| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Original Article
Volume 15, Number 2, February 2023, pages 90-98
Increased Serum Sodium at Acute Kidney Injury Onset Predicts In-Hospital Death
Benedikt Marahrensa, b, Leah Damscha, b, Rebecca Lehmanna, Igor Matyukhina, Susann Patschana, Daniel Patschana, c
aInnere Medizin 1, Kardiologie, Angiologie, Nephrologie, Universitatsklinikum Brandenburg, Medizinische Hochschule Brandenburg, Brandenburg, Germany
bThese authors contributed equally to the study.
cCorresponding Author: Daniel Patschan, Zentrum fur Innere Medizin 1, Universitatsklinikum Brandenburg, Medizinische Hochschule Brandenburg, Hochstraße 29, 14770 Brandenburg, Germany
Manuscript submitted November 16, 2022, accepted January 5, 2023, published online February 28, 2023
Short title: Sodium Predicts AKI Survival
doi: https://doi.org/10.14740/jocmr4845
| Abstract | ▴Top |
Background: Over the last decades, acute kidney injury (AKI) has been identified as a potentially fatal diagnosis which substantially increases in-hospital mortality in the short term and morbidity/mortality in the long term. However, reliable biomarkers for predicting AKI-associated outcomes are still missing. In this study, we assessed whether serum sodium, measured at different time points during the in-hospital treatment period, provided prognostic information in AKI.
Methods: This was a retrospective, observational cohort study. AKI subjects were identified via the in-hospital AKI alert system. Serum sodium and potassium levels were documented at five pre-defined time points: hospital admission, AKI onset, minimum estimated glomerular filtration rate, minimum and maximum of the respective electrolyte during the treatment period. In-hospital death, the need for kidney replacement therapy (KRT) and recovery of kidney function were defined as endpoints.
Results: Patients who suffered in-hospital death (n = 37, 23.1%) showed significantly higher serum sodium levels at diagnosis of AKI (survivors: 145.7 ± 2.13 vs. non-survivors: 138.8 ± 0.636 mmol/L, P = 0.003). A logistic regression model was significant for serum sodium levels in patients with in-hospital death (X2, P = 0.003; odds ratio = 1.08 (1.022 - 1.141); R2 = 0.082; d = 0.089). This suggests an increase of the relative risk for in-hospital death by 8% with every unit of serum sodium increase. Patients with a sodium above the upper normal range at AKI diagnosis were also more likely to suffer in-hospital death (P = 0.001).
Conclusion: In summary, we present evidence that serum sodium, measured at time of AKI diagnosis, potentially serves as a predictor for in-hospital death in patients with AKI.
Keywords: AKI; Serum sodium; Survival; Risk prediction
| Introduction | ▴Top |
Acute kidney injury (AKI), for many years known as “acute renal failure”, affects increasing numbers of patients treated in hospitals in Central Europe and in the USA [1]. The term “AKI” has officially been recommended since 2012 [2], which encompasses a broad spectrum of kidney damage patterns, ranging from exclusive tissue damage without functional impairment to bilateral cortical necrosis. Early AKI diagnosis remains difficult, although the field of biomarker research has significantly evolved during the last 15 - 20 years [3]. Risk prediction is also challenging. Several studies addressed the question on whether certain molecular markers potentially provide information about AKI progression after onset or if renal function would recover or not. Koyner and colleagues [4] for instance identified interleukin-18 (IL-18), urinary albumin to creatinine ratio, and plasma neutrophil gelatinase-associated lipocalin (NGAL) to be associated with a higher AKI progression risk. Caironi et al [5] measured plasma proenkephalin A 119-159 (PenKid) in more than 900 septic subjects (“Albumin Italian Outcome Sepsis” - ALBIOS - trial). The marker was shown as useful not only for AKI but also for post-AKI recovery prediction. In 2011, Singer and colleagues [6] analyzed both, the diagnostic and prognostic potential of urinary NGAL. Subjects that reached the primary endpoint of AKI progression, dialysis, and death showed higher NGAL levels at the time of inclusion. Survival prediction through both, urinary NGAL and kidney injury molecule-1 (KIM-1) was also shown by others [7]. Although some data are promising without doubt, it needs to be mentioned that many clinical laboratories still do not offer the opportunity to quantify proteins such as NGAL, KIM-1 or others yet. The advantage of sodium and potassium over other biomarker molecules tested or used in clinical practice is their general availability.
Electrolyte disturbances are a hallmark in established AKI. Namely hyperkalemia potentially endangers patients by inducing cardiac arrhythmias. However, few studies addressed the question on whether electrolyte disbalances, apparent before or at the time of AKI onset, are useful for risk prediction in the short term. Woitok and colleagues [8] for instance published a cross-sectional analysis of emergency department patients (January 2017 to December 2018). Eight percent were diagnosed with AKI, and hypo- and hypernatremia were found in 23.16% and 1.4%. Both electrolyte disturbances were associated with adverse outcomes. Ravioli et al [9] showed initial dyskalemia in AKI as an in-hospital mortality predictor also. Only very few studies found that sodium disbalance in AKI has predictive value [10, 11]. From the nephrologist’s perspective, three AKI-related outcome variables are particularly important: in-hospital death, the need for kidney replacement therapy (KRT), and recovery of kidney function until discharge.
