| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Case Report
Volume 14, Number 8, August 2022, pages 327-333
Urothelial Carcinoma With Pulmonary Tumor Thrombotic Microangiopathy After Chemotherapy for Hodgkin Lymphoma
Naomi Shimizua, e , Takuro Itob
, Shuji Satob
, Yoshiya Sugiurac
, Ichiro Tatsunod
aDepartment of Hematology, Toho University Sakura Medical Center, Chiba, Japan
bDepartment of Cardiology, Toho University Sakura Medical Center, Chiba, Japan
cDepartment of Surgical Pathology, Toho University Sakura Medical Center, Chiba 2858741, Japan
dCenter for Diabetes, Metabolism and Endocrinology, Toho University Sakura Medical Center, Chiba 2858741, Japan
eCorresponding Author: Naomi Shimizu, Department of Hematology, Toho University Medical Center Sakura Hospital, Sakura City, Chiba 2858741, Japan
Manuscript submitted July 26, 2022, accepted August 12, 2022, published online August 27, 2022
Short title: SMs After Chemotherapy for Classical HL
doi: https://doi.org/10.14740/jocmr4802
| Abstract | ▴Top |
Treatment-related second malignancies (SMs) remain a major concern in long-term survivors of Hodgkin lymphoma (HL). In this report, the autopsy findings of a patient with HL, who was in complete remission after chemotherapy but expired of pulmonary tumor thrombotic microangiopathy (PTTM) caused by urothelial carcinoma of the renal pelvis (UCRP), were described. A 78-year-old Japanese man with a history of classical HL developed irreversible heart failure about 2.5 years after chemotherapy. The patient expired shortly after being admitted due to ineffective treatment for heart failure. However, the cause of death was not determined. The patient’s autopsy findings revealed UCRP in the left kidney, as well as infiltration around the inferior vena cava and lungs, but no HL recurrence. The primary causes of mortality were respiratory and heart failure due to PTTM. Therefore, it is essential to consider the risk of SMs and search for them in patients with HL after chemotherapy.
Keywords: Pulmonary tumor thrombotic microangiopathy; Hodgkin lymphoma; Second malignancy; Urothelial carcinoma; Chemotherapy
| Introduction | ▴Top |
Classical Hodgkin lymphoma (HL) can be cured in up to 90% of patients [1]. Nevertheless, second malignancies (SMs) [2-8] and doxorubicin-related cardiac toxicity have been reported as causes of late-onset mortality after chemotherapy [9]. This report presented the autopsy findings of a patient with HL who maintained complete remission after chemotherapy for 2.5 years but developed irreversible heart and respiratory failure due to pulmonary tumor thrombotic microangiopathy (PTTM) with urothelial carcinoma of the renal pelvis (UCRP). Metastatic urothelial carcinoma has been historically associated with poor prognosis. Recently, Rizzo et al reported that immune checkpoint inhibitors (ICIs) have response in these some selected patients [10-12]. However unfortunately, UCRP was not diagnosed in this patient until the patient expired.
| Case Report | ▴Top |
A 75-year-old Japanese man came to the hospital of the current study for a checkup of the right inguinal lymph node that had swollen 2 months earlier and was causing night sweating but no fever or weight loss. A biopsy of the right inguinal lymph node revealed that the patient had classical HL. Fluorodeoxyglucose positron emission tomography (FDG-PET) revealed accumulations in the enlarged bilateral axillary and inguinal lymph nodes, surrounding the abdominal aorta and spleen, and in the bone marrow of the spine and right femur (Fig. 1a). It was classified as clinical stage IVB because HL invasion was detected in the bone marrow. After the first admission (day 0), the patient was given doxorubicin, bleomycin, vincristine, and dacarbazine (ABVD). The pretreatment ejection fraction was 52%, and it gradually decreased with therapy despite the reduction in doxorubicin (Fig. 2). After the fifth course of ABVD (day 162), FDG-PET revealed residual disease in the left axillary lymph nodes (Fig. 1b). Furthermore, the ejection fraction had declined to 46% on day 183 (Fig. 2), and a computed tomography (CT) scan revealed ground-glass opacity in both lungs. On day 211, the therapy was switched to brentuximab vedotin. The total doxorubicin dose up to this time was 226 mg/m2. The patient achieved complete remission after the fourth course of brentuximab vedotin, as detected by FDG-PET on day 281 (Fig. 1c). On day 973, the patient developed heart failure with a low ejection fraction (22%) and was diagnosed with angina pectoris. Consequently, he was given angiotensin-I-converting enzyme inhibitor, antiplatelet therapy, and diuretics, and then allowed to go home. On day 1,206, the patient was readmitted to the hospital with heart failure.
