| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Original Article
Volume 15, Number 3, March 2023, pages 148-160
Risk Factors Associated With Atrial Fibrillation in Elderly Patients
Jeeyong Shina , Megha Andrewsa, Lindsey DeJeana, Nicole Debskia, Alyssa Exarchakisa, Julia Fleminga, Roshni Gandhia, Christina Huma, Abyson Kalladanthyila, Rohini Maddiguntaa, Logan Napolia, Cynthia Nguyena, Reshma Paula, Nicole Schmalbacha, Joseph Sichela, Samuel Snydera, Matthew Sterna, Subhadra Thampia, Jesse Viggianoa, Gabriella Yaoa, Krystal Hunterb, Satyajeet Roya, c, d
aCooper Medical School of Rowan University, Camden, NJ, USA
bCooper Research Institute, Cooper Medical School of Rowan University, Camden, NJ, USA
cDepartment of Medicine, Cooper University Health Care, Camden, NJ, USA
dCorresponding Author: Satyajeet Roy, Cooper Medical School of Rowan University, Camden, NJ, USA
Manuscript submitted February 8, 2023, accepted March 23, 2023, published online March 28, 2023
Short title: Atrial Fibrillation in Elderly
doi: https://doi.org/10.14740/jocmr4884
| Abstract | ▴Top |
Background: Atrial fibrillation (AF) is the most common arrhythmia with a growing prevalence worldwide, especially in the elderly population. Patients with AF are at higher risk of serious life-threatening events and complications that may lead to long-term sequelae and reduce quality of life. The aim of our study was to examine the association of additional risk factors and comorbid medical conditions with AF in patients 65 years, or older.
Methods: We performed a retrospective electronic medical record review of patients aged 65 years and older, who visited our internal medicine office between July 1, 2020 and June 30, 2021.
Results: Among 2,433 patients, 418 patients (17.2%) had AF. Our analysis showed that for each unit increased in age, there was a 4.5% increase in the odds of AF (95% confidence interval (CI) 2.2-6.9%; P < 0.001). Compared to patients of Caucasian descent, African-American patients had significantly decreased odds of AF (odds ratio (OR) 0.274, 95% CI 0.141 - 0.531; P < 0.001). Patients with hypertension had 2.241 greater odds of AF (95% CI 1.421 - 3.534; P = 0.001). Additional comorbidities with significantly greater odds of AF included other cardiac arrhythmias (OR 2.523, 95% CI 1.720 - 3.720; P < 0.001), congestive heart failure (OR 3.111, 95% CI 1.674 - 5.784; P < 0.001), osteoarthritis (OR 3.014, 95% CI 2.138 - 4.247; P < 0.001), liver disease (OR 2.129, 95% CI 1.164 - 3.893; P = 0.014), and colorectal disease (OR 1.500 95% CI 1.003 - 2.243; P = 0.048). Comorbidities with significantly decreased odds of AF included other rheumatological disorder (OR 0.144, 95% CI 0.086 - 0.243; P < 0.001), non-steroidal anti-inflammatory drugs (NSAIDs) use (OR 0.206, 95% CI 0.125 - 0.338; P < 0.001), and corticosteroid use (OR 0.553, 95% CI 0.374 - 0.819; P = 0.003).
Conclusions: Increasing age, hypertension, presence of other cardiac arrhythmias, congestive heart failure, osteoarthritis, liver disease, and colorectal disease are associated with increased odds of having AF.
Keywords: Atrial fibrillation; Elderly population; Risk factors of atrial fibrillation
| Introduction | ▴Top |
Atrial fibrillation (AF) is the most common arrhythmia, with a growing prevalence worldwide, especially in the elderly population [1]. By 2050, the projections indicate that it will affect over 5 million individuals in the USA, more than half of whom will predictably be greater than 80 years old, or older [2]. AF also contributes a sizeable amount towards healthcare expenditures, with an estimated national incremental cost of $6 billion for AF alone [3]. In addition to inflicting a sizeable financial toll on the healthcare system, AF is a risk factor for serious life-threatening complications that may lead to long-term sequelae and reduced quality of life in patients. Patients with AF are at a higher risk of thromboembolic events such as stroke [4] and myocardial infarction [5], as well as cardiogenic complications such as sudden cardiac arrest and heart failure [6]. Thus, the management of AF includes anticoagulation therapy, pharmacological or electrical cardioversion, and ablation therapy [7], which aim to reduce the risk of adverse thromboembolic and cardiac events. The complications of AF prove to be especially challenging for the elderly population to recover from. For example, patients who suffered a stroke and who are over the age of 80, have higher risk-adjusted fatality, longer hospitalizations, and lesser likelihood of being discharged to their original residence [8].
The well-described mechanisms of the pathophysiology of AF posit that increased left atrial pressures and distension lead to fibrosis, and conduction defects [9]. Additionally, a growing body of evidence suggests that inflammation may also play a key role in the pathogenesis of AF [10]. After adjustment for confounding factors, increased levels of known inflammatory markers, including renin-angiotensin-aldosterone system, have been shown to be associated with AF [10]. Presence of inflammation has been documented in the atrial biopsies of patients with AF [10]. Accordingly, it is believed that the known risk factors for AF act through these mechanisms, such as congestive heart failure (CHF), valvular heart disease, and previous myocardial infarction [11]. Additionally, patients with a history of smoking, diabetes [11], and hypertension [12] are also known to be at risk for AF. Elderly patients, in particular, are at higher risk of development of AF, as prevalence and incidence increase with age, possibly due to increased comorbidities and health risks associated with the aging population [13]. As such, it is important to identify patient populations at risk for AF for the appropriate screening and initiation of therapy to prevent adverse outcomes associated with AF, especially in the more vulnerable elderly population with multiple comorbidities [8, 13]. The aim of our study was to examine the association of additional risk factors and comorbid medical conditions with AF in patients 65 years, or older.
| Materials and Methods | ▴Top |
Study design and setting
Our study was a retrospective, non-matched, case-control, convenience sampling of the existing electronic medical records of an entire of population of our elderly patients who visited our suburban internal medicine office, which is a part of a larger urban not-for-profit tertiary care healthcare center. The primary health insurance of the majority of our patients was Medicare (62.1%). The rest of the patients had either private health insurance (32.7%), or Medicaid (5.2%).
Participants
The inclusion criteria of our study were patients who were 65 years of age, or older, who visited our internal medicine office between July 1, 2020 and June 30, 2021. The exclusion criteria were the patients who were younger than 65 years of age; or patients 65 years of age, or older who visited our internal medicine office before July 1, 2020 and after June 30, 2021.
