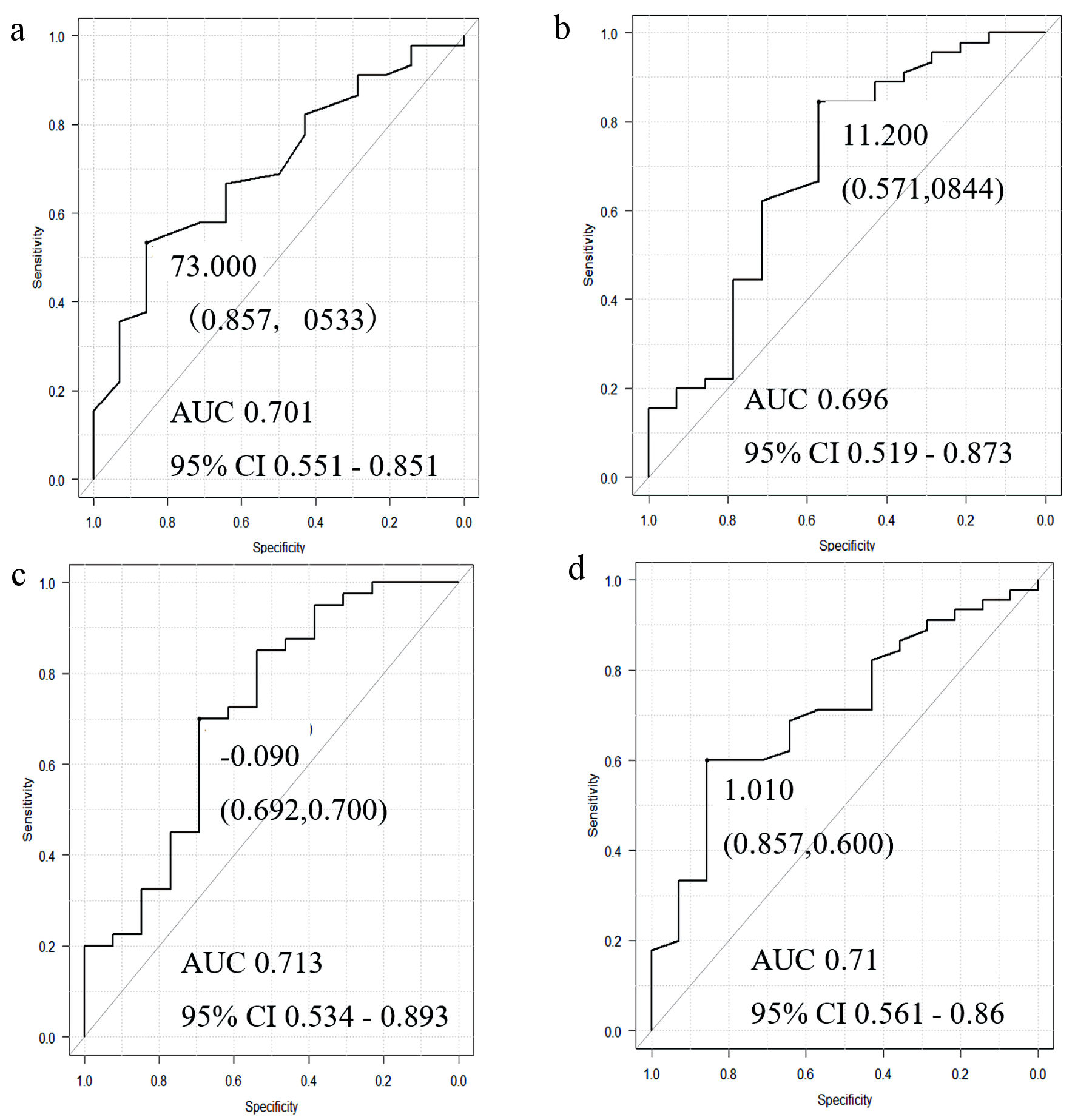 Click for large image | Figure 3. ROC curves generated by the factors of (a) age, (b) preoperative fat mass, (c) fat mass change rate, and (d) walking speed in the group with postoperative complications. AUC: area under the ROC curve; CI: confidence interval. |
| Discussion | ▴Top |
Few previous studies have investigated predictors of postoperative complications in pancreatic cancer patients based on nutrition and physical function. This present study demonstrated that preoperative nutritional status, body composition, and physical function such as walking speed and grip strength were key factors to predict the occurrence of postoperative complications in pancreatic surgeries. In this study, independent predictors were identified as age, fat mass, and walking speed. By identifying the predictive factors for the development of complications, at-risk patients can be identified in the preoperative period. Thereby, early intervention will be applied, and it would significantly contribute to attaining a shorter hospital stay and long-term prognosis in patients with pancreatic cancer.
