| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Original Article
Volume 15, Number 1, January 2023, pages 31-37
Rapid Cytological Diagnosis With Evaluation of Pre- and Post-Therapeutic Fungal Morphological Characteristics in Mucormycosis
Feroz Alama, d , Bushra Siddiquia, Naba Hasana, S.H. Arifa, Veena Maheshwaria, Kiran Alama, Mahboob Hasana, Roobina Khana, Parvez Anwer Khanb, Aftab Ahmedc, Surabhi Gautama
aDepartment of Pathology, Jawaharlal Nehru Medical College, Aligarh Muslim University, Aligarh, India
bDepartment of Microbiology, Jawaharlal Nehru Medical College, Aligarh Muslim University, Aligarh, India
cDepartment of Otorhinolaryngology, Jawaharlal Nehru Medical College, Aligarh Muslim University, Aligarh, India
dCorresponding Author: Feroz Alam, Department of Pathology, Jawaharlal Nehru Medical College, Aligarh Muslim University, Aligarh 202002, India
Manuscript submitted October 20, 2022, accepted November 23, 2022, published online January 24, 2023
Short title: Crush Smears in Mucormycosis
doi: https://doi.org/10.14740/jocmr4835
| Abstract | ▴Top |
Background: Mucormycosis necessitates rapid diagnosis and treatment. Microscopy and culture have been considered the gold standard for diagnosis but both take time of 3 - 5 days. KOH mount is another method for fungal identification that takes 1 - 2 h, but it has its own limitations. This study evaluated crush smear as a means of rapid cytological diagnosis.
Methods: Biopsy tissue (pre-treatment) from clinically suspicious mucormycosis patients (n = 52) was received in normal saline and crush/imprint smears were prepared; the remaining tissue was processed as routine biopsy specimen. After the rapid initial cytological identification, the patients were managed according to the standard clinical protocol. Random post-therapeutic biopsy samples of some of these patients (n = 19) were also obtained and again evaluated cytologically.
Results: Crush smears showed sensitivity/specificity of 77.7%/75.0% with histopathology and 72.2%/62.5% with culture, respectively, while KOH mount had values of 71.4%/70.5% with histopathology and 79.3%/69.5% with culture, respectively. Degenerative fungal morphological characteristics and cellular inflammatory infiltrate (predominantly neutrophilic) in the vicinity of fungal hyphae were compared in pre- and post-treatment groups, and we found a statistically significant difference (P < 0.05) between them.
Conclusion: Our preliminary results suggest that crush smear cytology is a simple, rapid, cost-effective and easily available method for diagnosing mucormycosis. Moreover, crush smears also demonstrated morphological alteration in hyphal structure and accompanying immune cell infiltration which may provide valuable insights into mechanism of therapy/host immune response against fungal pathogen.
Keywords: Crush smear; Fungal cytology; Culture; Histopathology; Mucormycosis; Neutrophils
| Introduction | ▴Top |
Second wave of COVID-19 pandemic in India was associated with an upsurge in the cases of an otherwise rare fungal infection called mucormycosis [1]. It is an angioinvasive disease caused by fungi belonging to the order mucorales of class zygomycetes [2]. The most common mode of infection is through inhalation of sporangiospores and could also occur through direct inoculation from disrupted skin or mucosal surfaces [3]. The fungus is highly vasotropic in nature causing tissue infarctions and becomes rapidly disseminated specially in immuno-compromised patients (diabetics, glucocorticoid therapy, patients with malignancy receiving chemotherapy, allogenic stem cell transplant recipients) [4]. The spectrum of infection ranges from rhino-orbito-cerebral (most common), sino-pulmonary, cutaneous, cerebral, gastrointestinal to even disseminated [5]. The disease requires prompt diagnosis and initiation of antifungal therapy. Microscopy (histopathology) and microbiological culture have been considered the gold standard for diagnosis [6]. Minimum turn-around time for tissue-based diagnosis by histopathology is around 3 - 5 days and culture also takes 3 - 5 days to yield results. Paradoxically only 50% cases with positive microscopy are culture positive owing to highly fragile nature of the fungus which may disintegrate during tissue manipulation [7]. Cheap and easily available corrosive alkali potassium hydroxide (KOH) is also used for identification of fungal hyphae from easily accessible superficial lesions. Treatment with KOH softens, digests, and removes cellular and keratin debris but leaves an intact hyphal cell wall allowing for visualization under a microscope. Preparation of mounts (KOH mount) is a rather quick and sensitive method of providing a preliminary diagnosis but has its own limitations [8]. Recently DNA-based molecular methods like polymerase chain reaction (PCR) have come up but they are very costly, not routinely available and moreover it also does not differentiate between dead and alive infective agent [9]. This study evaluated the role of crush smears prepared from biopsy material, as a means of rapid cytological diagnosis in clinically suspected cases of mucormycosis. Subsequently a comparative analysis of the results of crush smears and KOH mounts was done by comparing them with the gold standard methods of diagnosis (histopathology and culture).