Herein, we present a single-center retrospective observational study in which we assessed both serum sodium and potassium levels in AKI subjects at different time points after hospital admission and investigate whether the endpoints mentioned above can be predicted by serum sodium. In general, the study was planned in order to identify serum electrolytes as potential candidates for prognostic decisions in AKI. In other words, we analyzed the role of serum electrolytes as AKI biomarkers.
| Materials and Methods | ▴Top |
Design
The study was retrospective and observational in nature. The Ethics Committee of the Brandenburg Medical School formally approved the trial (E-01-20210510). The study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
Patients
All patients were recruited from the University Hospital Brandenburg of the Brandenburg Medical School. The observational period lasted from January to February 2019 (2 months). Only patients in whom the in-hospital AKI alert system identified a significant deterioration of excretory kidney function were screened for inclusion.
AKI diagnosis
Patients with AKI were identified through the in-hospital AKI alert system. The system is based on criteria 1 and 2 of the 2012 updated version of the “KDIGO clinical practice guidelines for acute kidney injury” (criterion 1: increase in serum creatinine of 0.3 mg/dL or more within 48 h; criterion 2: at least 1.5-fold increase in serum creatinine within 7 days [2]). If either criterion 1 and/or 2 is/are fulfilled, an automated message is transferred to the nephrologist in charge. It contains anonymized information about the respective individual. For further analyses, the in-hospital information system (MEDICO®-CGM) must be employed. Criterion 3 of the KDIGO guideline was not considered since information on urine output was missing in many subjects.
Analysis and time points
Serum sodium and potassium levels were documented at the following time points: 1) hospital admission, 2) AKI onset, and 3) at the time when the estimated glomerular filtration rate (eGFR) (Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) [12]) reached the minimum. Also, the respective maxima and minima of both serum sodium and potassium were documented.
Endpoints
The following endpoints were defined: in-hospital death (endpoint 1), the need for KRT (endpoint 2), and recovery of kidney function until discharge (endpoint 3). The need for KRT was fulfilled if a patient required at least one extracorporal procedure. Extracorporal therapy was initiated, if patients presented one or more of the following criteria: refractory hyperhydration including progressive dyspnea, refractory hyperkalemia of 6.5 mmol/L or above, refractory metabolic acidosis with a pH of 7.1 or below, neurological symptoms due to suspected uremia. KRT was either performed as intermittent hemodialysis, or slow extended daily dialysis (SLEDD), or continuous veno-venous hemodialysis. Recovery of kidney function was diagnosed if the last eGFR (CKD-EPI [12]) differed from the initial eGFR by no more than 10%. Data on endpoints 1 and 2 were available from all patients, and data on endpoint 3 were missing in 50 subjects.
Statistical analysis
We used Microsoft Excel, SPSS 26 and Prism to analyze and display our data. Normally distributed metric data of two groups were analyzed using a t-test, not normally distributed using Mann-Whitney U test. Comparisons between more than two groups in paired samples were performed with the Friedman’s test. Chi-square and Fisher’s exact tests were used to analyze two nominal values. To predict our outcomes by electrolyte concentrations at different time points, we used a logistic regression model. Multiple regression analysis was performed in order to identify independent predictors. For the identification of cut-off values, the Youden index was calculated (sensitivity + specificity - 1). The highest (positive) numerical result defined the final cut-off. A P-value < 0.05 was considered significant. The results are given as mean ± standard error of the mean (SEM) or as median ± interquartile range (IQR) as indicated.
| Results | ▴Top |
Patients
A total number of 160 individuals were included in the study (female 57; male 103). The mean age was 77.2 ± 12.4 years. The in-hospital treatment time was 16 ± 13.3 days. The in-hospital mortality was 23.1%. The following causes of death were identified: sepsis 43.8%, heart failure 25%, AKI 9.4%, volume/blood depletion 6.2%, liver failure 6.2%, cerebral hemorrhage 3.1%, lung cancer 3.1%, and pulmonary disease 3.1%. KRT became mandatory in 10.6%. Recovery of kidney function was observed in 60% of the individuals. Regarding the etiology of AKI, the following distribution of causes was identified (more to less frequent): sepsis 31.2%, cardiorenal 26.2%, post-surgery 10.6%, volume depletion (pre-renal) 8.8%, hepatorenal 5.6%, contrast-associated 3.8%, obstruction 1.2%, and others 9.4%. The distribution of AKI severity according to Acute Kidney Injury Network (AKIN) [13] was I 52.5%, II 21.2%, and III 26.3%. Pre-existing chronic kidney disease (CKD) was diagnosed in 49.7%, and arterial hypertension was pre-established in 81.3% of the subjects. Diabetes mellitus was prevalent with 39%, and pre-existing heart failure in 32.7%. Table 1 summarizes all relevant patients’ characteristics.
 Click to view | Table 1. Baseline Characteristics of All Patients Included in the Study |
Serum sodium
We measured serum sodium concentrations at five different time points: admission (137.3 ± 0.5 mmol/L), AKI onset (138.4 ± 0.61 mmol/L), minimal eGFR (138.2 ± 1.14 mmol/L), maximum serum sodium (143.8 ± 0.54 mmol/L), and minimum serum sodium (134.5 ± 0.52 mmol/L). The respective medians were not equal (P < 0.001) (Fig. 1).