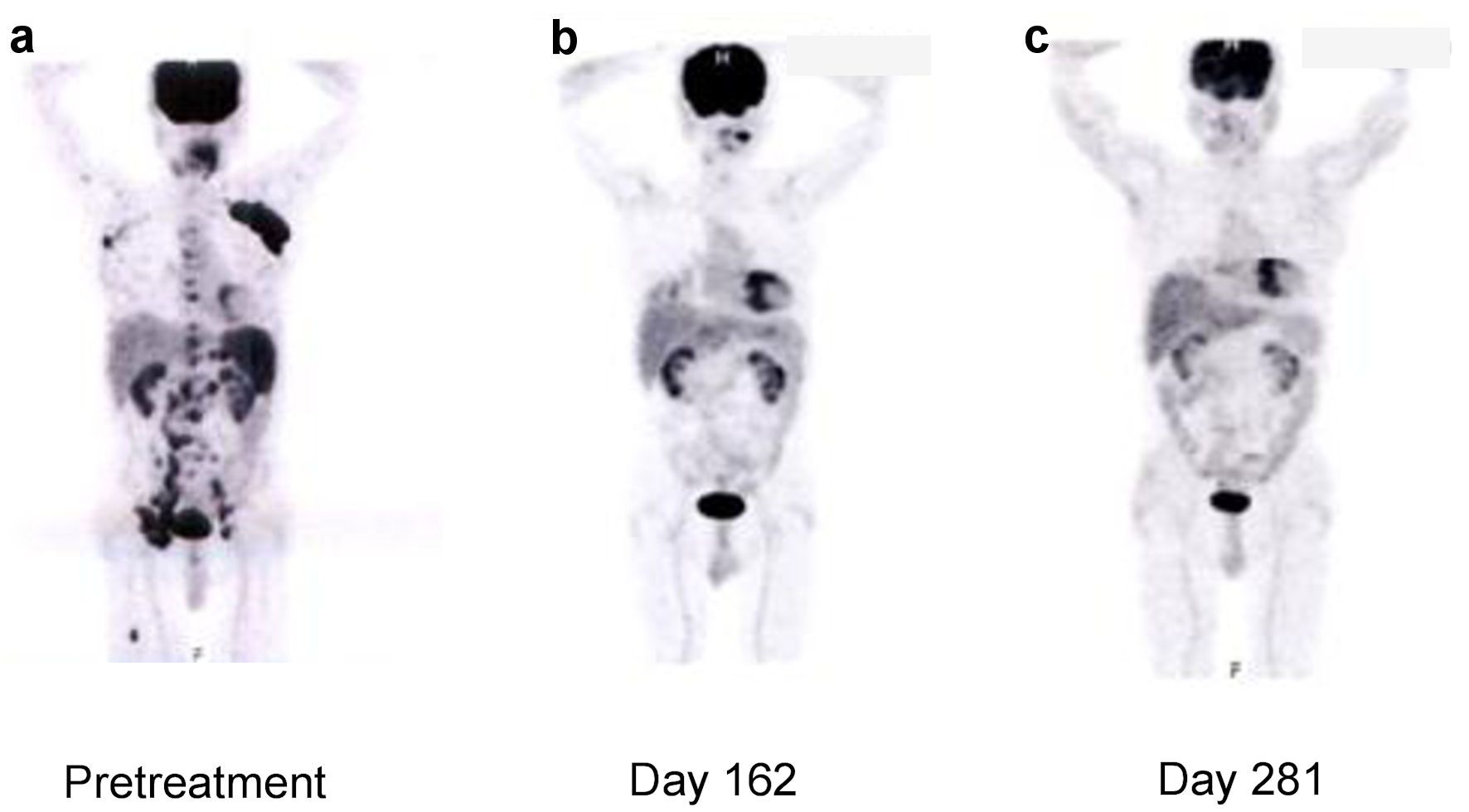 Click for large image | Figure 1. Fluorodeoxyglucose-positron emission tomography findings in the patient during HL treatment. FDG-PET: fluorodeoxyglucose-positron emission tomography; HL: Hodgkin lymphoma. |
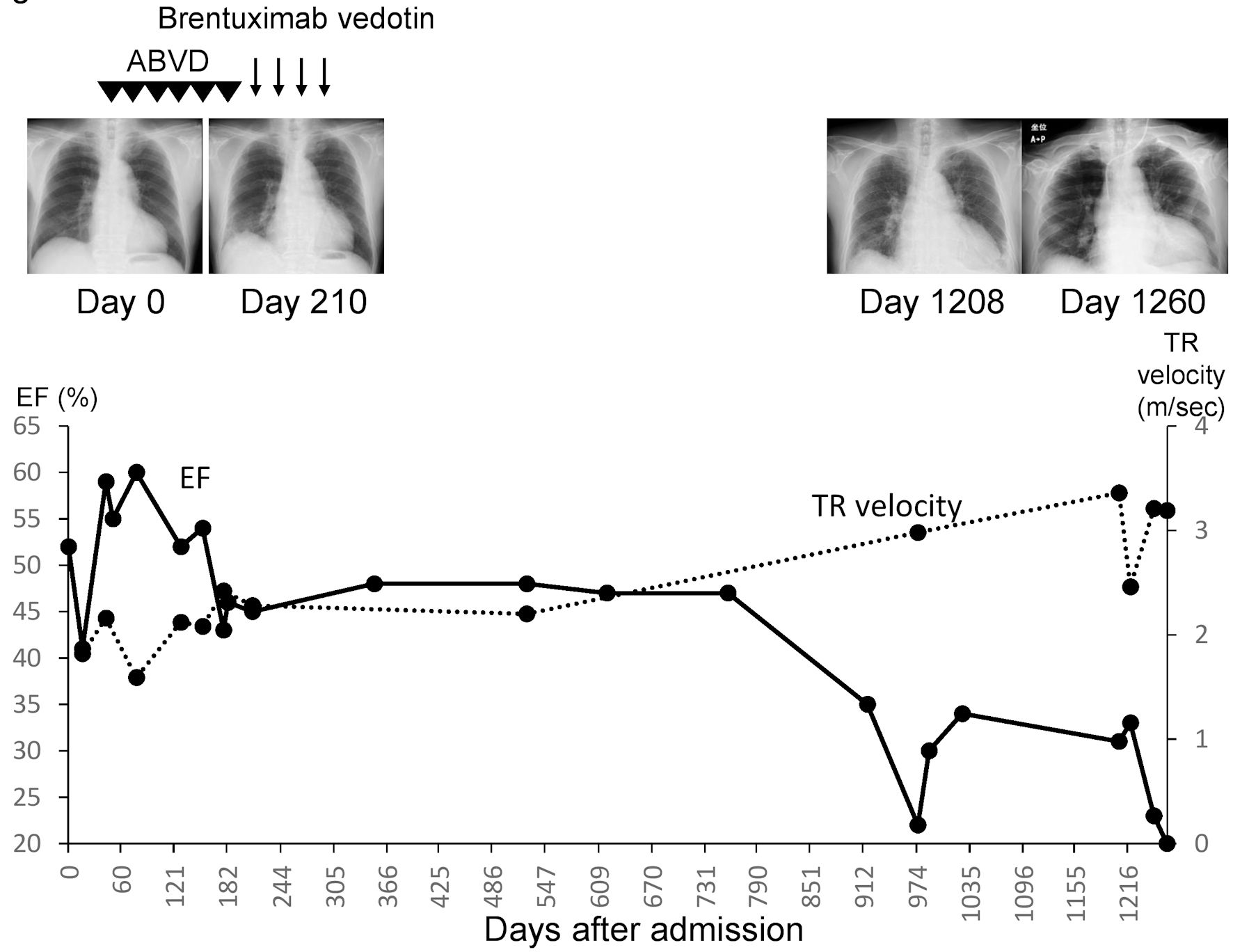 Click for large image | Figure 2. Patient’s clinical course. ABVD: doxorubicin, bleomycin, vincristine and dacarbazine; EF: ejection fraction; TR: tricuspid regurgitation. |
Medical history of previous illness
The patient had previously received treatment for hypertension and diabetes mellitus as well as chemotherapy for HL.