After early diagnosis, the second cornerstone of successful management is therapy monitoring, especially important in case of critically ill patients for deciding the appropriate timing and need for surgical debridement. Therapeutic drug monitoring (TDM) is used to monitor the blood levels of azole anti-fungals but not routinely recommended for polyenes (e.g., amphotericin B), which are the drugs of choice for treatment of mucormycosis [10]. In our study, during the course of treatment of diagnosed cases, some biopsy samples (n = 19) were again received which were also examined by crush smear examination. These post-therapeutic samples revealed subtle morphological changes in the fungal hyphae and accompanying immune cell infiltration when compared with earlier pre-therapeutic findings.
| Materials and Methods | ▴Top |
All the patients presenting to the fever clinic/oto-rhino-laryngology OPD from May 2021 till October 2021 with complaints suspicious of a clinical diagnosis of mucormycosis like facial pain and swelling, nasal obstruction, nasal discharge, headache, eye pain, swelling, etc. were included in the study. Pre-treatment biopsy tissue from the nasal black eschar was received in normal saline and crush/imprint smears were prepared for rapid cytological evaluation. It is a simple method wherein a small piece of tissue is gently crushed between two slides, and the resulting smear is fixed and stained with routine stains like hematoxylin and eosin (H&E), Papanicolaou (PAP) and periodic acid-Schiff (PAS) as per our lab protocol.
The processing of the biopsy tissue for histopathological examination was done according to the routine lab protocol wherein after overnight fixation tissue pieces were processed in Yorko automated tissue processor for a 24-h cycle. This comprised treating the tissue with increasing concentrations of ethyl alcohol, i.e., 70%, 80% and 95% for 1 h each. After adequate dehydration, the tissue was given two changes of 1 h each in absolute alcohol followed by copper alcohol. Then the tissue was treated with aniline for 6 h followed by two changes of 1 h each in xylene, then two changes of 1.25 h each in paraffin wax. Processed tissue was transferred from final wax bath to rectangular moulds filled with molten paraffin wax to form blocks. Once solidified long ribbons of thin sections (4 - 5 µm in thickness) were cut using a Rotary Microtome (LEICA RM 2125 RT) and immediately floated in water bath at 60 °C. The sections were mounted onto clean glass slides and stained with H&E and PAS stain as per lab protocol. The sample for culture and KOH mount included scraping from necrotic lesions that were received in the Department of Microbiology. For preparation of KOH mounts, a part of the specimen was placed on a clean glass slide and one drop of 20% potassium hydroxide was added. A cover slip was placed and gently pressed to get rid of any air bubbles. Slide was then examined at low illumination. For microbiological culture, clinical specimen was inoculated onto Sabouraud dextrose agar and incubated at 37 °C. If positive for mucorales, the growth became evident in 3 - 5 days. The colonies demonstrated varying coloration from white to tan brown or black.
After the initial diagnosis, the patients were managed according to the clinical protocol which included antifungal therapy and surgical debridement of involved tissue. Intravenous lipid formulation of amphotericin B was the drug of choice for initial therapy. Posaconazole and isavuconazole were also used as step down therapy after adequate response was achieved [11]. Some post-therapeutic biopsy samples were also received after 5 - 7 days of antifungal therapy. These samples were also evaluated cytologically by preparing crush smears. All the afore-mentioned methods were carried out in accordance with relevant standard guidelines and regulations. The study was carried according to the Declaration of Helsinki. All the experimental (cytopathological) protocols were approved by the J.N. Medical College Institutional Committee.
| Results | ▴Top |
A total of 52 pre-therapeutic crush smears prepared from biopsy sample of patients with suspected mucormycosis were evaluated. Patients presented over a wide age range (21 - 80 years) and both young and old were affected. The most common presenting complaint was facial pain and swelling seen in 37 patients (71.1%), followed by eye pain and swelling seen in 13 patients (25%). Other minor complaints included diminution of vision, headache, dental problems (tooth loosening and pain), nasal obstruction and discharge. Diabetes was the most common co-morbid condition present in 35 patients (67.3%) followed by hypertension in 11 patients (21.1%), and one case was associated with hypothyroidism (1.9%). A recent history of indiscriminate steroid use was present in 38 patients (73.0%). Forty-one out of 52 patients (79%) had positive RT-PCR for COVID-19 on presentation, seven patients (14%) however, had a negative RT-PCR but gave history of fever/sore throat/anosmia/weakness in recent past putting them in the category of suspected COVID-19. Four patients (7%) did not have an RT-PCR done at the time of presentation. Twenty-nine out of 52 (55.7%) samples were positive for fungal infection on crush smear, 20 cases (38.4%) did not show any fungal elements on crush smear and were reported as negative for fungal infection. Three samples (5.7%) showed mostly necrotic tissue debris along with some highly degenerated small ribbon like fragments with loss of morphological details and hence were reported as suspicious for a fungal infection on crush cytology. The fungi were identified on crush smear according to their morphological characteristics and the most common fungus reported was mucor (21/29, 72.4%), followed by aspergillus and mucor co-infection (5/29, 17.2%) and candida plus mucor co-infection (3/29, 10.3%). Thirty out of 52 (57.6%) samples showed fungal hyphae on KOH mount, while 22/52 (42.3%) did not show any fungal hyphae. Upon subsequent histopathological evaluation of the biopsy specimens, 40/52 (76.9%) cases were found to be positive for fungal infection and 12/52 (23%) did not show any fungal hyphae even after extensive search. The results of microbiological culture showed fungal growth in 36/52 (69.2%) cases, while 16/52 cases were culture negative (Table 1). Comparison of sensitivity, specificity, positive predictive value and negative predictive value of crush cytology and KOH mount with histopathology and culture is shown in Table 2.