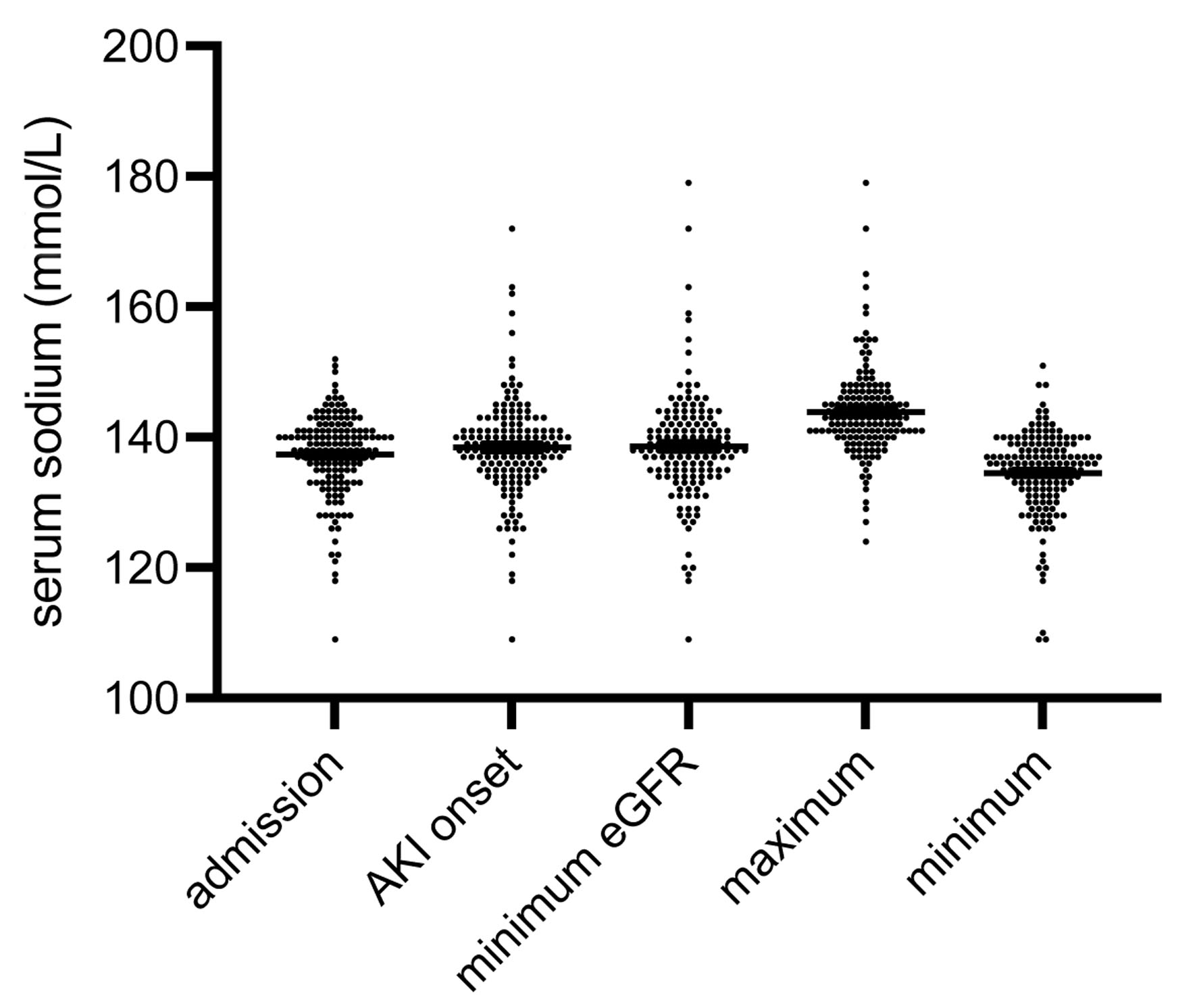 Click for large image | Figure 1. Distribution of serum sodium at five pre-defined time points: admission to the hospital, diagnosis of AKI (AKI onset), minimum eGFR, maximum and minimum of serum sodium, respectively. The medians were not equal (P < 0.001). The numbers of all outliers per group were: admission 1, AKI onset 6, minimum eGFR 6, maximum 5, minimum 3 (data are shown as median ± IQR). AKI: acute kidney injury; eGFR: estimated glomerular filtration rate; IQR: interquartile range. |
Logistic binary regression was used for prediction analysis. Serum sodium at AKI diagnosis predicted in-hospital death (X2, P = 0.003; odds ratio = 1.08 (1.022 - 1.141); R2 = 0.082). The effect by Cohen was weak (d = 0.089). With an odds ratio of 1.08, the chances of in-hospital death increase by 8% with every unit of serum sodium increase. Multiple regression analysis was additionally performed with the following variables: age, gender, pre-existing CKD, serum sodium and potassium initially, and sodium and potassium at AKI onset. Serum sodium at AKI onset remained survival predictive (P = 0.037). The cut-off value for serum sodium at AKI onset was identified with 145.5 mmol/L. Interestingly, not only was serum sodium at AKI diagnosis markedly higher in patients who suffered in-hospital death (145.7 ± 2.13 vs. 138.8 ± 0.64 mmol/L, P = 0.003) (Fig. 2), but also significantly more patients from this group presented with a serum sodium above the normal range (P = 0.001). This finding generally suggests a serum sodium above the normal range to be associated with an increased in-hospital mortality. Additional analyses of systolic and diastolic blood pressure values in survivors and non-survivors at AKI onset did not show any significant differences (systolic values: survivors 120 ± 1.9 mm Hg vs. non-survivors 125 ± 4.4 mm Hg; P = 0.27; diastolic values: survivors 68 ± 1.2 mm Hg vs. non-survivors 69 ± 2.6 mm Hg; P = 0.56). Serum sodium at AKI diagnosis also predicted poor renal recovery (X2, P = 0.024; odds ratio = 0.937 (0.883 - 0.995); R2 = 0.063; d = 0.067), suggesting that with every unit of sodium increase, the chances of renal recovery decrease by 6%. Finally, maximum serum sodium was predictive for in-hospital death as well (X2, P = 0.002).