Personal and family history
The patient had smoked for 20 years.
Physical examination upon admission
The patient’s height and weight were 158.1 cm and 62.8 kg, respectively. Moreover, his blood pressure and heart rate were 156/99 mm Hg and 100 bpm, respectively. His level of consciousness was normal, and a third heart sound was heard on auscultation. Furthermore, he presented with pretibial edema and carotid artery distension.
Laboratory investigations and imaging
Compared to earlier X-ray findings taken on days 0 and 210, chest X-rays on 210 day revealed significant cardiomegaly. On day 1,208, echocardiography revealed a marked rise in tricuspid regurgitation (TR) velocity (3.36 m/s) and a low ejection fraction (31%; Fig. 2). Moreover, laboratory results upon admission revealed elevated brain natriuretic peptide (662.2 pg/mL) and D-dimer (16.06 µg/mL) levels, as well as poor glycemic control (Table 1).
 Click to view | Table 1. Laboratory Findings Upon Admission on Day 1,208 |
Treatment
After admission, the patient was given tolvaptan on day 1,208. A contrast-enhanced chest CT revealed pulmonary artery thromboembolism (Fig. 3a), without abnormal findings in the kidneys (Fig. 3b). Based on these findings, apixaban was given as thrombolytic therapy. The patient was also given heparin and rivaroxaban on day 1,269, due to an elevated D-dimer level (96.01 µg/mL). Despite these treatments, the patient’s general condition deteriorated, and he eventually expired on day 1,269.
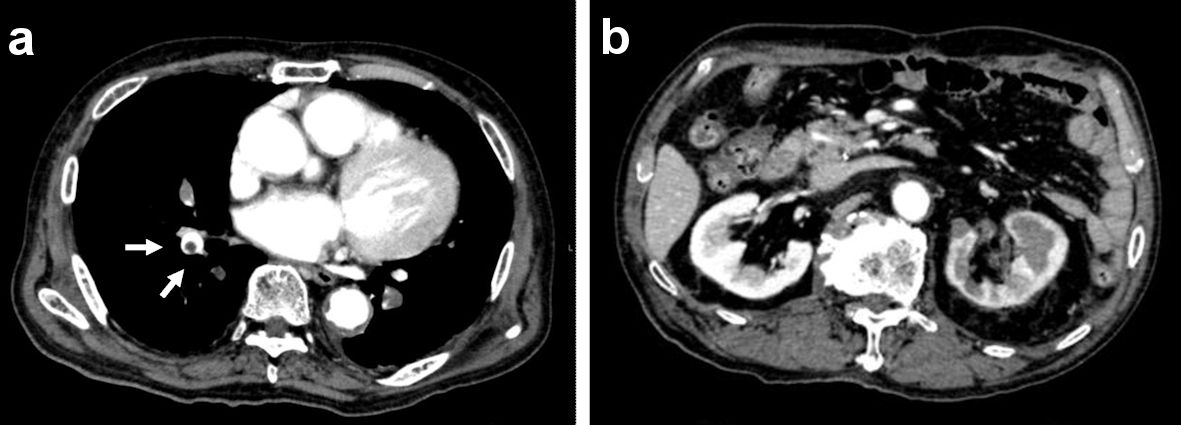 Click for large image | Figure 3. Contrast-enhanced CT scan findings upon admission on day 1,208. (a) Chest CT scan. (b) Abdominal CT scan. CT computed tomography. |
Autopsy findings
The hematoxylin and eosin (H&E)-stained section findings revealed no HL recurrence but invasive urothelial carcinoma with high-grade dysplasia (latent cancer) in the left renal pelvis (Fig. 4a-c), as well as infiltration around the inferior vena cava, lungs, and left kidney. Lung histology revealed some cancer cells and a small number of thrombi (Fig. 5a), with fibrocellular intimal proliferation of small pulmonary arteries (Fig. 5b). The heart showed marked left ventricular hypertrophy (Fig. 6a), while histology showed focal myocardial necrosis (Fig. 6b, indicated by the arrows in the H&E-stained section) and mild fibrosis around the vessels with Masson’s trichrome staining (Fig. 6c, shown as the blue staining). The primary causes of mortality, in this case, were respiratory and heart failure due to PTTM.