Variables
We collected the following data for each patient from their existing electronic medical records: demographics, such as age, sex, race; personal and social factors, such as tobacco use, alcohol use, recreational drug use; family history of coronary artery disease or AF; presence or absence of AF; associated medical conditions, such as hypertension, diabetes mellitus, hyperlipidemia, hypothyroidism, hyperthyroidism, coronary artery disease, QT-prolongation, other cardiac arrhythmias, cerebrovascular accident, peripheral artery disease, carotid artery stenosis, CHF, arthritis, other rheumatological disorder, depression, bipolar disorder, anxiety disorder, schizophrenia, chronic obstructive pulmonary disease, asthma, chronic kidney disease, liver disease, colorectal disorder, frail (assessed by Katz index of risk assessment of activities of daily living, in which a score of 6 was interpreted as independent or not-frail, while a score of 0 to 5 was interpreted as non-independent or frail), human immunodeficiency virus (HIV) infection, immunodeficiency disorder, immune-suppressant therapy post-transplant or post-chemotherapy, reduced immunity due to monoclonal antibody based treatment against inflammation, cancer, other endocrine disorder, sleep apnea; systolic blood pressure, diastolic blood pressure, body mass index (BMI); laboratory indicators, such as high-sensitivity C-reactive protein (hs-CRP), white blood cell count (WBC), erythrocyte sedimentation rate (ESR), thyroid-stimulating hormone (TSH), glycosylated hemoglobin (HbA1c), antinuclear antibody (ANA), rheumatoid factor, total cholesterol, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglyceride (TG) level; and medication use, such as aspirin, antihypertensive medication, non-steroidal anti-inflammatory drugs (NSAIDs), statin, steroid, levothyroxine, selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), central nervous system stimulants, benzodiazepines, immunosuppressant, warfarin, non-vitamin K antagonist oral and anticoagulants. Being a single office study site with an attached laboratory service, the devices used for the measurement of blood pressures, weights and other vital signs, and the laboratory equipment used for the diagnostic laboratory tests, were the same for all the patients who were included in this study. Additionally, we collected the last three consecutive international normalized ratio (INR) values for the patients who were on warfarin.
Data source and access
This study was reviewed and approved by the Institutional Review Board of the Cooper University Health Care (CUHC), Camden, New Jersey, USA (IRB: 21-261). Permission was granted to use materials that were collected solely for research study purposes as per the Health Insurance Portability and Accountability Act (HIPPA) requirements, and the informed consent waivers were granted by the Institutional Review Board. This study was fully compliant with the ethical standards set forth by the CUHC institutional review board. All investigators had full access to the data available only in the electronic medical records of the list of patients approved by the medical informatics of the CUHC, who were selected based on the selection criteria of the study.
Bias
To address the potential for inaccurate or inconsistent diagnosis of AF, we excluded patients who had a documentation of AF in their problem list, but lacked consistent documentation of AF or management of AF in progress notes, or in medical decision-making documentation, during their multiple office visits.
Study size
The entire population of our 2,433 patients who were 65 years of age, or older, who visited our internal medicine office between July 1, 2020 and June 30, 2021. Being a retrospective study, the rationale behind the sample size depended upon the total number of patients of age 65 and older, who visited our internal medicine office between July 1, 2020 and June 30, 2021. Based on our patient volume, we estimated that at least 1,600 patients, who were 65 years and older, were seen in the office during our study period. Our office patients were also estimated to be approximately 75% White and 25% Black. Studies indicate that the lifetime risk of AF is estimated to be one in three in White individuals older than 40 years, and one in five for Black individuals [14]. Based on our patient population, one in three among White patients would have been 1,200/3 = 400, and one in five among Black patients would have been 400/5 = 80, which provided us an estimated 480 patients who would have AF. This sample size was selected for the analysis in order to achieve the sample that would provide 80% power, a large effect size (d ≥ 0.8) and 5% alpha error.
Quantitative variables
We selected the most recent values for the quantitative variables, such as age, BMI, hs-CRP, WBC, ESR, TSH, HbA1c, total cholesterol, LDL-C, HDL-C, TG, and the last three consecutive INR values.
Statistical methods
We recorded the collected data into a Microsoft Excel (2016, Redmond, Washington, USA) spreadsheet. Statistical analysis was computed by using SPSS (Statistical Package for the Social Sciences, version 15.01, IBM, Armonk, New York, USA) software. The patients were divided into two groups. The first group represented the patients who had AF, and the second group represented the patients who did not have AF. AF group included patients with long-term or longstanding persistent AF, or permanent AF. We excluded paroxysmal AF, transient AF, and atrial flutter. We compared each collected independent variable data between the two groups and studied their association with AF in order to find any significant difference. We applied univariate analysis using an independent t-test, Mann Whitney U-test and Chi-square tests. We conducted a multivariate analysis as per the following steps. We examined those variables that were found to be significant at the P < 0.05 level when the univariate analysis was done. We then examined the sample sizes within each explanatory variable to be sure that it could be supported within the model. These explanatory variables were placed in the logistic regression model using the enter procedure. The dependent variable was AF. In this study, significance was defined as a P < 0.05.
| Results | ▴Top |
A total of 2,433 patients were of age 65 years and older who were included in this study. Four hundred and eighteen patients (17.2%) had AF and 2015 patients (82.8%) did not have AF. The mean age of patients with AF was significantly higher than that of patients without AF (80.1 vs. 75.7 years; P < 0.001) (Table 1). The ratio of males to females was significantly greater in the group of patients with AF compared to that of patients without AF. The majority of patients in both groups were White, but the group with AF had a significantly higher proportion of Whites compared to that of the group without AF (86.3% vs. 76.2%; P < 0.001). On the other hand, the group with AF had a significantly lower proportion of Blacks (6.5% vs. 13.0%; P < 0.001) and other races (7.2% vs. 10.8%; P < 0.001) (Table 1). Analysis of social factors did not yield significant differences between patients with AF and patients without (Table 1).
 Click to view | Table 1. Baseline Characteristics |
Patients with AF exhibited a significantly lower systolic blood pressure (mean of 126 vs. 129 mm Hg; P = 0.002) and lower diastolic blood pressure (mean of 72 vs. 74 mm Hg; P < 0.001). The mean BMI for patients with AF was greater than patients without AF (29.8 vs. 28.2 kg/m2), but the difference was not statistically significant (Table 1).
Comparison of the laboratory values showed that the mean HbA1c of patients with AF was equal to that of patients without AF (6.9% vs. 6.9%). Additionally, analysis of hs-CRP, ESR, and ANA yielded no significant difference between patients with AF and patients without AF. However, patients with AF were found to have significantly decreased total cholesterol (151.8 vs. 174.1 mg/dL; P < 0.001), significantly decreased LDL (80.3 vs. 96.4 mg/dL; P < 0.001), significantly decreased HDL (53.5 vs. 56.9 mg/dL; P = 0.009), and significantly decreased TG (102.7 vs. 112.0 mg/dL; P = 0.006) compared to the patients without AF. Although both the groups had their mean TSH levels and mean WBC in the normal ranges, the patients with AF exhibited significantly increased mean TSH levels (2.8 vs. 2.3 mIU/L; P < 0.001) and significantly increased mean WBC (6.79 × 109/L vs. 6.40 × 109/L; P = 0.030) compared to the patients without AF (Table 1). Only 7.7% (n = 32) patients in the AF group were on amiodarone. Analysis of family history showed that patients with AF had a significantly increased reported family history of AF (1.5% vs. 0.5%; P = 0.038) compared to the patients without AF. Additionally, patients with AF had a significantly increased reported family history of coronary artery disease compared to patients without AF (31.3% vs. 25.2%; P = 0.011) (Table 1).