 Click to view | Table 1. Comparison of Crush Smear and KOH Mount Diagnosis With Histopathology and Culture |
 Click to view | Table 2. Comparison of Sensitivity, Specificity, Positive Predictive Value and Negative Predictive Value of Crush Smears and KOH Mount With Histopathology and Culture |
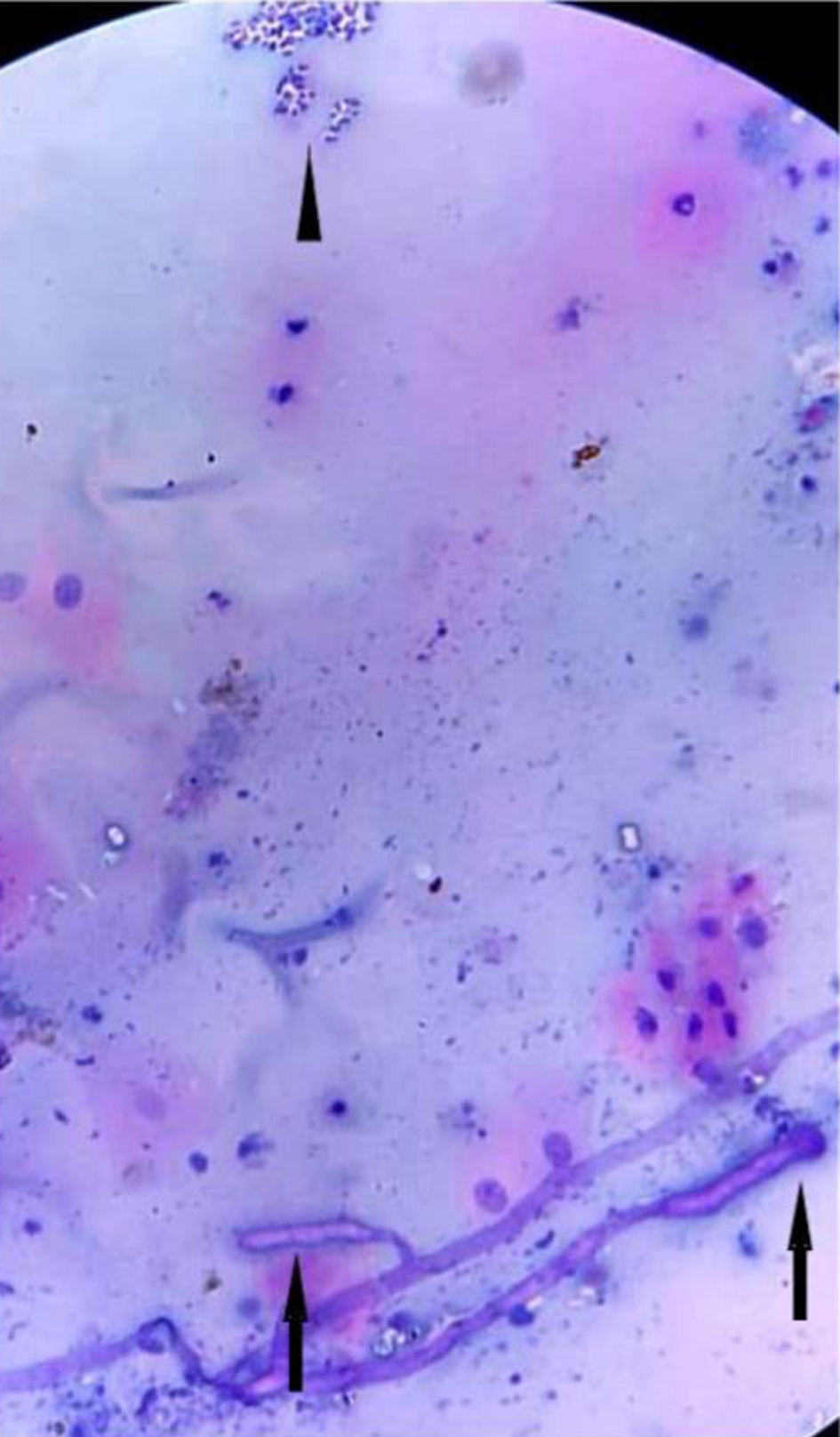
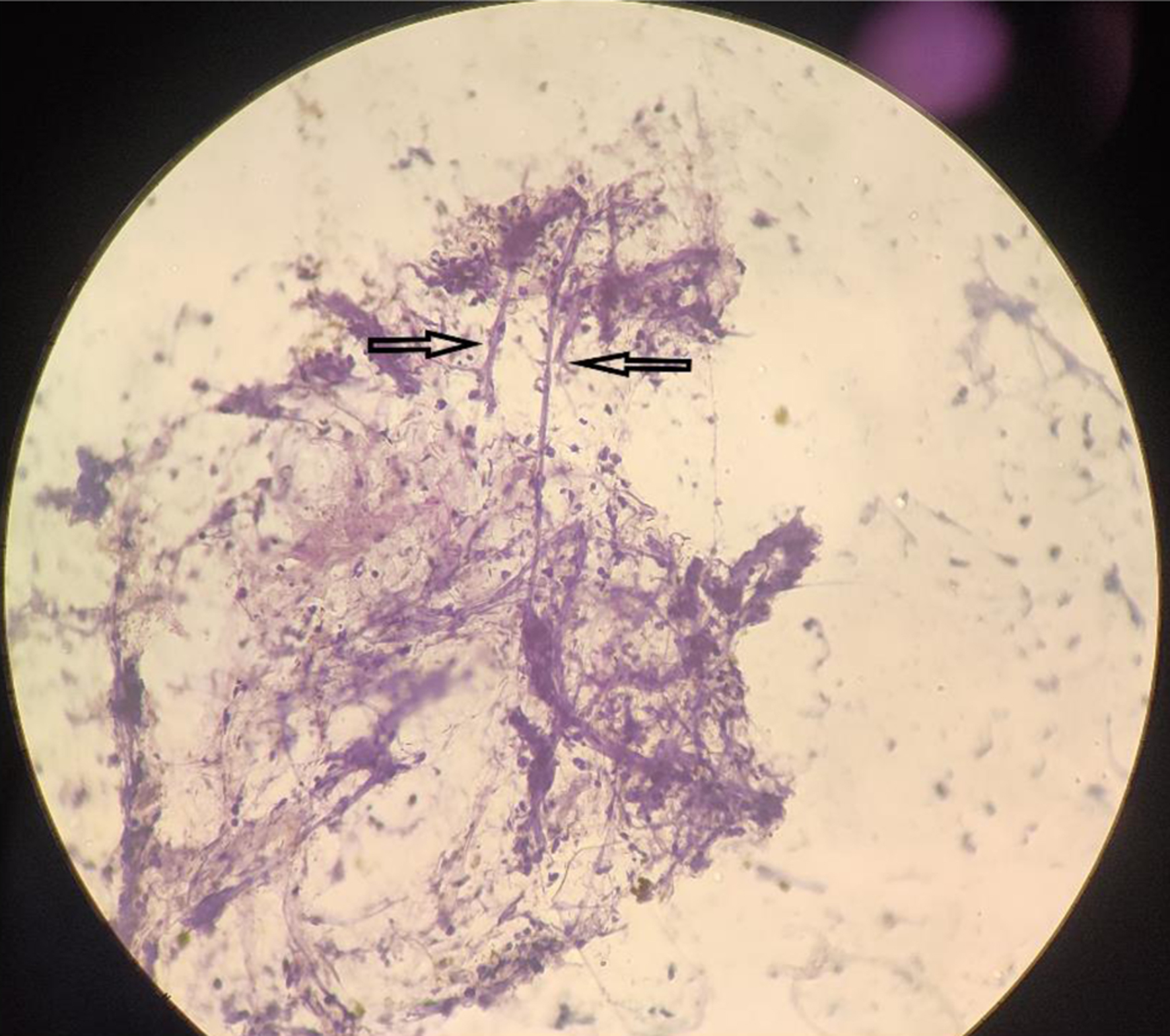
Twenty-six out of 29 (89.6%) positive pre-therapeutic samples showed broad proliferating fungal hyphae with branching and 7/29 (24.1%) cases even showed intact fruiting bodies (sporangia and spores) (Figs. 1, 2). An absence of inflammatory cells was also noted in the vicinity of such proliferating hyphae. Nineteen post-therapeutic samples from mucor-positive patients were also received after 5 - 7 days of antifungal therapy. Thirteen out of 19 samples (68.4%) showed degenerated, broken hyphae with loss of morphological details along with attack on such degenerated hyphae by inflammatory cells (Figs. 3, 4). The inflammatory infiltrate predominantly comprised of neutrophils along with some lymphocytes. Three cases also showed presence of giant cells in addition to the inflammatory cells (Fig. 5). Three out of 19 (15.7%) post-therapeutic samples did not reveal any fungal hyphae after thorough search on crush smears and 3/19 cases (15.7%) showed some degenerated and highly fragmented hyphae like structures with loss of morphological details but absence of any inflammatory cells. A comparison of cytological features of pre- and post-therapeutic samples on crush smear is shown in Table 3.