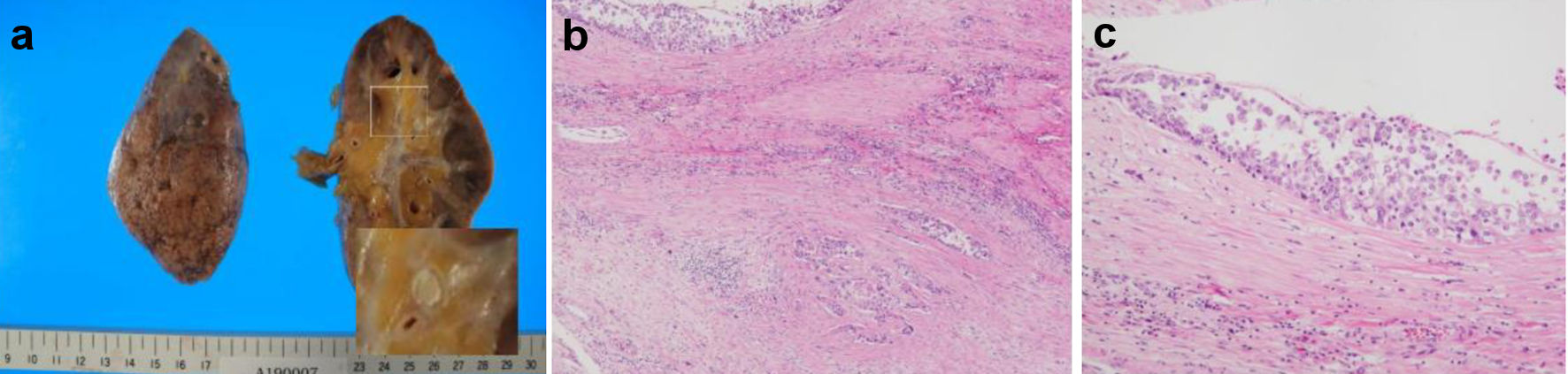 Click for large image | Figure 4. Patient’s autopsy findings of the kidney. (a) Gross findings of the left kidney. (b, c) Urothelial carcinoma of the renal pelvis in hematoxylin and eosin-stained sections. |
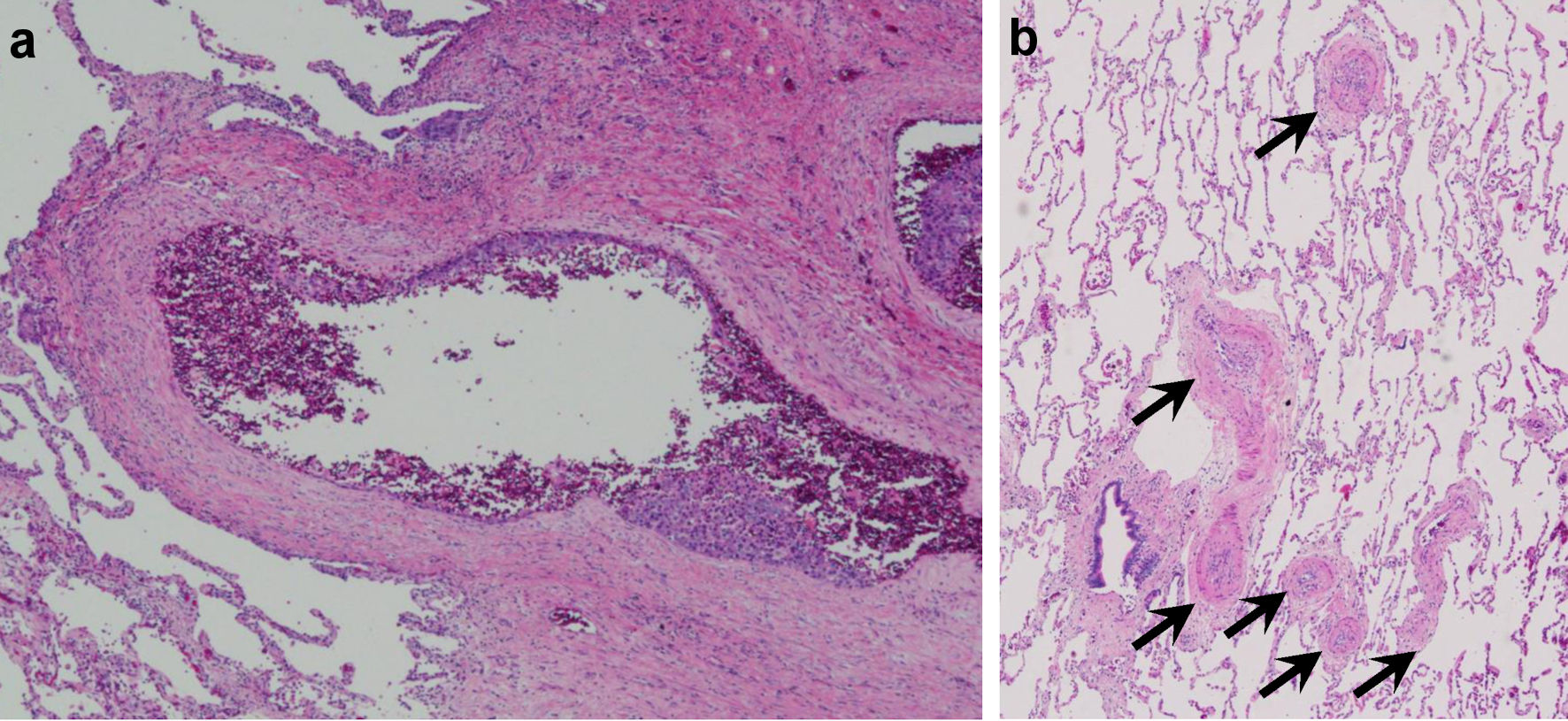 Click for large image | Figure 5. Patient’s autopsy findings of the lung showing (a) some cancer cells and small number of thrombi, (b) fibrocellular intimal proliferation of small pulmonary arteries. |
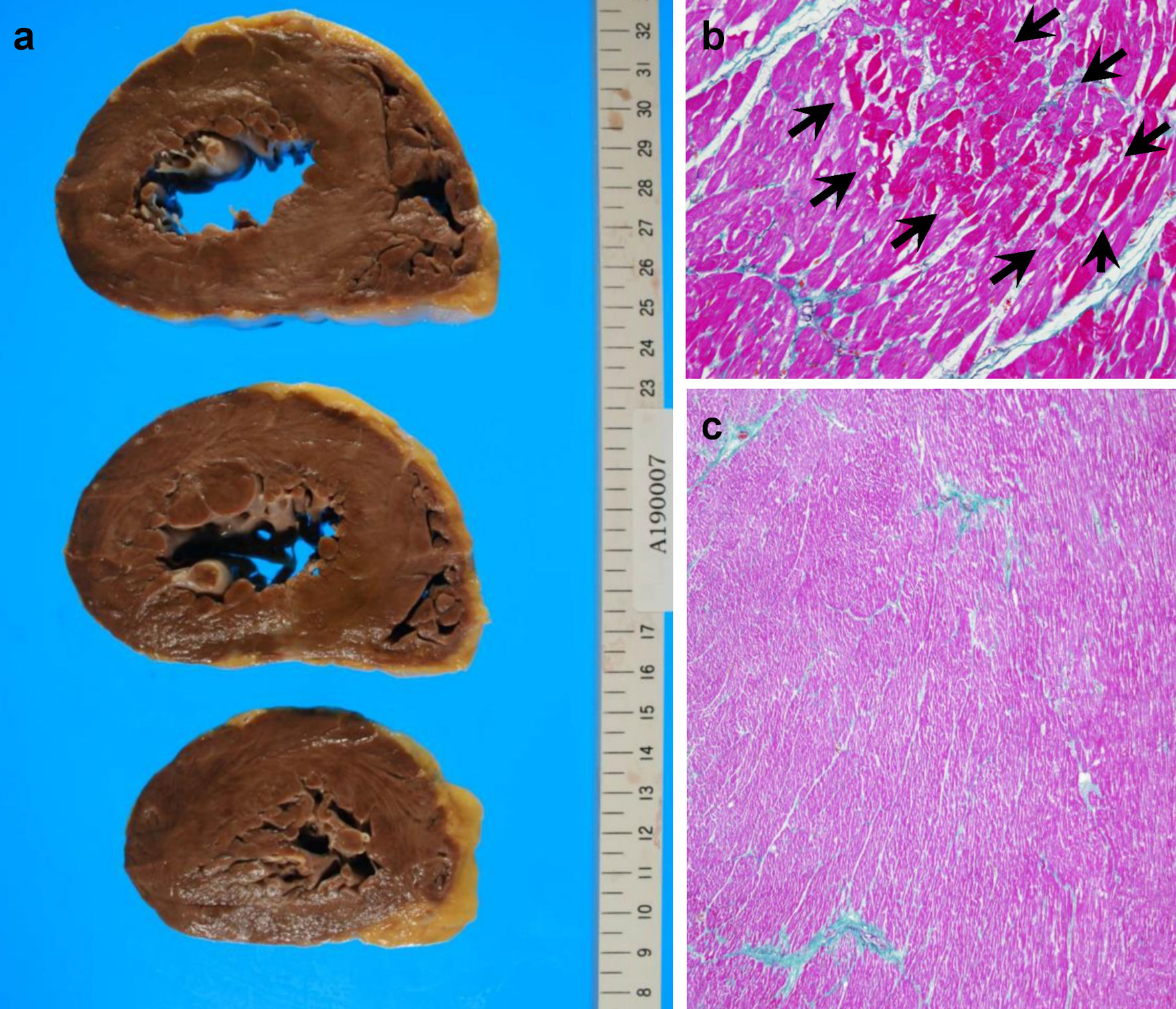 Click for large image | Figure 6. Patient’s autopsy findings of the heart. (a) Gross findings of the heart. (b) Focal myocardial necrosis (arrows) in the hematoxylin and eosin-stained section. (c) Mild fibrosis in myocardial tissues around vessels, shown as blue Masson’s trichrome staining. |
Ethics approval
The Ethics Committee of Toho University approved this study.
| Discussion | ▴Top |
HL can effectively be treated with combination chemotherapy [1]. However, a rigorous assessment should be conducted for late complications, which mostly involve the development of SMs, the cumulative incidence of which ranges from 7% to 26.3% [2-8]. Ng et al reported that 50 of 1,319 patients with HL developed hematological SMs, e.g., acute leukemia, non-HL, and multiple myeloma, while 131 patients developed solid tumors, including 39 with breast cancer, 22 with lung cancer, 24 with gastrointestinal cancer, and 11 with genitourinary cancer [8]. Foss et al reported that 194 of 1,024 patients with HL developed SMs, including non-HL and lung, breast, and stomach cancer [4]. These reports indicate that the incidence rate of UCRP as SMs is limited; hence, the patient in the current study was a very rare case who developed UCRP as an SM.