We found significantly higher rates of frequencies of several associated comorbid medical conditions in patients with AF, such as hypertension (85.9% vs. 68.5%; P < 0.001), hyperlipidemia (73.4% vs. 64.5%; P = 0.021), diabetes mellitus (32.5% vs. 27.0%; P < 0.001), coronary artery disease (37.3% vs. 16.9%; P < 0.001), cerebrovascular accident (14.1% vs. 4.4%; P = 0.015), CHF (17.5% vs. 2.8%; P < 0.001), carotid artery stenosis (7.2% vs. 4.4%; P < 0.001), aortic aneurysm (5.4% vs. 2.0%; P = 0.019), peripheral artery disease (10.3% vs. 3.2%; P < 0.001), other arrhythmias (which included sinus tachycardia, sinus bradycardia, atrial flutter, premature atrial complexes, premature ventricular complexes, multifocal atrial tachycardia, first degree heart block, and Mobitz type 1 and type 2 heart blocks) (36.6% vs. 15.1%; P = 0.001), obstructive sleep apnea (17.0% vs. 10.2%; P = 0.001), chronic obstructive pulmonary disease (11.5% vs. 5.6%; P < 0.001), osteoarthritis (OA) (51.7% vs. 26.8%; P < 0.001), chronic kidney disease (25.4% vs. 11.0%; P < 0.001), liver disease (9.3% vs. 5.5%; P = 0.005), and cancer (36.6% vs. 26.6%; P < 0.001) (Table 1, Fig. 1). We also found that the patients with AF had significantly decreased rates of frequencies of other associated comorbidities, such as other rheumatological disorders (which included rheumatoid arthritis (RA), systemic lupus erythematosus, gouty arthritis, mixed connective tissue disorder, and unspecified rheumatological disorders) (1.0% vs. 30.1%; P < 0.001), and monoclonal antibody treatment for inflammatory systemic disorders (such as Crohn’s disease, ulcerative colitis, psoriatic arthritis, and RA) (0% vs. 1.8%; P = 0.005). Of note, comorbidities with no significant difference between the two groups included hypothyroidism, hyperthyroidism, other endocrine disorder, long QTc, depression, bipolar disorder, anxiety disorder, schizophrenia, asthma, being frail, HIV infection, other immunodeficiency diseases, and post-immunosuppressive therapy (Table 1, Fig. 1).
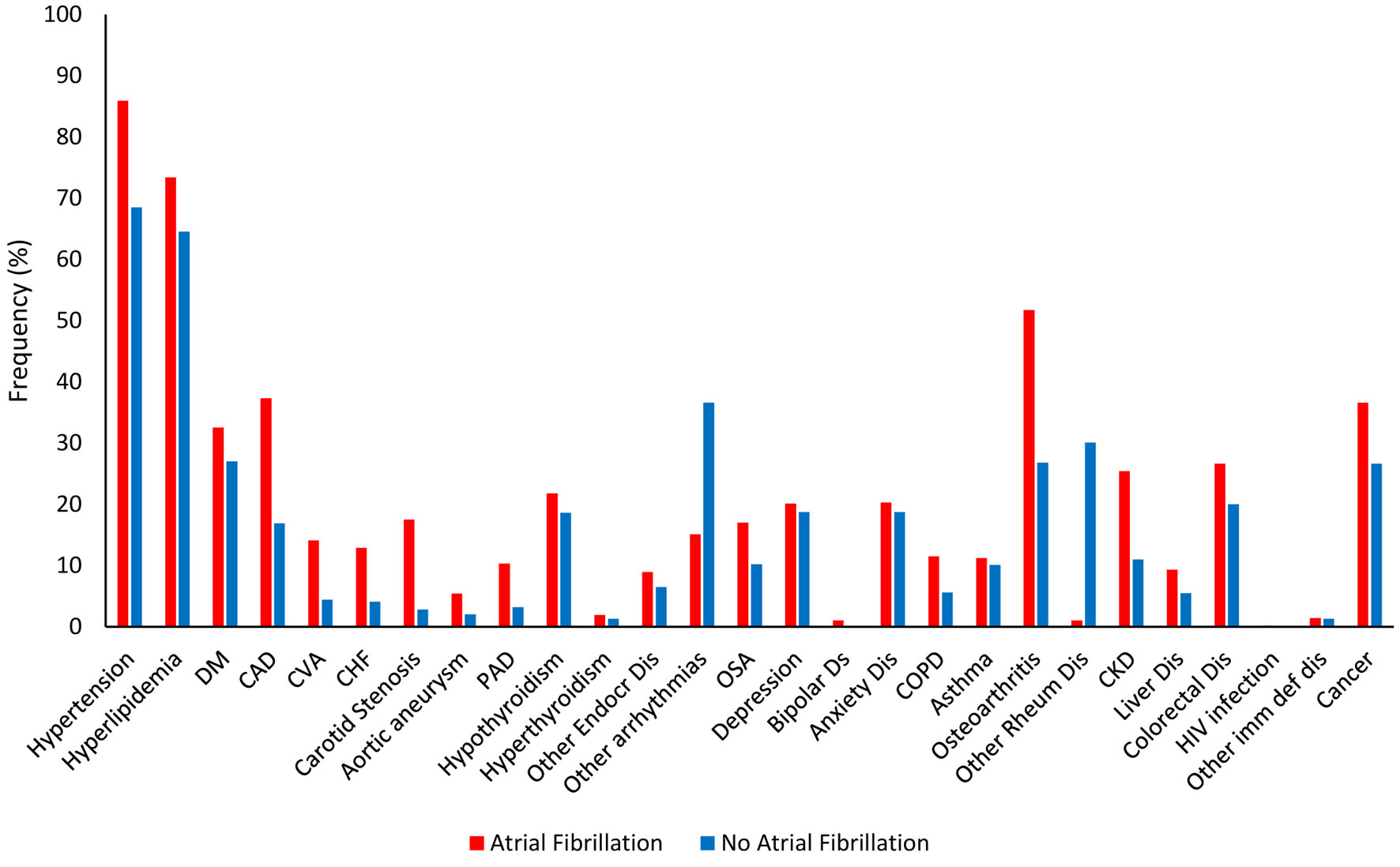 Click for large image | Figure 1. Frequencies of associated medical diagnoses. |
Analysis of medications showed patients with AF had significantly increased frequencies of use of antihypertensive medication (85.9% vs. 67.5%; P < 0.001), statins (73.4% vs. 62.2%; P < 0.001), warfarin (12.5% vs. 2.0%; P < 0.001), and novel oral anticoagulants (52.5% vs. 2.6%; P < 0.001). On the other hand, patients with AF exhibited significantly decreased rates of use of NSAIDs (12.0% vs. 31.2%; P < 0.001) and steroids (27.1% vs. 34.2%; P = 0.005). Medications that did not yield statistically significant differences in our analysis included aspirin, levothyroxine, digoxin, SSRI, SNRI, CNS stimulants, and benzodiazepines (Table 1). In the patients with AF group, 12.5% patients were on warfarin. All of them had their last three consecutive INR values in the therapeutic rage based on their therapeutic target for anticoagulation (INR = 2.0 - 3.0). In the group of patients without AF, 2% patients were on warfarin for various thrombophilic indications, such as deep venous thrombosis or venous thromboembolism. All of them had their last three consecutive INR values in the therapeutic rage based on their therapeutic target for anticoagulation. In the remaining patients with AF group, 52.5% patient were on NOACs, while the remaining 35% patients were not on anticoagulation therapy, either due to a low CHA2DS2-VASc score of 1 (98%), or they opted against anticoagulation due to other reasons, such as frequent falls, or frailty (2%).