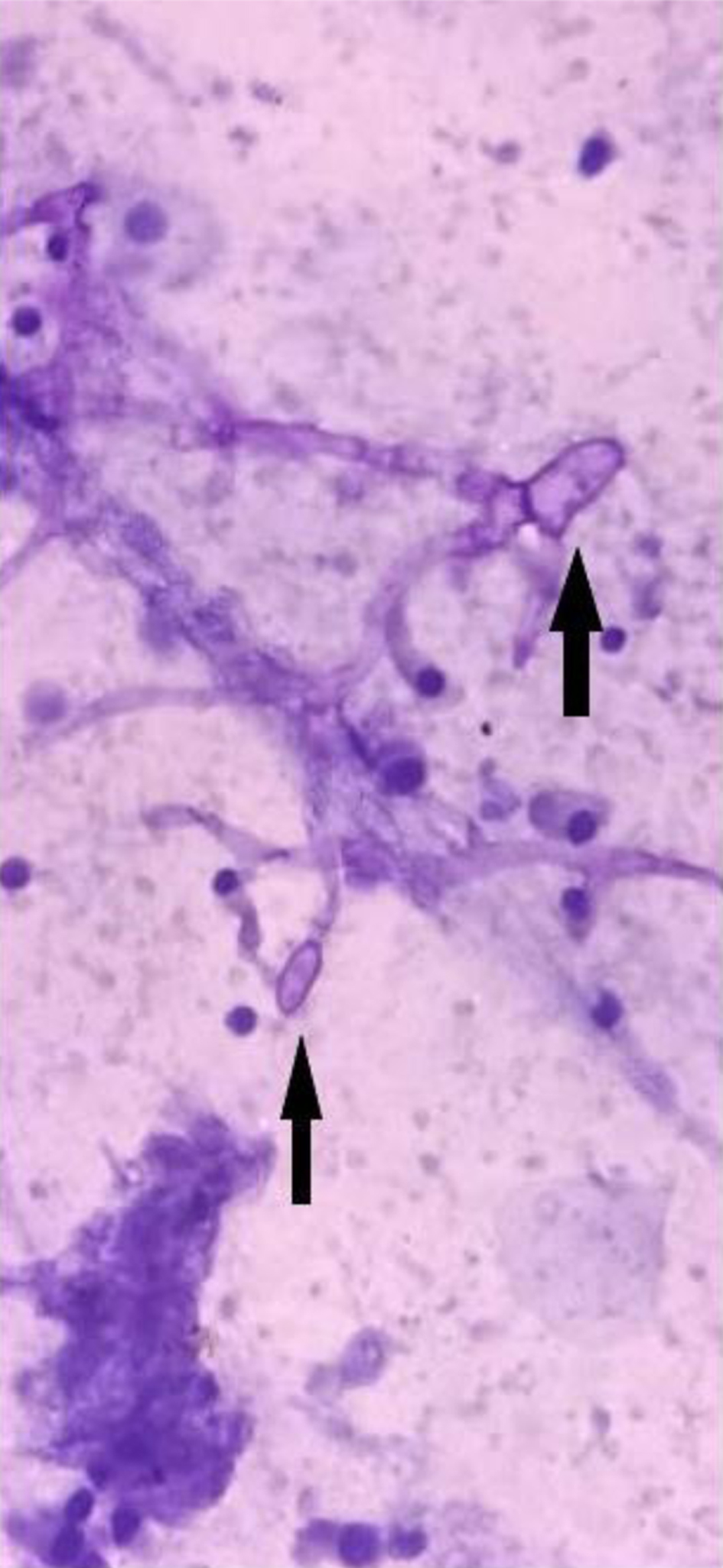 Click for large image | Figure 1. Pre-treatment sample showing viable branching hyphae with sporangia and few degenerated columnar cells (PAP, × 40). PAP: Papanicolaou. |
 Click for large image | Figure 2. Pre-treatment sample showing viable hyphae with sporangia, absence of inflammatory cells in vicinity and mature superficial squamous cells (H&E, × 40). H&E: hematoxylin and eosin. |
 Click for large image | Figure 3. Post-treatment sample showing a degenerated hyphae being attacked by neutrophils (H&E, × 40). H&E: hematoxylin and eosin. |
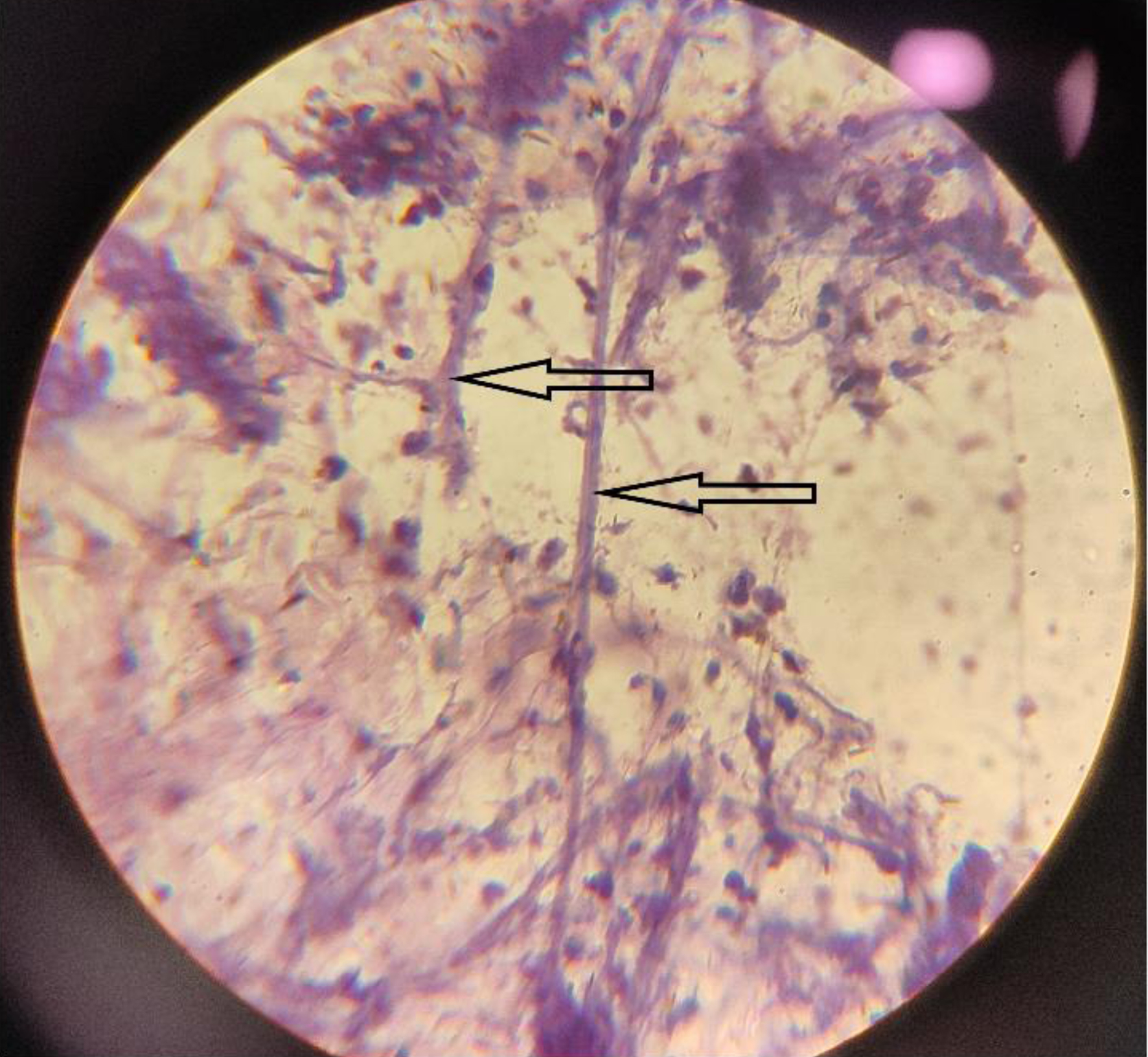 Click for large image | Figure 4. Post-treatment sample showing entangled degenerated hyphae being attacked by inflammatory cells (H&E, × 40). H&E: hematoxylin and eosin. |
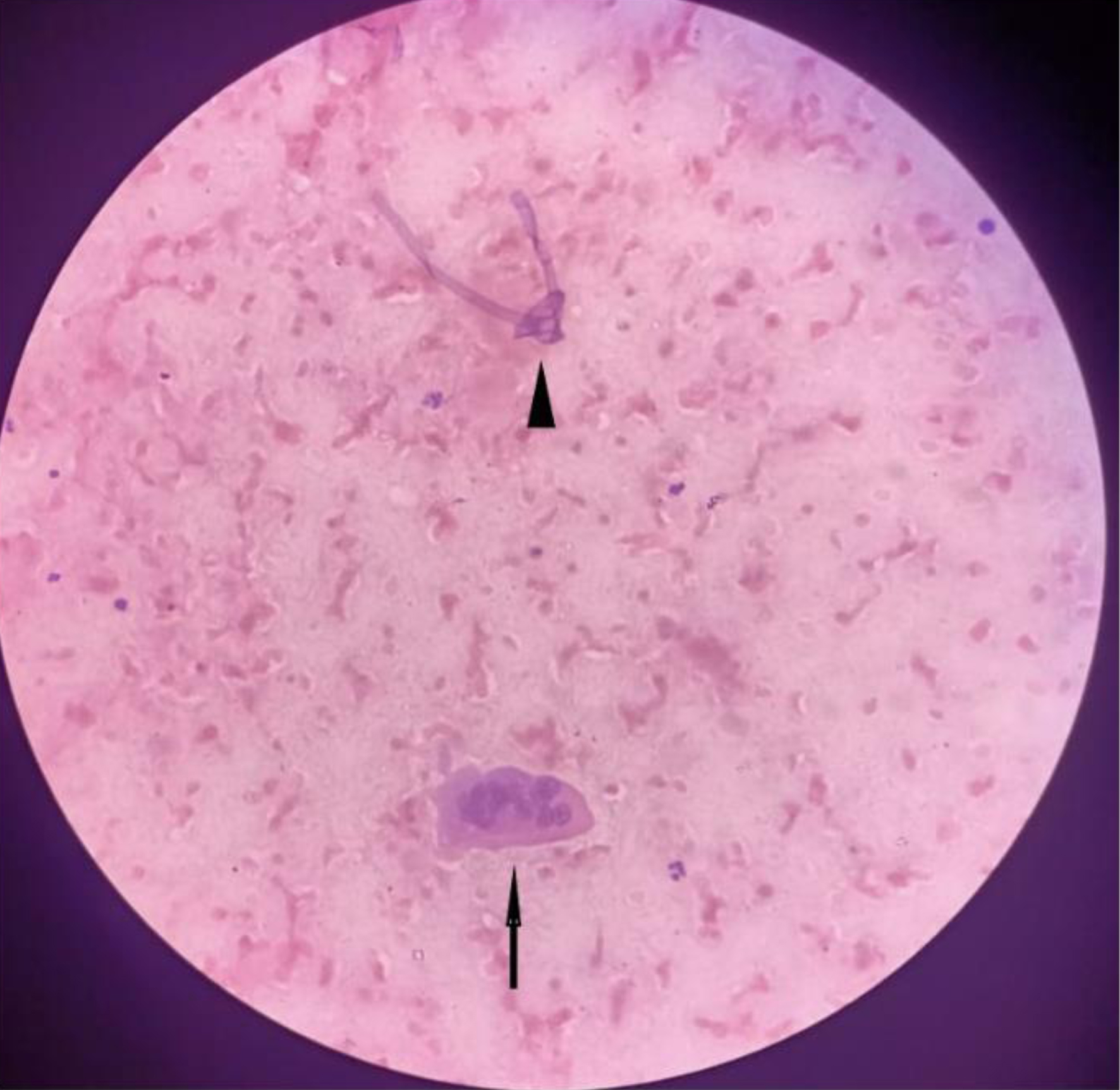 Click for large image | Figure 5. Post-treatment sample showing degenerated hyphal fragment along with a multi-nucleated giant cell (H&E, × 40). H&E: hematoxylin and eosin. |
 Click to view | Table 3. Comparison of Pre- and Post-Therapeutic Fungal Morphological Characteristics on Crush Smears |
The different microscopic findings on crush smear of pre- and post-therapeutic samples were statistically compared by applying the McNemar Chi-square test. The presence of viable hyphae and fruiting bodies in pre-therapeutic samples when compared to that of post-therapeutic samples was found to be highly significant with a P value of < 0.0001 and < 0.004, respectively. Similarly, the frequent presence of degenerated hyphae and inflammatory cells in the vicinity of degenerated hyphae in post-therapeutic samples as compared to pre-therapeutic samples was also found to be significant with a P value of 0.0003. However, the presence of giant cells in the two groups was not found to be significant with a P value of 0.083 (Table 3).