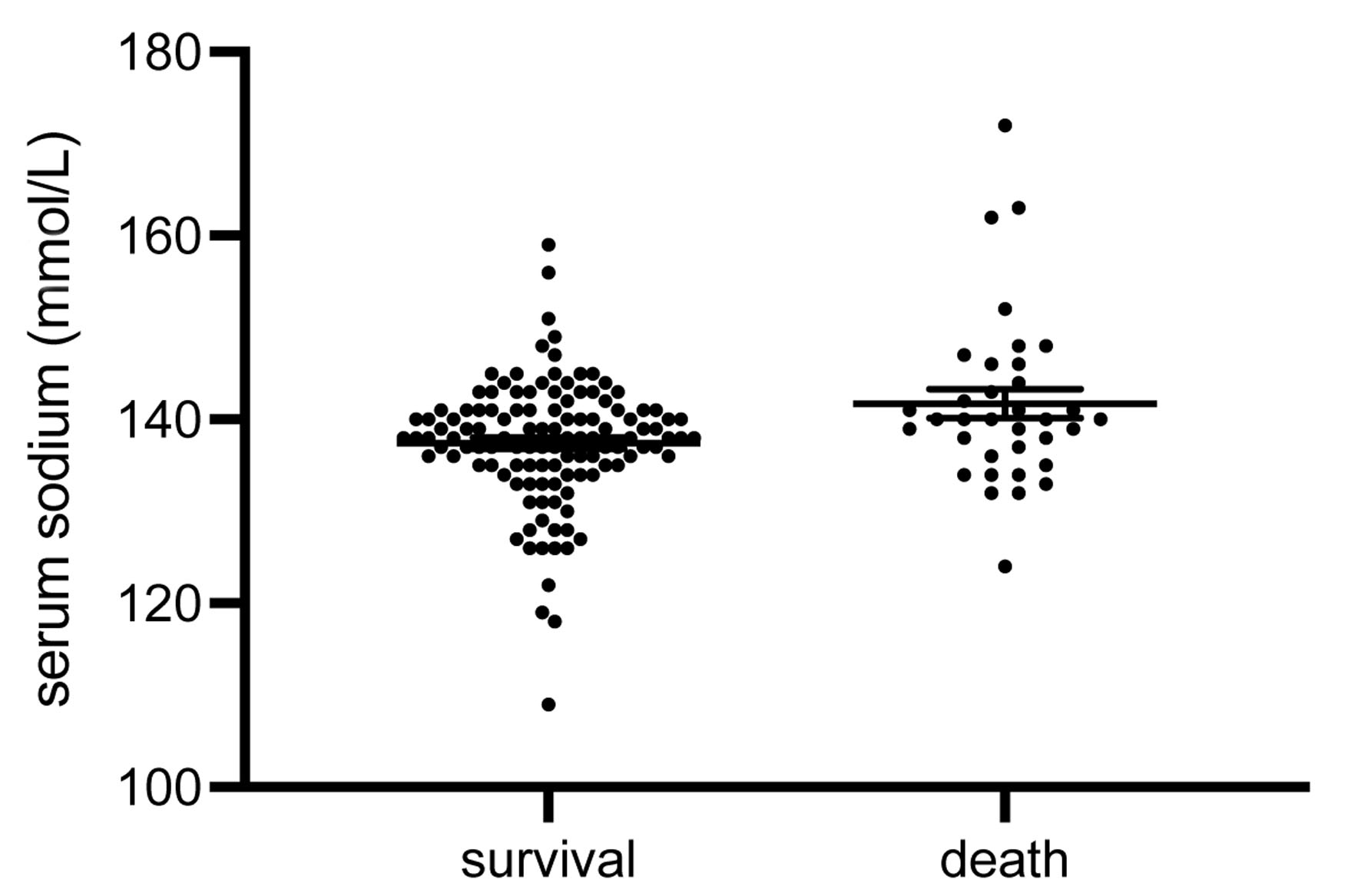 Click for large image | Figure 2. Serum sodium at AKI diagnosis. Subjects that did not survive the in-hospital treatment period showed significantly higher serum sodium levels at AKI onset (P = 0.003). The numbers of all outliers per group were: survival 4, death 1 (data are shown as median ± IQR). AKI: acute kidney injury; IQR: interquartile range. |
Using a receiver operating characteristic (ROC) curve, we analyzed the prognostic value for serum sodium for in-hospital death. ROC showed a sensitivity of 0.6 and a specificity of 0.6. The area under the curve (AUC) of the ROC was 0.622, suggesting that our model for our data has prognostic value as the AUC is > 0.5 but cannot be considered a good model for which the AUC value should be above 0.7 (Fig. 3).