Logistic regression analysis showed significant findings for age, Black vs. White race, hypertension, other cardiac arrhythmias, CHF, OA, other rheumatological disorder, liver disease, colorectal disease, use of NSAIDs, and use of corticosteroids. Our analysis showed that for each unit increased in age, there was a 4.5% increase in the odds of AF (95% confidence interval (CI) 2.2-6.9%; P < 0.001). Compared to patients of White race, Black patients had significantly decreased odds of AF with an odds ratio (OR) of 0.274 (95% CI 0.141 - 0.531; P < 0.001). Patients with hypertension had 2.241 greater odds of AF (95% CI 1.421 - 3.534; P = 0.001). Additional comorbidities with significantly greater odds of AF included other cardiac arrhythmias (OR 2.523, 95% CI 1.720 - 3.720; P < 0.001), CHF (OR 3.111, 95% CI 1.674 - 5.784; P < 0.001), OA (OR 3.014, 95% CI 2.138 - 4.247; P < 0.001), liver disease (OR 2.129, 95% CI 1.164 - 3.893; P = 0.014), and colorectal disease (OR 1.500, 95% CI 1.003 - 2.243; P = 0.048). Comorbidities with significantly decreased odds of AF included other rheumatological disorder (OR 0.144, 95% CI 0.086 - 0.243; P < 0.001), NSAIDs use (OR 0.206, 95% CI 0.125 - 0.338; P < 0.001), and corticosteroid use (OR 0.553, 95% CI 0.374 - 0.819; P = 0.003) (Table 2).
 Click to view | Table 2. Influence of Risk Factors on Atrial Fibrillation |
| Discussion | ▴Top |
We found that risk factors for AF include increasing age, hypertension, presence of other cardiac arrhythmias, CHF, OA, liver disease, and colorectal disease. The risk of AF was associated with increasing age in our study, supporting several previous studies which have established age a significant risk factor for AF [2, 13, 15-19]. In the USA, approximately 70% of individuals living with AF fall between the ages of 65 and 85 years [15]. Among the US population that is 80 years of age or older, 8.8% are affected by AF [19]. In a cross-sectional study from Go and associates involving 17,974 adults, prevalence of AF increased from 0.1% in adults aged 55 years or younger to 9.0% in adults aged 80 years or older. Additionally, it was projected that 5.6 million people in the USA will be living with AF by the year 2050, with half of cases predicted to occur in individuals older than 80 years [16]. AF often develops secondarily to comorbidities such as hypertension, heart failure, and valvular heart disease which predispose the atrium to myocardial damage [17]. These conditions are also found in higher rates among the elderly and may partly explain the increased prevalence of AF in older patients [18]. However, studies show that cases remain disproportionately higher in older populations even after accounting for comorbidities [18]. Furthermore, different pathophysiological mechanisms have been proposed explaining AF in younger and older patients [17, 18, 20]. In younger patients, AF is thought to occur due to ectopic electrophysiological beats initiated from the pulmonary veins, often without accompanying structural changes of the atria. Conversely, atrial dilation, cardiac fibrosis, and other electrical and structural atrial changes contribute to AF pathogenesis in older patients [17, 20]. Increasing age remains a major risk factor for the development of AF as observed in our study.
In our study, we found an increased odds of AF for those with hypertension (OR 2.241, 95% CI 1.421 - 3.534; P = 0.001). Hypertension has numerous adverse effects on cardiovascular health [21]. The increased afterload from hypertension can lead to left ventricular concentric hypertrophy and left atrial enlargement because of elevated left ventricular pressures [21, 22] which is associated with AF. The increased odds of AF with hypertension are concerning because AF is associated with an increased risk of stroke [23]. Unfortunately, about one in four adults with hypertension have their condition under control [24] which highlights the importance of treatment to prevent cardiovascular events including AF. Research has shown that many of the structural and functional changes that lead to AF can be attenuated or reversed through treatment with specific antihypertensive therapy [25].
In our study, patients with other pre-existing cardiac arrhythmias had 2.5 times greater odds of AF (95% CI 1.720 - 3.720; P < 0.001), which included sinus tachycardia, sinus bradycardia, atrial flutter, premature atrial complexes, premature ventricular complexes, multifocal atrial tachycardia, first degree heart block, and Mobitz type 1 and type 2 heart blocks. In a nationwide population-based analysis in the Republic of Korea, patients with a prior premature ventricular contraction (PVC) showed a 2.7 times greater risk of developing AF (95% CI 2.428 - 3.013) [26]. A similar study from Taiwan measuring 24-h electrocardiography monitoring noted that patients with multiform PVCs had a 1.55 (95% CI 1.058 - 2.258) increased incidence of new-onset AF [27]. However, both studies could be limited as these studies were based exclusively on East Asian populations. Additionally, a meta-analysis including six cohort studies summarized that patients with PVCs have a 1.90 (95% CI 1.51 - 2.39) increased risk of developing AF compared to patients without PVCs [28]. These studies all show that PVCs, a type of cardiac arrhythmia, can increase the risk of developing AF, but it is unclear how PVCs can induce AF. One theory states that retrograde ventricular-atrial conduction can act as atrial ectopic beats, inducing AF [26]. Additionally, other theories state that during PVCs, it has been demonstrated that there is both a decrease in the flow velocity through the left atrial appendage, as well as an increase in the left atrial pressure, leading to atrial remodeling and a more habitable environment for developing AF [29, 30]. Premature atrial contractions (PACs) can also increase the risk of developing AF. Durmaz and associates performed a retrospective review of ambulatory 24-h Holter monitoring of frequent PACs, defined as more than 720 PACs per 24 h, and found that frequent PACs are strongly associated with risk of future AF [31]. Additionally, patients with very frequent PACs, such as more than 3,000 PACs per 24 h, have a 11-fold higher risk of new-onset AF than those without frequent PAC [31]. A meta-analysis conducted in 2018 demonstrated that frequent PACs on 24 - 48 h Holter monitors were strongly associated with AF (hazard ratio (HR) 2.96 (95% CI 2.33 - 3.76)) [32]. Similarly, the ASSERT study, showed that subclinical atrial tachyarrhythmias were associated with a 5.5-fold increase in the risk of future clinical AF [33]. Thus, these studies regarding PACs also demonstrate an increased risk of AF. These PACs may originate in the pulmonary veins, spontaneously initiating AF [34]. Overall, both PVCs and PACs are generically termed focal tachycardias that can promote atrial remodeling, which can trigger a re-entry circuit. Our findings of increased association of AF with pre-existing cardiac arrhythmias align with the aforementioned studies.