| Discussion | ▴Top |
Mucormycosis is an angio-invasive fungal disease which could rapidly become disseminated and life-threatening. The mainstay of management is an early diagnosis with prompt initiation of antifungal therapy. Crush smear preparation is a rapid and cost-effective method of diagnosis with a turn-around time of just 1 - 2 h. The technique is simple and does not require much of logistics. On comparison with the gold standard histopathological method of diagnosis, the specificity and positive predictive value of crush smear in diagnosing mucormycosis were 75% and 87.5% respectively in this study; however, the sensitivity rested at 77.7% and a negative predictive value of 60%. Similarly, compared with the alternative method of culture for diagnosis, the specificity and positive predictive value of crush smear were 62.5% and 81.2% respectively, sensitivity was found to be 72.2% and the negative predictive value came out to be 50%. It has been shown earlier that histopathological examination of biopsy material is a better method to diagnose mucormycosis than culture as under normal laboratory conditions, sporulation fails and culture results are often negative due to unviable organism in necrotic tissues [12]. On comparison with either histopathology or culture, the positive predictive value of crush smears being > 80% emphasizes the importance of it being used in such emergency situations.
Examination of wet KOH mounts is another direct microscopic method of diagnosis with a turn-around time similar to crush smear, i.e., 1 - 2 h. But KOH mount being an unstained preparation, the fungal morphology is not clear making it difficult to type the fungus. Various unstained artefacts can also give a false impression of being elongated fungal hyphae [13]. Degeneration of the cell wall of accompanying leukocytes and epithelial cells takes place in KOH mounts preventing documentation of host immune response against the fungal infection [14]. Finally, these wet mounts cannot be archived also. The sensitivity (71.4%), specificity (70.5%), positive predictive value (83.3%) and negative predictive value (54.5%) of KOH mount were all found to be lower than crush smear when histopathology was taken as gold standard. However, sensitivity (79.3%), specificity (69.5%) and negative predictive value (72.7%) were better, but positive predictive value (76.6%) was again found to be lower than crush smear when culture was taken as the gold standard for diagnosis.
Mucormycosis in addition to the then on-going COVID-19 pandemic posed an additional burden on an already overwhelmed health care system. Hence, it became necessary to monitor patients who may require an early aggressive surgical intervention. In this regard also, crush smears could be useful as we found changes in fungal morphology and immune cell infiltration pattern in post-therapeutic samples. The pre-therapeutic samples showed mostly viable proliferating fungal hyphae with intact fruiting bodies and paucity/absence of inflammatory cells (neutrophils) in the vicinity of the viable fungal hyphae. This sparse inflammatory response could be an in vivo reflection of the process of fungal biofilm formation [15]. The post-therapeutic samples on the other hand showed only degenerated hyphae with loss of morphological details and attack by inflammatory cells. It has been shown that amphotericin B at high concentrations is the only anti-fungal agent that is known to inhibit the metabolic activity of the hyphal clumps [16]. The morphological changes brought by amphotericin B on fungal hyphae are abnormal configuration, fragmentation, roughening, shrinkage and fracture [17]. Post-therapeutic samples again had a predominantly neutrophilic infiltrate but this time the neutrophils were seen attacking/lying in proximity to the degenerated fungal hyphae. Few lymphocytes were also appreciated in addition to the neutrophils and three cases also showed the presence of multi-nucleated giant cells. Diabetic patients secondarily affected by mucormycosis had been shown to exhibit an inflammatory infiltrate comprising of neutrophils, multinucleated giant cells and phagocytes [18]. Three post-therapeutic samples showed only degenerated hyphae with absence of inflammatory cells in close proximity to the hyphae which may be because of severe underlying immune-suppression in the patient. Similar findings were seen in immune-compromised cancer patients suffering from mucormycosis, where only 30% of the patients showed an inflammatory infiltrate [19].