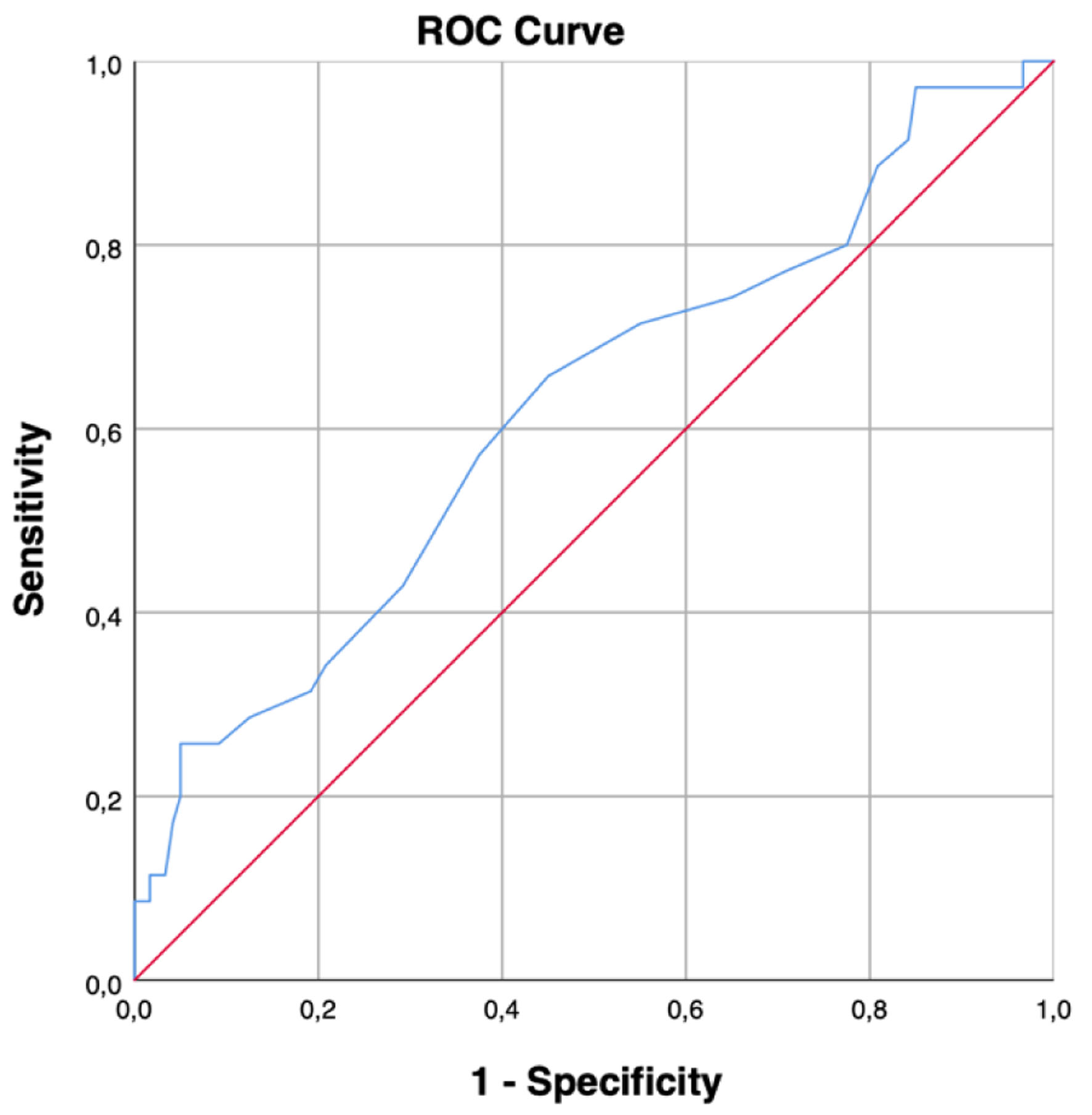 Click for large image | Figure 3. ROC curve for serum sodium at AKI diagnosis. AUC > 0.5 as the ROC lies above the diagonal line. ROC: receiver operating characteristic; AKI: acute kidney injury; AUC: area under the curve. |
Serum potassium
Comparable to serum sodium, the medians of serum potassium were not equal at the five pre-defined time points: admission (4.5 ± 0.06 mmol/L), AKI onset (4.4 ± 0.06 mmol/L), minimum eGFR (4.5 ± 0.07 mmol/L), maximum serum potassium (5.0 ± 0.06 mmol/L), and minimum serum potassium (3.7 ± 0.04 mmol/L) (P < 0.001) (Fig. 4). As expected, serum potassium was predictive at various time points. Initial, peak, minimum eGFR (AKI peak) predicted KRT (initial: X2, P = 0.042; peak: X2, P = 0.001; AKI peak: X2, P = 0.029). In-hospital death was also reliably predictable by both maximum potassium (X2, P < 0.001) and potassium at AKI peak (X2, P < 0.001).
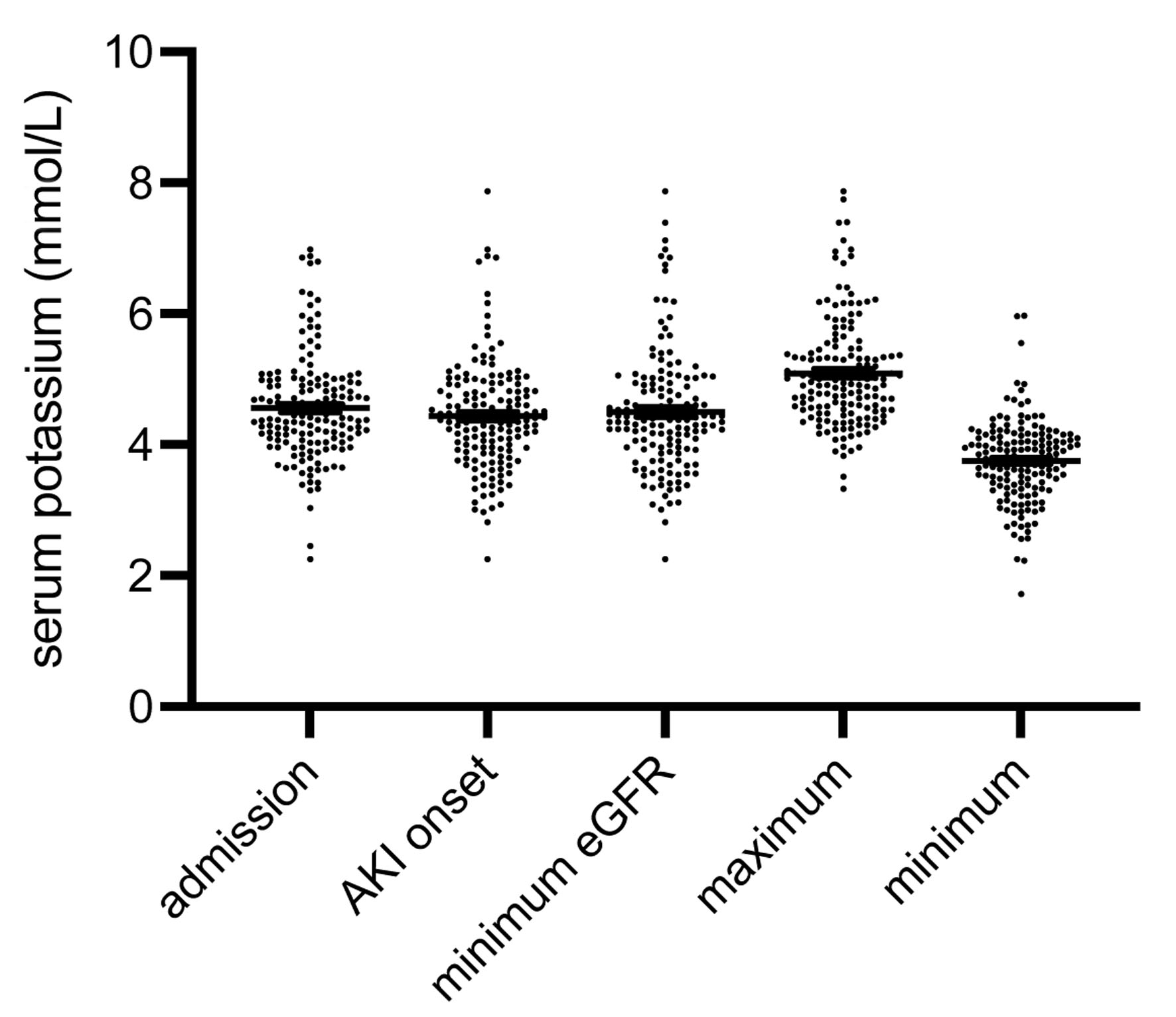 Click for large image | Figure 4. Distribution of serum potassium at the five time points (Fig. 2). Comparable to serum sodium, the respective medians were not equal (P < 0.001). The numbers of all outliers per group were: admission 5, AKI onset 1, minimum eGFR 2, maximum 2, minimum 3 (data are shown as median ± IQR). AKI: acute kidney injury; eGFR: estimated glomerular filtration rate; IQR: interquartile range. |
Analysis of confounding variables
Regarding the endpoint in-hospital death, non-surviving individuals met the diagnostic criteria for more severe AKI significantly more often (AKI stages I/II/III (n and %) in survivors vs. non-survivors: 58.5/22.8/18.7 vs. 32.4/16.2/51.4; P < 0.001). The same observation applied for the need for KRT (AKI stages I II/III (%) in KRT vs. no KRT: 11.8/11.8/76.4 vs. 57.3/22.4/20.3; P < 0.001). Subjects requiring KRT were also treated for longer periods at the hospital (25.3 ± 3.4 vs. 14.9 ± 1 days; P < 0.001). In addition, the prevalence of pre-existing CKD was higher in dialyzed patients (76.5% vs. 46.5%; P = 0.019). Regarding the third endpoint, subjects without recovery of kidney function were older (82 ± 1.2 vs. 75.6 ± 1.6 years; P = 0.007). Non-recovering patients underwent shorter in-hospital treatment periods (9.7 ± 1.1 vs. 18.7 ± 1.9 days; P < 0.001) and suffered more often from hypertension (95.2% vs. 81.5%; P = 0.04) (Table 2).
 Click to view | Table 2. Analysis of Confounding Factors Regarding the Endpoints In-Hospital Death, KRT and Recovery of Kidney Function |
| Discussion | ▴Top |