In this study, we found that there were increased odds of having AF for those with a co-morbidity of CHF (OR 3.111, 95% CI 1.674 - 5.784; P ≤ 0.001). Both AF and CHF are prevalent cardiac disorders that often present together in patients [35]. However, the prognostic significance of using AF as a risk factor in predicting CHF is still debated [36]. Many previous studies have attempted to investigate their correlation to help determine treatment options when patients present with both conditions, especially since one will often exacerbate the other resulting in a continuous cycle. While the pathophysiology showing a causative relationship has not been fully determined, it is believed that CHF can induce AF due to a variety of mechanisms including intracellular calcium dysregulation, increased filling pressures, and neurohormonal activation [37]. On the other hand, it is believed that AF can intensify CHF, especially CHF with diastolic dysfunction, due to the ineffective atrial contraction leading to decreased cardiac output. Also unclear is the most effective clinical practice for treating patients with both AF and CHF. Previous literature shows that treatment has been shifting from rhythm control to rate control strategies for patients with both AF and CHF, likely due to the results of the Atrial Fibrillation and Congestive Heart Failure (AF-CHF) trial [38], but other data have revealed rhythm control drugs to be more effective [39]. This presents an area for future studies with strong clinical implications.
We found that OA was associated with increased odds of AF (OR 3.014, 95% CI 2.138 - 4.247; P < 0.001). The relationship between OA and AF is not well-documented, but there have been many previous studies and meta-analyses examining the relationship between OA and cardiovascular diseases (CVDs) in general. A meta-analysis found a significant increased risk of CVD, specifically myocardial infarction (MI) and stroke, associated with a pro-atherogenic lipid and glycemic profile as well as atherosclerotic biomarkers [40]. A thorough systematic review demonstrated that OA was correlated with an increased risk of CVD. On the other hand, one study using a retrospective cohort analysis investigated the incidence rate of AF in patients with RA and in patients with OA, and it concluded no increased risk in either the inflammatory RA condition nor in OA [41]. Suggested mechanisms that may link OA and AF include shared risk factors of CVD and OA [41], such as hypertension, diabetes, hypercholesterolemia, and obesity [42]. Furthermore, NSAIDs are frequently prescribed for OA, and NSAIDs have been linked to a higher risk of AF [43]. OA patients are also less likely to stay physically active due to their joint pains, and lack of exercise might be a contributing factor for CVD and AF, as well [44].
We found that the comorbidity of liver disease significantly increased the odds of AF in our patients. Prior studies have suggested that there is a protective effect of liver disease on developing AF [45]. However, more recent studies have shown that even after controlling for atherosclerosis, an increased advanced fibrosis index is associated with an increased risk for AF [46]. Non-alcoholic fatty liver disease (NAFLD) is associated with increased arterial stiffness in association with histological findings of hepatic fibrosis [47]. Sanbul and associates measured arterial stiffness using an arteriograph system and found increased values in NAFLD patients compared to controls [47]. Liver fibrosis was found to be a positive independent predictor for increased arterial thickness [47]. Eun and associates found that increasing severity of NAFLD was associated with increased arterial stiffness [48]. The mechanism between the association between liver disease and AF is not well understood. However, there are multiple proposed theories. One such theory is that the increased fibrosis from liver disease increases risk of diastolic dysfunction, which may induce AF [49].
Our study found an association between positive history of colorectal disease and increased risk of AF. Such colorectal diseases encountered during our chart review included cancer, Crohn’s disease, ulcerative colitis, and pathological polyps; for purposes of discussion, we may broadly categorize these into cancerous and inflammatory processes. Numerous studies examining the association between specifically colorectal-type cancer and AF have produced conflicting results. A retrospective chart review found AF prevalence was almost three times greater in colorectal cancer patients versus controls [50]; however, multiple studies found elevated AF incidence in the first 90 days after colorectal cancer diagnosis but not later, suggesting it may be attributable to surgery and other acute factors [51-53]. In the reverse relationship, cancer risk remained elevated at 1 year following AF diagnosis [53, 54] while another study did not find increased incidence of colorectal cancer in AF patients [55]. It is supported that AF pathogenesis is linked to an inflammatory state, with common occurrence of CRP elevation [56-58]. Many theorize that inflammation is the link between cancer and AF; this mechanism explains our finding that inflammatory colorectal diseases and colorectal cancer were in combination associated with increased risk of AF, and parallels our results that use of corticosteroids decreased the association of AF. In one study, comorbid AF was associated with worse colorectal cancer outcomes [59], whereas another concluded from multivariate analysis that, despite an overall association with worse colorectal cancer survival rates, AF was not a significant independent factor in morbidity and mortality [58].
We found that Black race, presence of other rheumatological disorders, NSAID use, and corticosteroid used were protective factors against AF. With Black race having the highest prevalence of hypertension in the world (59% in males and 56% in females) partly due to higher association of hypertension [60, 61], one would infer that they would also have the highest prevalence of AF; however, our data show that this is not the case. In a cross-sectional study, Heckbert and associates found that the African American population had a much smaller incidence of clinically detected AF compared to the Caucasians; however, the difference in unbiased ambulatory detection of AF was not statistically significant between the two groups [61]. They also noted differences in symptom perception, clinical recognition, disparities in access to health care or the possibility of differences of completeness of clinical event ascertainment as possible reasons for these findings. It is well accepted that there are immense inequalities in access to health care across socioeconomic groups. Whether or not the issue of not going to the hospital during episodes of AF, or not receiving adequate workup, it is apparent that there are certain barriers creating this underestimation and the gap between clinically diagnosed and monitor detected AF in the African American population.
In our study, we identified that the presence of a rheumatological disorder decreased the odds of AF. The mechanism and pathophysiology behind this are unclear. In a retrospective study, patients with rheumatological disorders were studied from October 2015 to December 2017. It was noted that enteropathic arthropathy, scleroderma, ankylosing spondylitis, and Sjogren’s syndrome all had a decreased association of AF [62]. One possible explanation is the aggressive treatments used to treat such rheumatological disorders. These intense treatments may decrease a patient’s risk of cardiovascular issues such as AF. RA represents a large portion of the rheumatological disorders occurring in the population. One group reports a decreased risk of CVD in RA patients who were treated with TNF blockers and methotrexate [63]. With disease modifying antirheumatic drugs, particularly methotrexate, inflammation and lipid profile can be reduced, which can decrease patients’ risk of CVD [7]. Another aspect to consider is the body mass index of the patients studied. Body mass index was observed to have a protective effect on the level of joint destruction in RA patients [64]. As such, we could consider the possibility of BMI having an inverse relationship with the severity of RA. Additionally, all that was noted in chart reviews was either the presence or absence of a rheumatological disorder, so we were unable to identify the severity. Low disease activity of RA was found to reduce the risk of a first cardiovascular event [65]. It would be worthwhile to investigate the frequency of AF in patients with rheumatological disorders separated based on whether they were being treated, and if they were being treated, how they were being treated. A vast majority of the patients who have rheumatological diseases are being treated and the treatment affects their inflammatory state, and perhaps inverse association with AF.