Effects of age on the occurrence of postoperative complications
Aging is associated with decreased functional reserves in all organ systems. Age-related decline in circulatory, respiratory, renal, and other organ functions are associated with increased postoperative complications [15]. A systematic review of postoperative complications after PD by Prashant et al also indicated that patients > 75 years old had a higher incidence of complications than younger patients [16]. In this study, the mean age of the patients with complications was 74.0 years (range: 44 - 88; P = 0.02). Therefore, the results of this study are generally consistent with those of previous studies and showed that the risk of developing complications was increased in patients more than 70 years old undergoing surgery. This study revealed a logistic regression analysis of the relationship between preoperative age and postoperative complications (odds ratio: 27.6; 95% CI: 1.34 - 569.0). This relationship was elucidated in a multivariable logistic regression analysis, which showed no association, but a significant difference was found in a univariate analysis (odds ratio: 6.86; 95% CI: 1.370 - 34.2).
Relationship between preoperative fat mass and postoperative complications in surgical patients
In a meta-analysis by Saravana-Bawan et al, patients with obesity were more likely to have postoperative complications, such as surgical site infection, pancreatic fistula, and pneumonia [17]; the incidence of wound infection is directly related to tissue perfusion and oxygenation. In particular, excess adipose tissue is exposed to the surgical site and may prolong operative time [18-20]. Surgical retractors decrease adipose tissue perfusion [21]. Uchida et al found high concentrations of free fatty acids in the drainage of patients with postoperative pancreatic fistulas, suggesting a causal relationship between intra-abdominal lipolysis and leakage into the pancreatic fistula, it is believed to be related to fat [22]. In this study, the mean preoperative fat mass of patients with complications was 16.5 kg (range: 4.7 - 46.2; P = 0.02). The cut-off value calculated from the ROC curve was 11.2 kg, indicating that patients with complications had more preoperative fat mass. Compared to the mean preoperative fat mass of 14.3 kg (standard deviation: 7.1) in pancreatic cancer patients in the study by Trestini et al [6], patients in this study had more preoperative fat mass. Therefore, our patients with more fat mass would cause more postoperative complications.
Furthermore, in the group with complications, the mean change in fat mass before and after surgery was -0.14% (range: -0.8 - 0.1; P = 0.02), indicating that fat was utilized in the postoperative period (Table 6). The body is prone to hypermetabolism after the surgical invasion. Proteins in the body are used as substrates for glycogenesis. As a result, glycogen stores in the liver are greatly reduced and the body is in a state of starvation. Furthermore, if the bicarbonate contained in the pancreatic juice is not secreted, gastric acid is not neutralized, Thereby, the pH of the digestive tract decreases, and glycine-conjugated bile acid precipitates. Finally, fatty acid micelle formation failure occurs, resulting that bile acids not absorbed through the ileum and excreted in large amounts in the feces. This not only results in impaired fatty acid digestion and absorption but also increases cholesterol catabolism in the liver, which contributes to malnutrition [23, 24]. In this study, the mean change in fat mass from preoperative to postoperative was 0% (range: -0.2 - 1.1) in the uncomplicated group. In contrast, there was a -0.14% (range: -0.8 - 0.1) (P = 0.02) change in fat mass in the group with complications. These findings indicate that patients with complications would impair digestion and absorption [24, 25].