Reliable AKI outcome predictors are yet to be found. In general, they are needed without doubt. Most biomarker-related studies focused (and still focus) on markers for diagnostic purposes. Prognostic parameters however are limited. A study published in 2012 by Nickolas et al [7] investigated the predictive ability of urinary NGAL, KIM-1, liver-fatty acid binding protein (L-FABP), IL-18, and cystatin C. Over 1,600 individuals were included, whose urine samples were collected and analyzed at the time of emergency department admission. Two markers, NGAL and KIM-1, predicted a higher risk for dialysis/in-hospital death. Another more recent study was published by Sun and colleagues [14]. The authors performed LC-MS analyses in critically ill patients, recruited from “Veteran’s Affairs/National Institutes of Health Acute Renal Failure Trial Network study”. The term LC-MS stands for “liquid chromatography coupled to mass spectrometry” [15]. It allows the detection of non-volatile and complex molecules. Blood samples were collected at days 1 and 8 after admission. The study revealed models of mortality prediction in AKI patients that required KRT, based on differences between certain serum metabolites at both days. LC-MS procedures have however not been established for clinical routine use on a daily basis yet. Xia and colleagues [16] finally analyzed the predictive role of the fibrinogen level to the albumin level ratio (FAR) in more than 5,000 critically ill AKI subjects. The FAR had been evaluated in other diseases also (e.g., malignant disorders [17-19], myocardial infarction [20]). The data were extracted from the “Multiparameter Intelligent Monitoring in Intensive Care Database III”. Higher FARs were in fact predictive for mortality. The findings showed that all FARs were employed from the time of intensive care unit (ICU) admission. Under clinical circumstances, both proteins, fibrinogen and albumin are measured quite often, particularly in ICU-treated subjects. They are usually needed for certain clinical decisions (e.g., coagulable state in sepsis, assessment of nutritional situation and others). A recently published study from our group [21] included 151 subjects with de novo AKI according to KDIGO [2]. Almost 17% of the patients died during in-hospital treatment, and 40% (39.7%) required KRT. Two parameters were quantified, serum IL-33 and its circulating receptor isoform sST2. The latter allowed to discriminate surviving individuals from non-survivors (higher in non-survivors); in addition, the receptor was increased in patients that required transient ICU therapy/ventilatory treatment/vasopressor infusion. In summary, sST2 was identified as a potential survival predictor in AKI. It also needs to be mentioned that “classical” surrogate parameters such as proteinuria/albuminuria have been identified as AKI risk factors in the past. In a larger cohort study from 2015 [22] including more than 1.2 million patients, both diminished eGFR and increased albumin-to-creatinine-ratio were associated with risk of AKI. Similar results were reported in 2018 and 2022 [23, 24]. Pre-operative dipstick albuminuria was identified as an AKI risk factor, respectively. The study from 2018, published by Park and colleagues [23], also showed an association between albuminuria and the risk of death during the following year.