Conclusion
Crush smear preparation is a rapid and cost-effective method of diagnosing fungal infection with a turn-around time of 1 - 2 h, and the positive cases showed a high degree of conformity with both gold standard methods of histopathology and culture. Comparison with KOH mount showed that crush smear demonstrates higher positive predictive value thus making it a better diagnostic tool in emergency situations. Degenerating fungal morphology with characteristic proximal immune cell infiltration (predominantly neutrophils + few lymphocytes) on post-therapeutic samples could provide a hint towards the mechanism of therapy/immune response in susceptible patients.
Acknowledgments
The authors are highly thankful to the staff of Cytopathology and Histopathology sections of the Department of Pathology and the Hospital administration, JNMC, AMU, for their efforts during the pandemic.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained from the patients to obtain biopsy tissue and prepare crush smears.
Author Contributions
Feroz Alam: concept, design and data acquisition. Feroz Alam, Bushra Siddiqui, and Naba Hasan: analysis and interpretation of data, drafting of article. Kiran Alam, Roobina Khan, and Surabhi Gautam: critical revision of article. S.H. Arif, Veena Maheshwari, and Mahboob Hasan: administrative and logistic support, final approval. Aftab Ahmed and Parvez Anwer Khan: provision of study material.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Singh AK, Singh R, Joshi SR, Misra A. Mucormycosis in COVID-19: A systematic review of cases reported worldwide and in India. Diabetes Metab Syndr. 2021;15(4):102146.
doi pubmed - Prakash H, Chakrabarti A. Epidemiology of mucormycosis in India. Microorganisms. 2021;9(3):523.
doi pubmed - Radotra B, Challa S. Pathogenesis and pathology of COVID-associated mucormycosis: what is new and why. Curr Fungal Infect Rep. 2022.
doi pubmed - Ibrahim AS, Spellberg B, Walsh TJ, Kontoyiannis DP. Pathogenesis of mucormycosis. Clin Infect Dis. 2012;54 Suppl 1:S16-22.
doi pubmed - Petrikkos G, Skiada A, Lortholary O, Roilides E, Walsh TJ, Kontoyiannis DP. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. 2012;54(Suppl 1):S23-34.
doi pubmed - Lackner N, Posch W, Lass-Florl C. Microbiological and molecular diagnosis of mucormycosis: from old to new. Microorganisms. 2021;9(7):1518.
doi pubmed - Skiada A, Lass-Floerl C, Klimko N, Ibrahim A, Roilides E, Petrikkos G. Challenges in the diagnosis and treatment of mucormycosis. Med Mycol. 2018;56(suppl_1):93-101.
doi pubmed - Gautam M, Bhatia S. Mount the Menace-Potassium Hydroxide in superficial fungal infections. Indian J Pediatr Dermatol. 2020;21:343-346. https://www.ijpd.in/text.asp?2020/21/4/343/296851.
doi - Cangelosi GA, Meschke JS. Dead or alive: molecular assessment of microbial viability. Appl Environ Microbiol. 2014;80(19):5884-5891.
doi pubmed - John J, Loo A, Mazur S, Walsh TJ. Therapeutic drug monitoring of systemic antifungal agents: a pragmatic approach for adult and pediatric patients. Expert Opin Drug Metab Toxicol. 2019;15(11):881-895.
doi pubmed - Cornely OA, Alastruey-Izquierdo A, Arenz D, Chen SCA, Dannaoui E, Hochhegger B, Hoenigl M, et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis. 2019;19(12):e405-e421.
- Badiee P, Arastefar A, Jafarian H. Comparison of histopathological analysis, culture and polymerase chain reaction assays to detect mucormycosis in biopsy and blood specimens. Iran J Microbiol. 2013;5(4):406-410.
- Kurade SM, Amladi SA, Miskeen AK. Skin scraping and a potassium hydroxide mount. Indian J Dermatol Venereol Leprol. 2006;72(3):238-241.
doi pubmed - Arora S, Pal R, Suhag DK, Dabas R, Arora G, Chand S. KOH wet mount versus cellophane strip without mounting medium for rapid diagnosis in superficial mycoses. Indian J Dermatopathol Diagn Dermatol. 2021;8:1-5.
doi - Kernien JF, Snarr BD, Sheppard DC, Nett JE. The interface between fungal biofilms and innate immunity. Front Immunol. 2017;8:1968.
doi pubmed - van de Sande WW, Tavakol M, van Vianen W, Bakker-Woudenberg IA. The effects of antifungal agents to conidial and hyphal forms of Aspergillus fumigatus. Med Mycol. 2010;48(1):48-55.
doi pubmed - Han Y, Zhao J, Zhang B, Shen Q, Shang Q, Li P. Effect of a novel antifungal peptide P852 on cell morphology and membrane permeability of Fusarium oxysporum. Biochim Biophys Acta Biomembr. 2019;1861(2):532-539.
doi pubmed - Montano DE, Voigt K. Host immune defense upon fungal infections with mucorales: pathogen-immune cell interactions as drivers of inflammatory responses. J Fungi (Basel). 2020;6(3):173.
doi pubmed - Ben-Ami R, Luna M, Lewis RE, Walsh TJ, Kontoyiannis DP. A clinicopathological study of pulmonary mucormycosis in cancer patients: extensive angioinvasion but limited inflammatory response. J Infect. 2009;59(2):134-138.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