In this study, the mean preoperative BMI was 22 kg/m2, and complications occurred in patients who were obese (≥ 25 kg/m2), as defined by the World Health Organization (WHO). Therefore, it is necessary to evaluate not only body weight, but also body composition as part of the preoperative evaluation. Comparing with other tools for estimating body composition (computed tomography (CT) scan, magnetic resonance, imaging (MRI), dual-energy X-ray absorptiometry (DEXA), body composition has the advantages of being inexpensive and noninvasive and offers the option of performing measurements at the bedside. Angrisani et al validated the utility of bioimpedance analysis for assessing anthropometry, hydration status, and fluid shifts. The authors demonstrated its ability to predict the development of major complications after pancreatic surgery [26, 27]. The present study clarified the relationship between preoperative fat mass and postoperative complications (odds ratio: 15.9; 95% CI: 1.49 - 168.0). This relationship was elucidated in the multivariable logistic regression analysis, where no association was found, but significant differences were found in the univariate analysis.
Relationship between preoperative walking speed and postoperative complications in surgical patients
Aging causes a decrease in walking speed due to a decrease in muscle mass, muscle strength, and physical ability [28]. Compared to healthy people, sick people walk slower, and their walking speed tends to decrease with increasing age [29]. A systematic review by Pamoukdjian et al showed that slow walking speed is a predictor of early death, disability, and falls [30] and is associated with a higher risk of mortality [31]. Elderly individuals with walking speeds faster than 1.0 m/s have generally been found to have a better functional status, better health, and higher survival rates [32]. In this study, walking speed was measured during a 6-min walk [28, 33, 34]. Walking speed was significantly slower in the group with complications than in the group without complications in this study. The AWG2019 defined a gait speed of less than 1 m/s as slowing down [35], which supported the results of the present study.
Limitations
This study had several limitations. First, it was a single-center retrospective study and was a very small retrospective study compared to previous studies; thus, the interpretation of the results may be associated with bias. In addition, we investigated the predictors of postoperative complications and included all complications that could be observed. The postoperative complications included pulmonary thromboembolism, peroneal nerve palsy, and other non-infectious and inflammatory complications. All the patients in this study underwent laparotomy. Thus, the results of this study may not be applicable in the current era of minimally invasive surgery, such as laparoscopic surgery and robot-assisted surgery. Physical measurements were not taken by the physical therapist. Therefore, it is possible that the measurements were not taken properly. Although this study is novel, the sample size is small. It was also those performed on pancreatic patients for preoperative risk assessment, not for prognostic purposes. The type of surgery was not considered, as preoperative blood tests showed no difference. Because of the small number of cases in this study, it was not possible to include confounding factors in the multivariable logistic regression analysis. Therefore, more cases need to be analyzed in the future.
Conclusions
Age, preoperative fat mass, and decreased walking speed are risk factors for postoperative complications in pancreatic surgeries, and appropriate preoperative assessment and nutritional and exercise interventions are necessary for the outpatient setting and early in hospitalization.
Acknowledgments
We appreciate the support of the Japanese Red Cross Medical Center.
Financial Disclosure
This work was supported by JSPS KAKENHI Grant (number: JP1919577).
Conflict of Interest
The authors have no conflict of interest to disclose.
Informed Consent
As a retrospective observational study, patients were deemed as exposed to no more than minimal risk and the need for individual written informed consent was waived.
Author Contributions
NK and YM designed and performed this study. NH drafted the manuscript and performed critical editing. NK and YM assisted and supported sample collection and subsequent statistical analysis. YI, TM, and TH carefully supervised the manuscript preparation and writing.
Data Availability
Data collected during this study was stored in a secure location and only the collaborators directly involved in this study have access. The authors declare that data supporting the findings of this study are available in the article.
| References | ▴Top |
- Matsuki R, Arai T, Kogure M, Suzuki Y, Sakamoto Y. Trends in the treatment of pancreatic cancer in Japan. Biosci Trends. 2021;15(3):135-137.
doi pubmed - Yokoyama S, Hamada T, Higashi M, Matsuo K, Maemura K, Kurahara H, Horinouchi M, et al. Predicted prognosis of patients with pancreatic cancer by machine learning. Clin Cancer Res. 2020;26(10):2411-2421.