Serum electrolytes such as sodium and potassium on the other hand are available in almost every in- and out-clinic patient and can be immediately determined by blood gas analysis. Our study particularly aimed to evaluate the predictive role of serum sodium in AKI.
Interestingly, not only patients with established hypernatremia were more likely to die within the hospital, but even patients with a sodium value within the normal range but ≥ 139 mmol/L were also at significantly higher risk for death. Adverse outcomes in hypernatremic patients have already been documented in the past. For instance, Peres et al [25] identified the electrolyte disturbance as an independent risk factor for ICU mortality. In a more recent study, hypernatremia predicted AKI but not death in septic individuals [26]. Finally, Woitik and colleagues showed both hyper- and hyponatremia as independent risk factors for mortality in AKI [8]. Whether hypernatremia worsens clinical outcome variables in a mechanistical manner or not is speculative. In most cases, hypernatremia occurs secondary to the depletion of free water but less frequently to the accumulation of water and sodium combined [27]. Thus, hypernatremia reflects hypovolemia and inadequate systemic perfusion. On the other hand, our analysis also showed higher serum sodium at AKI onset to be predictive for recovery of kidney function. These discrepant findings are even harder to explain. Nevertheless, the study was retrospective in nature, which at the most allows to identify associations rather than to provide pathophysiological explanations. Correctly predicted deaths in the logistic regression were only 9% for serum sodium at AKI diagnosis; however, 98% of survivals were correctly predicted.
Limitations
The ROC AUC exceeds 0.5 (0.62) which underlines its prognostic values although not being considered a good model with an AUC < 0.7. The reason is most likely the relatively small sample size and especially the small size of the in-hospital death group. Another limitation is the exclusive follow-up observation within the hospital. Data on mortality after discharge were not available. It also needs to be mentioned that the diagnosis of AKI did not consider urine output (KDIGO criterion 3 [2]). Therefore, some patients may have been missed. Finally, the retrospective design is a disadvantage, although the data in whole justify a prospective follow-up analysis without doubt.
We however must conclude that serum sodium, measured at the time of AKI diagnosis (AKI onset), provides prognostic information. These are not only related to the risk of death but also to the chance of kidney recovery. Larger cohorts must be studied, and future designs should be prospective.
Conclusions
Serum electrolytes, measured during the treatment course of AKI subjects, provide substantial prognostic information.
For instance, serum sodium, measured at the time of AKI diagnosis (AKI onset), is associated with a higher risk of death during follow-up. Serum sodium also allows to distinguish subjects with a higher chance of kidney recovery post-AKI.
At various time points, serum potassium is associated with higher risk of dialysis and death during follow-up.
Thus, certain serum electrolytes are potentially useful in AKI risk prediction of hospitalized patients.
Larger cohorts must be studied and future investigations should of course be designed in a prospective manner.
Acknowledgments
None to declare.
Financial Disclosure
Funded by the Brandenburg Medical School (Medizinische Hochschule Brandenburg, MHB) publication fund supported by the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG).
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
It was not required to obtain written consent due to the retrospective nature of the study. This decision was made by the Ethics Committee of the Brandenburg Medical School (No.: E-01-20210510).
Author Contributions
BM performed extended data analysis and wrote the article. LD collected all data. RL helped in data analysis and assisted in article writing. IM prepared figures and assisted in writing. SP prepared figures and helped in data analysis. DP designed the study and assisted in writing. All authors approved the final version of the article.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Bienholz A, Wilde B, Kribben A. From the nephrologist's point of view: diversity of causes and clinical features of acute kidney injury. Clin Kidney J. 2015;8(4):405-414.
doi pubmed - Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179-184.
doi pubmed - Schrezenmeier EV, Barasch J, Budde K, Westhoff T, Schmidt-Ott KM. Biomarkers in acute kidney injury - pathophysiological basis and clinical performance. Acta Physiol (Oxf). 2017;219(3):554-572.
doi pubmed - Koyner JL, Garg AX, Coca SG, Sint K, Thiessen-Philbrook H, Patel UD, Shlipak MG, et al. Biomarkers predict progression of acute kidney injury after cardiac surgery. J Am Soc Nephrol. 2012;23(5):905-914.
doi pubmed - Caironi P, Latini R, Struck J, Hartmann O, Bergmann A, Bellato V, Ferraris S, et al. Circulating Proenkephalin, Acute Kidney Injury, and Its Improvement in Patients with Severe Sepsis or Shock. Clin Chem. 2018;64(9):1361-1369.
doi pubmed - Singer E, Elger A, Elitok S, Kettritz R, Nickolas TL, Barasch J, Luft FC, et al. Urinary neutrophil gelatinase-associated lipocalin distinguishes pre-renal from intrinsic renal failure and predicts outcomes. Kidney Int. 2011;80(4):405-414.