Our data revealed a significant negative correlation between patients diagnosed with AF and chronic NSAID use or corticosteroid use. Our findings contrast the findings of studies which reported that chronic use of NSAIDs, especially in patients suffering from pre-existing heart conditions, exhibit an increased risk of developing AF. Schmidt and associates conducted a population-based case-control study that found a 40-70% risk increase in AF with chronic NSAID use [43]. One potential reason for this discrepancy between our findings and the existing literature is that the patients might have been recommended by their physicians to refrain from NSAIDs for the treatment of their musculoskeletal pain due to the well-documented increased risk of cardiovascular events and gastrointestinal effects [66]. Also, patients may be under-reporting their NSAID use to their healthcare providers. Hensrud and associates studied a cohort of 200 patients and found that nearly half of those patients did not report their usage of NSAIDs to their healthcare providers [67]. Moreover, our elderly patient population might have led to different results. A study in Denmark studied patient population aged 30 years and older, and they concluded that NSAIDs led to an increased risk of AF with an HR of 1.27 (95% CI 2.0 - 2.4) [68]. In contrast, our study population was significantly older with differing demographics. These possibilities could explain the discrepancy between our observed data and other existing literature. One study reported that giving corticosteroids to patients who experienced a pulmonary vein isolation demonstrated a lower frequency of AF recurrence. It is assumed this occurred because pulmonary vein isolation typically causes inflammation in the body, which is what the corticosteroids were used for [69]. However, other studies have found that use of corticosteroids was associated with AF. For instance, in one study, it was found that out of 20,221 patients who had AF, 1,288 (6.4%) were glucocorticoid users and 2,375 (11.7%) were former users [70]. One reason for this might be that high doses of corticosteroids regulate a potassium efflux by directly affecting the cell membrane. As a result, the change in the membrane potential can induce arrhythmogenesis. Also, corticosteroids can cause retention of fluid as well as sodium, which can lead to hypertension, CHF, and left atrial enlargement, all of which are risk factors for AF [71]. However, we found a negative correlation between corticosteroid use and AF.
We also found a significantly lower mean total cholesterol, LDL-C and TG levels in the group of the patients with AF compared to the patients without AF, which could be explained by the fact that a significantly greater number of patients in the AF group were on statin therapy which lowered their mean cholesterol indices compared to the group without AF (73.4% vs. 62.2%; P < 0.001).
Our study had a few limitations. Being a retrospective chart review study, we had to rely solely on the documentation entered in the electronic medical record by the patient care teams, hence certain items, such as the exact onset of AF in relation to the onset of comorbidities, could not be reliably ascertained. Additionally, being a suburban office setting limits the generalizability of our findings in other settings. Having a large sample size from one primary care office was the major strength of our study. Additional strength was that the study subjects received care from a small group of primary care physicians, and were followed by their specific physicians for a long time which allowed them to chronologically document associated comorbidities and the emerging disorders over a long time period.
Conclusions
We conclude that increasing age, hypertension, presence of other cardiac arrhythmias, CHF, OA, liver disease, and colorectal disease are associated with increased odds of having AF. When assessing the elderly patients, identification of these risk factors along with established risk factors, may assist clinicians to have a high index of suspicion for AF to formulate appropriate therapeutic plans. Optimal management of the identified risk factors may play a role in preventing AF in the elderly population.
Acknowledgments
The authors thank Christine Rickette, RN (study coordinator) for her contribution to this study.
Financial Disclosure
The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
The authors declared no potential conflict of interest with respect to the research, authorship, and/or publication of this article.
Informed Consent
Not applicable. Being a retrospective chart review study, the Institutional Review Board waived the need for informed consent.
Author Contributions
JS and SR made substantial contributions to the study design, drafting, data acquisition and analysis, and manuscript writing. All authors contributed in data collection and manuscript writing and review. KH analyzed the data. SR contributed in revising the manuscript critically for improved intellectual content, and final approval for the version to be published.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129(8):837-847.
doi pubmed pmc - Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285(18):2370-2375.
doi - Kim MH, Johnston SS, Chu BC, Dalal MR, Schulman KL. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes. 2011;4(3):313-320.
doi - Alkhouli M, Friedman PA. Ischemic Stroke Risk in Patients With Nonvalvular Atrial Fibrillation: JACC Review Topic of the Week. J Am Coll Cardiol. 2019;74(24):3050-3065.
doi - Ruddox V, Sandven I, Munkhaugen J, Skattebu J, Edvardsen T, Otterstad JE. Atrial fibrillation and the risk for myocardial infarction, all-cause mortality and heart failure: A systematic review and meta-analysis. Eur J Prev Cardiol. 2017;24(14):1555-1566.
doi pubmed pmc - Bosch NA, Cimini J, Walkey AJ. Atrial Fibrillation in the ICU. Chest. 2018;154(6):1424-1434.
doi pubmed pmc - Gutierrez C, Blanchard DG. Diagnosis and Treatment of Atrial Fibrillation. Am Fam Physician. 2016;94(6):442-452
- Saposnik G, Cote R, Phillips S, Gubitz G, Bayer N, Minuk J, Black S, et al. Stroke outcome in those over 80: a multicenter cohort study across Canada. Stroke. 2008;39(8):2310-2317.
doi - Carlisle MA, Fudim M, DeVore AD, Piccini JP. Heart Failure and Atrial Fibrillation, Like Fire and Fury. JACC Heart Fail. 2019;7(6):447-456.
doi - Boos CJ, Anderson RA, Lip GY. Is atrial fibrillation an inflammatory disorder? Eur Heart J. 2006;27(2):136-149.
doi - Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271(11):840-844
- Huxley RR, Lopez FL, Folsom AR, Agarwal SK, Loehr LR, Soliman EZ, Maclehose R, et al. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2011;123(14):1501-1508.
doi pubmed pmc - Heeringa J, van der Kuip DA, Hofman A, Kors JA, van Herpen G, Stricker BH, Stijnen T, et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J. 2006;27(8):949-953.
doi - Mou L, Norby FL, Chen LY, O'Neal WT, Lewis TT, Loehr LR, Soliman EZ, et al. Lifetime Risk of Atrial Fibrillation by Race and Socioeconomic Status: ARIC Study (Atherosclerosis Risk in Communities). Circ Arrhythm Electrophysiol. 2018;11(7):e006350.