doi pubmed - Kimura W, Miyata H, Gotoh M, Hirai I, Kenjo A, Kitagawa Y, Shimada M, et al. A pancreaticoduodenectomy risk model derived from 8575 cases from a national single-race population (Japanese) using a web-based data entry system: the 30-day and in-hospital mortality rates for pancreaticoduodenectomy. Ann Surg. 2014;259(4):773-780.
doi pubmed - Aoki S, Miyata H, Konno H, Gotoh M, Motoi F, Kumamaru H, Wakabayashi G, et al. Risk factors of serious postoperative complications after pancreaticoduodenectomy and risk calculators for predicting postoperative complications: a nationwide study of 17,564 patients in Japan. J Hepatobiliary Pancreat Sci. 2017;24(5):243-251.
doi pubmed pmc - La Torre M, Ziparo V, Nigri G, Cavallini M, Balducci G, Ramacciato G. Malnutrition and pancreatic surgery: prevalence and outcomes. J Surg Oncol. 2013;107(7):702-708.
doi pubmed - Trestini I, Paiella S, Sandini M, Sperduti I, Elio G, Pollini T, Melisi D, et al. Prognostic impact of preoperative nutritional risk in patients who undergo surgery for pancreatic adenocarcinoma. Ann Surg Oncol. 2020;27(13):5325-5334.
doi pubmed - Kagifuku Y, Tohara H, Wakasugi Y, Susa C, Nakane A, Toyoshima M, Nakakuki K, et al. What factors affect changes in body composition and swallowing function in patients hospitalized for oral cancer surgery? Clin Interv Aging. 2020;15:1-7.
doi pubmed pmc - Ignacio de Ulibarri J, Gonzalez-Madrono A, de Villar NG, Gonzalez P, Gonzalez B, Mancha A, Rodriguez F, et al. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp. 2005;20(1):38-45.
pubmed - Watanabe J, Otani S, Sakamoto T, Arai Y, Hanaki T, Amisaki M, Tokuyasu N, et al. Prognostic indicators based on inflammatory and nutritional factors after pancreaticoduodenectomy for pancreatic cancer. Surg Today. 2016;46(11):1258-1267.
doi pubmed - Kanda M, Mizuno A, Tanaka C, Kobayashi D, Fujiwara M, Iwata N, Hayashi M, et al. Nutritional predictors for postoperative short-term and long-term outcomes of patients with gastric cancer. Medicine (Baltimore). 2016;95(24):e3781.
doi pubmed pmc - Chou MY, Nishita Y, Nakagawa T, Tange C, Tomida M, Shimokata H, Otsuka R, et al. Role of gait speed and grip strength in predicting 10-year cognitive decline among community-dwelling older people. BMC Geriatr. 2019;19(1):186.
doi pubmed pmc - Choi JY, Kim KI, Choi Y, Ahn SH, Kang E, Oh HK, Kim DW, et al. Comparison of multidimensional frailty score, grip strength, and gait speed in older surgical patients. J Cachexia Sarcopenia Muscle. 2020;11(2):432-440.
doi pubmed pmc - Soltani A, Abolhassani N, Marques-Vidal P, Aminian K, Vollenweider P, Paraschiv-Ionescu A. Real-world gait speed estimation, frailty and handgrip strength: a cohort-based study. Sci Rep. 2021;11(1):18966.
doi pubmed pmc - Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48(3):452-458.
doi pubmed pmc - Canet J, Gallart L, Gomar C, Paluzie G, Valles J, Castillo J, Sabate S, et al. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology. 2010;113(6):1338-1350.
doi pubmed - Sukharamwala P, Thoens J, Szuchmacher M, Smith J, DeVito P. Advanced age is a risk factor for post-operative complications and mortality after a pancreaticoduodenectomy: a meta-analysis and systematic review. HPB (Oxford). 2012;14(10):649-657.
doi pubmed pmc - Saravana-Bawan B, Goplen M, Alghamdi M, Khadaroo RG. The relationship between visceral obesity and post-operative complications: a meta-analysis. J Surg Res. 2021;267:71-81.
doi pubmed - Liu JM, Deng HL, Chen XY, Zhou Y, Yang D, Duan MS, Huang SH, et al. Risk factors for surgical site infection after posterior lumbar spinal surgery. Spine (Phila Pa 1976). 2018;43(10):732-737.