doi pubmed - Nickolas TL, Schmidt-Ott KM, Canetta P, Forster C, Singer E, Sise M, Elger A, et al. Diagnostic and prognostic stratification in the emergency department using urinary biomarkers of nephron damage: a multicenter prospective cohort study. J Am Coll Cardiol. 2012;59(3):246-255.
doi pubmed - Woitok BK, Funk GC, Walter P, Schwarz C, Ravioli S, Lindner G. Dysnatremias in emergency patients with acute kidney injury: A cross-sectional analysis. Am J Emerg Med. 2020;38(12):2602-2606.
doi pubmed - Ravioli S, Pluess E, Funk GC, Walter P, Schwarz C, Exadaktylos AK, Woitok BK, et al. Dyskalemias in patients with acute kidney injury presenting to the emergency department are common and independent predictors of adverse outcome. Int J Clin Pract. 2021;75(1):e13653.
doi pubmed - Gao XP, Zheng CF, Liao MQ, He H, Liu YH, Jing CX, Zeng FF, et al. Admission serum sodium and potassium levels predict survival among critically ill patients with acute kidney injury: a cohort study. BMC Nephrol. 2019;20(1):311.
doi pubmed - Li Q, Wang Y, Mao Z, Kang H, Zhou F. Serum sodium levels predict mortality in elderly acute kidney injury patients: a retrospective observational study. Int J Gen Med. 2021;14:603-612.
doi pubmed - Michels WM, Grootendorst DC, Verduijn M, Elliott EG, Dekker FW, Krediet RT. Performance of the Cockcroft-Gault, MDRD, and new CKD-EPI formulas in relation to GFR, age, and body size. Clin J Am Soc Nephrol. 2010;5(6):1003-1009.
doi pubmed - Bagshaw SM, George C, Bellomo R, Committe ADM. A comparison of the RIFLE and AKIN criteria for acute kidney injury in critically ill patients. Nephrol Dial Transplant. 2008;23(5):1569-1574.
doi pubmed - Sun J, Cao Z, Schnackenberg L, Pence L, Yu LR, Choudhury D, Palevsky PM, et al. Serum metabolite profiles predict outcomes in critically ill patients receiving renal replacement therapy. J Chromatogr B Analyt Technol Biomed Life Sci. 2021;1187:123024.
doi pubmed - Theodoridis G, Gika HG, Wilson ID. Mass spectrometry-based holistic analytical approaches for metabolite profiling in systems biology studies. Mass Spectrom Rev. 2011;30(5):884-906.
doi pubmed - Xia W, Li C, Yao X, Chen Y, Zhang Y, Hu H. Prognostic value of fibrinogen to albumin ratios among critically ill patients with acute kidney injury. Intern Emerg Med. 2022;17(4):1023-1031.
doi pubmed - Zhang J, Ding Y, Wang W, Lu Y, Wang H, Wang H, Teng L. Combining the fibrinogen/albumin ratio and systemic inflammation response index predicts survival in resectable gastric cancer. Gastroenterol Res Pract. 2020;2020:3207345.
doi pubmed - Zheng Y, Wu C, Yan H, Chen S. Prognostic value of combined preoperative fibrinogen-albumin ratio and platelet-lymphocyte ratio score in patients with breast cancer: A prognostic nomogram study. Clin Chim Acta. 2020;506:110-121.
doi pubmed - Xu Q, Yan Y, Gu S, Mao K, Zhang J, Huang P, Zhou Z, et al. A Novel Inflammation-based prognostic score: the fibrinogen/albumin ratio predicts prognoses of patients after curative resection for hepatocellular carcinoma. J Immunol Res. 2018;2018:4925498.
doi pubmed - Xiao L, Jia Y, Wang X, Huang H. The impact of preoperative fibrinogen-albumin ratio on mortality in patients with acute ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Clin Chim Acta. 2019;493:8-13.
doi pubmed - Erfurt S, Hoffmeister M, Oess S, Asmus K, Patschan S, Ritter O, Patschan D. Soluble IL-33 receptor predicts survival in acute kidney injury. J Circ Biomark. 2022;11:28-35.
doi pubmed - Grams ME, Sang Y, Ballew SH, Gansevoort RT, Kimm H, Kovesdy CP, Naimark D, et al. A meta-analysis of the association of estimated GFR, albuminuria, age, race, and sex with acute kidney injury. Am J Kidney Dis. 2015;66(4):591-601.
doi pubmed - Park S, Lee S, Lee A, Paek JH, Chin HJ, Na KY, Chae DW, et al. Preoperative dipstick albuminuria and other urine abnormalities predict acute kidney injury and patient outcomes. Surgery. 2018;163(5):1178-1185.
doi pubmed - Yang JN, Li Z, Wang ML, Li XY, Li SL, Li N. Preoperative dipstick albuminuria is associated with acute kidney injury in high-risk patients following non-cardiac surgery: a single-center prospective cohort study. J Anesth. 2022;36(6):747-756.
doi pubmed - Peres LA, Wandeur V, Matsuo T. Predictors of acute kidney injury and mortality in an Intensive Care Unit. J Bras Nefrol. 2015;37(1):38-46.
doi pubmed - Zhi D, Lin J, Dong L, Ji X, Zhuang H, Liu Z, Liu J, et al. Risk predictive role of hypernatremia for occurrence of sepsis-induced acute kidney injury. Ann Palliat Med. 2021;10(4):4705-4715.
doi pubmed - Adrogue HJ, Madias NE. Hypernatremia. N Engl J Med. 2000;342(20):1493-1499.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