doi pubmed pmc - Feinberg WM, Blackshear JL, Laupacis A, Kronmal R, Hart RG. Prevalence, age distribution, and gender of patients with atrial fibrillation. Analysis and implications. Arch Intern Med. 1995;155(5):469-473
- Lloyd-Jones DM, Wang TJ, Leip EP, Larson MG, Levy D, Vasan RS, D'Agostino RB, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110(9):1042-1046.
doi - Pandit SV, Jalife J. Aging and atrial fibrillation research: where we are and where we should go. Heart Rhythm. 2007;4(2):186-187.
doi pubmed pmc - Sankaranarayanan R, Kirkwood G, Dibb K, Garratt CJ. Comparison of Atrial Fibrillation in the Young versus That in the Elderly: A Review. Cardiol Res Pract. 2013;2013:976976.
doi pubmed pmc - Ryder KM, Benjamin EJ. Epidemiology and significance of atrial fibrillation. Am J Cardiol. 1999;84(9A):131R-138R.
doi - Wasmer K, Eckardt L, Breithardt G. Predisposing factors for atrial fibrillation in the elderly. J Geriatr Cardiol. 2017;14(3):179-184.
doi pubmed pmc - Bundy JD, Mills KT, He J. Comparison of the 2017 ACC/AHA Hypertension Guideline with Earlier Guidelines on Estimated Reductions in Cardiovascular Disease. Curr Hypertens Rep. 2019;21(10):76.
doi pubmed pmc - Appleton CP, Galloway JM, Gonzalez MS, Gaballa M, Basnight MA. Estimation of left ventricular filling pressures using two-dimensional and Doppler echocardiography in adult patients with cardiac disease. Additional value of analyzing left atrial size, left atrial ejection fraction and the difference in duration of pulmonary venous and mitral flow velocity at atrial contraction. J Am Coll Cardiol. 1993;22(7):1972-1982.
doi - Wolf PA, Dawber TR, Thomas HE, Jr., Kannel WB. Epidemiologic assessment of chronic atrial fibrillation and risk of stroke: the Framingham study. Neurology. 1978;28(10):973-977.
doi - Centers for Disease Control and Prevention. Hypertension cascade: hypertension prevalence, treatment and control estimates among U.S. adults aged 18 years and older applying the criteria from the American College of Cardiology and American Heart Association’s 2017 Hypertension Guideline-NHANES 2015-2018. Atlanta, GA: U.S. Department of Health and Human Services; 2021. Accessed November 30, 2022. Available at: https://millionhearts.hhs.gov/data-reports/hypertension-prevalence.html.
- Healey JS, Connolly SJ. Atrial fibrillation: hypertension as a causative agent, risk factor for complications, and potential therapeutic target. Am J Cardiol. 2003;91(10A):9G-14G.
doi - Kim YG, Han KD, Choi JI, Choi YY, Choi HY, Shim J, Kim YH. Premature ventricular contraction is associated with increased risk of atrial fibrillation: a nationwide population-based study. Sci Rep. 2021;11(1):1601.
doi pubmed pmc - Lin CY, Chang SL, Lin YJ, Lo LW, Chung FP, Chen YY, Chao TF, et al. Long-term outcome of multiform premature ventricular complexes in structurally normal heart. Int J Cardiol. 2015;180:80-85.
doi - Rujirachun P, Wattanachayakul P, Phichitnitikorn P, Charoenngam N, Winijkul A. Risk of atrial fibrillation among patients with premature ventricular complexes: a systematic review and meta-analysis of cohort studies. Minerva Cardiol Angiol. 2022.
doi - Siostrzonek P, Koppensteiner R, Gossinger H, Zangeneh M, Heinz G, Kreiner G, Stumpflen A, et al. Hemodynamic and hemorheologic determinants of left atrial spontaneous echo contrast and thrombus formation in patients with idiopathic dilated cardiomyopathy. Am Heart J. 1993;125(2 Pt 1):430-434.
doi - Alizadeh A, Maleki M, Bassiri H, Alasti M, Emkanjoo Z, Haghjoo M, Arya A, et al. Evaluation of atrial thrombus formation and atrial appendage function in patients with pacemaker by transesophageal echocardiography. Pacing Clin Electrophysiol. 2006;29(11):1251-1254.
doi - Durmaz E, Ikitimur B, Kilickiran Avci B, Atici A, Yurtseven E, Tokdil H, Ebren C, et al. The clinical significance of premature atrial contractions: How frequent should they become predictive of new-onset atrial fibrillation. Ann Noninvasive Electrocardiol. 2020;25(3):e12718.
doi pubmed pmc - Himmelreich JCL, Lucassen WAM, Heugen M, Bossuyt PMM, Tan HL, Harskamp RE, van Etten-Jamaludin FS, et al. Frequent premature atrial contractions are associated with atrial fibrillation, brain ischaemia, and mortality: a systematic review and meta-analysis. Europace. 2019;21(5):698-707.
doi - Healey JS, Connolly SJ, Gold MR, Israel CW, Van Gelder IC, Capucci A, Lau CP, et al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366(2):120-129.
doi - Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339(10):659-666.
doi - Eysenck W, Saba M. Rhythm Control in Heart Failure Patients with Atrial Fibrillation. Arrhythm Electrophysiol Rev. 2020;9(3):161-166.
doi pubmed pmc - Naccarelli GV, Hynes BJ, Wolbrette DL, Bhatta L, Khan M, Samii S, Luck JC. Atrial fibrillation in heart failure: prognostic significance and management. J Cardiovasc Electrophysiol. 2003;14(12 Suppl):S281-S286.
doi - Anter E, Jessup M, Callans DJ. Atrial fibrillation and heart failure: treatment considerations for a dual epidemic. Circulation. 2009;119(18):2516-2525.
doi - Roy D, Talajic M, Dubuc M, Thibault B, Guerra P, Macle L, Khairy P. Atrial fibrillation and congestive heart failure. Curr Opin Cardiol. 2009;24(1):29-34.
doi - Khan MA, Ahmed F, Neyses L, Mamas MA. Atrial fibrillation in heart failure: The sword of Damocles revisited. World J Cardiol. 2013;5(7):215-227.
doi pubmed pmc - Mathieu S, Couderc M, Tournadre A, Soubrier M. Cardiovascular profile in osteoarthritis: a meta-analysis of cardiovascular events and risk factors. Joint Bone Spine. 2019;86(6):679-684.
doi - Kim SC, Liu J, Solomon DH. The risk of atrial fibrillation in patients with rheumatoid arthritis. Ann Rheum Dis. 2014;73(6):1091-1095.
doi pubmed pmc - Wang H, Bai J, He B, Hu X, Liu D. Osteoarthritis and the risk of cardiovascular disease: a meta-analysis of observational studies. Sci Rep. 2016;6:39672.
doi pubmed pmc - Schmidt M, Christiansen CF, Mehnert F, Rothman KJ, Sorensen HT. Non-steroidal anti-inflammatory drug use and risk of atrial fibrillation or flutter: population based case-control study. BMJ. 2011;343:d3450.