doi pubmed - Gondo T, Ohno Y, Nakashima J, Hashimoto T, Takizawa I, Tanaka A, Shimodaira K, et al. Factors predicting incisional surgical site infection in patients undergoing open radical cystectomy for bladder cancer. Int J Clin Oncol. 2014;19(5):935-939.
doi pubmed - Kabon B, Nagele A, Reddy D, Eagon C, Fleshman JW, Sessler DI, Kurz A. Obesity decreases perioperative tissue oxygenation. Anesthesiology. 2004;100(2):274-280.
doi pubmed pmc - Mehta AI, Babu R, Karikari IO, Grunch B, Agarwal VJ, Owens TR, Friedman AH, et al. 2012 Young Investigator Award winner: The distribution of body mass as a significant risk factor for lumbar spinal fusion postoperative infections. Spine (Phila Pa 1976). 2012;37(19):1652-1656.
doi pubmed - Uchida Y, Masui T, Nakano K, Yogo A, Sato A, Nagai K, Anazawa T, et al. Clinical and experimental studies of intraperitoneal lipolysis and the development of clinically relevant pancreatic fistula after pancreatic surgery. Br J Surg. 2019;106(5):616-625.
doi pubmed - Powell-Brett S, de Liguori Carino N, Roberts K. Understanding pancreatic exocrine insufficiency and replacement therapy in pancreatic cancer. Eur J Surg Oncol. 2021;47(3 Pt A):539-544.
doi pubmed - Traub J, Reiss L, Aliwa B, Stadlbauer V. Malnutrition in patients with liver cirrhosis. Nutrients. 2021;13(2):540.
doi pubmed pmc - Kato H, Nakao A, Kishimoto W, Nonami T, Harada A, Hayakawa T, Takagi H. 13C-labeled trioctanoin breath test for exocrine pancreatic function test in patients after pancreatoduodenectomy. Am J Gastroenterol. 1993;88(1):64-69.
pubmed - Angrisani M, Sandini M, Cereda M, Paiella S, Capretti G, Nappo G, Roccamatisi L, et al. Preoperative adiposity at bioimpedance vector analysis improves the ability of Fistula Risk Score (FRS) in predicting pancreatic fistula after pancreatoduodenectomy. Pancreatology. 2020;20(3):545-550.
doi pubmed - Sandini M, Paiella S, Cereda M, Angrisani M, Capretti G, Casciani F, Famularo S, et al. Perioperative interstitial fluid expansion predicts major morbidity following pancreatic surgery: appraisal by bioimpedance vector analysis. Ann Surg. 2019;270(5):923-929.
doi pubmed - Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412-423.
doi pubmed pmc - Fukuchi CA, Fukuchi RK, Duarte M. Effects of walking speed on gait biomechanics in healthy participants: a systematic review and meta-analysis. Syst Rev. 2019;8(1):153.
doi pubmed pmc - Pamoukdjian F, Paillaud E, Zelek L, Laurent M, Levy V, Landre T, Sebbane G. Measurement of gait speed in older adults to identify complications associated with frailty: A systematic review. J Geriatr Oncol. 2015;6(6):484-496.
doi pubmed - Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146-156.
doi pubmed - Afilalo J, Kim S, O'Brien S, Brennan JM, Edwards FH, Mack MJ, McClurken JB, et al. Gait speed and operative mortality in older adults following cardiac surgery. JAMA Cardiol. 2016;1(3):314-321.
doi pubmed - Bohannon RW, Crouch R. Minimal clinically important difference for change in 6-minute walk test distance of adults with pathology: a systematic review. J Eval Clin Pract. 2017;23(2):377-381.
doi pubmed - Nakashima Y, Iwaki D, Kawae T, Fudeyasu K, Uemura K, Kimura H. Case-control study of the correlation between the five times sit to stand and 6-min walk distance in patients with pancreatic cancer. Support Care Cancer. 2022;30(12):9743-9749.
doi pubmed pmc - Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, Jang HC, et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020;21(3):300-307.e302.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