doi - Elliott AD, Maatman B, Emery MS, Sanders P. The role of exercise in atrial fibrillation prevention and promotion: Finding optimal ranges for health. Heart Rhythm. 2017;14(11):1713-1720.
doi - Zamirian M, Sarmadi T, Aghasadeghi K, Kazemi MB. Liver cirrhosis prevents atrial fibrillation: A reality or just an illusion? J Cardiovasc Dis Res. 2012;3(2):109-112.
doi pubmed pmc - Park HE, Lee H, Choi SY, Kim HS, Chung GE. The risk of atrial fibrillation in patients with non-alcoholic fatty liver disease and a high hepatic fibrosis index. Sci Rep. 2020;10(1):5023.
doi pubmed pmc - Sunbul M, Agirbasli M, Durmus E, Kivrak T, Akin H, Aydin Y, Ergelen R, et al. Arterial stiffness in patients with non-alcoholic fatty liver disease is related to fibrosis stage and epicardial adipose tissue thickness. Atherosclerosis. 2014;237(2):490-493.
doi - Chung GE, Choi SY, Kim D, Kwak MS, Park HE, Kim MK, Yim JY. Nonalcoholic fatty liver disease as a risk factor of arterial stiffness measured by the cardioankle vascular index. Medicine (Baltimore). 2015;94(12):e654.
doi pubmed pmc - Chung GE, Lee JH, Lee H, Kim MK, Yim JY, Choi SY, Kim YJ, et al. Nonalcoholic fatty liver disease and advanced fibrosis are associated with left ventricular diastolic dysfunction. Atherosclerosis. 2018;272:137-144.
doi - Guzzetti S, Costantino G, Vernocchi A, Sada S, Fundaro C. First diagnosis of colorectal or breast cancer and prevalence of atrial fibrillation. Intern Emerg Med. 2008;3(3):227-231.
doi - Saliba W, Rennert HS, Gronich N, Gruber SB, Rennert G. Association of atrial fibrillation and cancer: Analysis from two large population-based case-control studies. PLoS One. 2018;13(1):e0190324.
doi pubmed pmc - Erichsen R, Christiansen CF, Mehnert F, Weiss NS, Baron JA, Sorensen HT. Colorectal cancer and risk of atrial fibrillation and flutter: a population-based case-control study. Intern Emerg Med. 2012;7(5):431-438.
doi - Conen D, Wong JA, Sandhu RK, Cook NR, Lee IM, Buring JE, Albert CM. Risk of malignant cancer among women with new-onset atrial fibrillation. JAMA Cardiol. 2016;1(4):389-396.
doi pubmed pmc - Ostenfeld EB, Erichsen R, Pedersen L, Farkas DK, Weiss NS, Sorensen HT. Atrial fibrillation as a marker of occult cancer. PLoS One. 2014;9(8):e102861.
doi pubmed pmc - Wassertheil-Smoller S, McGinn AP, Martin L, Rodriguez BL, Stefanick ML, Perez M. The associations of atrial fibrillation with the risks of incident invasive breast and colorectal cancer. Am J Epidemiol. 2017;185(5):372-384.
doi pubmed pmc - Chung MK, Martin DO, Sprecher D, Wazni O, Kanderian A, Carnes CA, Bauer JA, et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001;104(24):2886-2891.
doi - Guzzetti S, Costantino G, Fundaro C. Systemic inflammation, atrial fibrillation, and cancer. Circulation. 2002;106(9):e40.
doi - Walsh SR, Gladwish KM, Ward NJ, Justin TA, Keeling NJ. Atrial fibrillation and survival in colorectal cancer. World J Surg Oncol. 2004;2:40.
doi pubmed pmc - Patel N, Amgai B, Chakraborty S, Hajra A, Binit A, Patel Z, Ashish K, et al. Impact of atrial fibrillation in patients with colorectal cancer: a national inpatient sample database analysis. European Heart Journal. 2021;42(Supplement_1).
- Deere BP, Ferdinand KC. Hypertension and race/ethnicity. Curr Opin Cardiol. 2020;35(4):342-350.
doi - Heckbert SR, Austin TR, Jensen PN, Floyd JS, Psaty BM, Soliman EZ, Kronmal RA. Yield and consistency of arrhythmia detection with patch electrocardiographic monitoring: The Multi-Ethnic Study of Atherosclerosis. J Electrocardiol. 2018;51(6):997-1002.
doi pubmed pmc - Khan MZ, Patel K, Patel KA, Doshi R, Shah V, Adalja D, Waqar Z, et al. Burden of atrial fibrillation in patients with rheumatic diseases. World J Clin Cases. 2021;9(14):3252-3264.
doi pubmed pmc - Roman MJ, Salmon JE. Cardiovascular manifestations of rheumatologic diseases. Circulation. 2007;116(20):2346-2355.
doi - van der Helm-van Mil AH, van der Kooij SM, Allaart CF, Toes RE, Huizinga TW. A high body mass index has a protective effect on the amount of joint destruction in small joints in early rheumatoid arthritis. Ann Rheum Dis. 2008;67(6):769-774.
doi - Arts EE, Fransen J, Den Broeder AA, van Riel P, Popa CD. Low disease activity (DAS28</=3.2) reduces the risk of first cardiovascular event in rheumatoid arthritis: a time-dependent Cox regression analysis in a large cohort study. Ann Rheum Dis. 2017;76(10):1693-1699.
doi - Bennett JS, Daugherty A, Herrington D, Greenland P, Roberts H, Taubert KA. The use of nonsteroidal anti-inflammatory drugs (NSAIDs): a science advisory from the American Heart Association. Circulation. 2005;111(13):1713-1716.
doi - Hensrud DD, Engle DD, Scheitel SM. Underreporting the use of dietary supplements and nonprescription medications among patients undergoing a periodic health examination. Mayo Clin Proc. 1999;74(5):443-447.
doi - Schjerning Olsen AM, Fosbol EL, Pallisgaard J, Lindhardsen J, Lock Hansen M, Kober L, Hansen PR, et al. NSAIDs are associated with increased risk of atrial fibrillation in patients with prior myocardial infarction: a nationwide study. Eur Heart J Cardiovasc Pharmacother. 2015;1(2):107-114.
doi - van der Hooft CS, Heeringa J, Brusselle GG, Hofman A, Witteman JC, Kingma JH, Sturkenboom MC, et al. Corticosteroids and the risk of atrial fibrillation. Arch Intern Med. 2006;166(9):1016-1020.
doi - Christiansen CF, Christensen S, Mehnert F, Cummings SR, Chapurlat RD, Sorensen HT. Glucocorticoid use and risk of atrial fibrillation or flutter: a population-based, case-control study. Arch Intern Med. 2009;169(18):1677-1683.
doi - Koyama T, Tada H, Sekiguchi Y, Arimoto T, Yamasaki H, Kuroki K, Machino T, et al. Prevention of atrial fibrillation recurrence with corticosteroids after radiofrequency catheter ablation: a randomized controlled trial. J Am Coll Cardiol. 2010;56(18):1463-1472.
doi
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