Radiation therapy duration and dosage, combination chemotherapy, alkylating agents, chemotherapeutic drug accumulation, age at diagnosis, and splenectomy have all been identified as risk factors for SMs [4, 5, 7, 8, 13, 14]. Therefore, using brentuximab vedotin, an anti-CD30 monoclonal antibody, as well as developing a new regimen combining brentuximab vedotin with other medications to suppress SM development would be preferable.
The cumulative dose of the drug has been linked to congestive heart failure, and discontinuing doxorubicin after a total dose of 550 mg/m2 has been shown to reduce the incidence of doxorubicin-induced cardiomyopathy [15]. The total doxorubicin dose, in this case, was 370 mg/body. Dilated cardiomyopathy with diffuse atrophy and fibrosis of myocardial tissues is a common clinical finding in doxorubicin-induced cardiomyopathy [16]. However, the pathology findings of the patient of the current study did not reveal these findings. Therefore, his cardiomyopathy is believed to be unrelated to doxorubicin administration.
In this case, the PTTM due to SMs was not recognized to be the cause of heart failure during admission. The autopsy findings indicated that his UCRP was invasive and nonmass-forming, and the CT scan did not reveal any evident UCRP (Fig. 3b). Therefore, clear evidence of malignancy was not established because the cytology findings revealed only class III on several occasions.
A TR velocity > 2.8 m/s suggests pulmonary hypertension [17]. On day 16, the TR velocity of the patient was 1.82 m/s and increased to 3.36 m/s on day 1,206 upon admission. Therefore, the cause of pulmonary hypertension should be thoroughly investigated.
Herbay et al first described the pathogenesis of PTTM, a fatal disease characterized by tumor microemboli associated with fibrocellular and fibromuscular intimal proliferation in small arteries in the lungs, resulting in elevated pulmonary artery pressure and right-sided heart failure [18]. PTTM is most commonly linked to gastric adenocarcinoma and lung carcinoma [18, 19]. However, cases of PTTM linked to UCRP are rarely reported.
Our patient had kept long remission after the chemotherapy with HL. However, he developed irreversible heart failure about 2.5 years after chemotherapy. The cause of his heart failure was PTTM associated with UCRP. As shown in Table 1, he showed hematuria on day 1,208. However, because he showed microscopic hematuria about 1 year before development of Hodgkin disease, urologists had examined him already in past. Urologists examined with cystoscopy of bladder and three times cytology in urine at the last hospitalization. However, we could not diagnose for his disease during he alive.
Recently, the usefulness of ICIs has been reported with the metastatic UCRP patients [10-12]. However, the identification of biomarkers for response to ICIs is very important. So, the effort to obtain the adequate histopathological specimens and examination of the expression of programmed cell death ligand 1 in tumor will be needed to improve prognosis in these patients.
Conclusions
The current study presents the findings of a patient with PTTM and UCRP diagnosed during the autopsy. The possibility of developing SMs other than doxorubicin-induced cardiotoxicity should be considered in patients with HL receiving ABVD. Thus, long-term SM screening is required to ensure adequate care for such patients.
Acknowledgments
None to declare.
Financial Disclosure
This report received no external funding.
Conflict of Interest
The authors report no conflict of interest in this work.
Informed Consent
Informed consent was obtained from the patient’s family.
Author Contributions
Naomi Shimizu collected clinical data and drafted the manuscript. Takuro Ito and Shuji Sato carried out medical care. Yoshiya Sugiura took pathological photographs. Ichiro Tatsuno checked the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
CT: computed tomography; DBVD: doxorubicin, bleomycin, vincristine, and dacarbazine; HL: Hodgkin lymphoma; PTTM: pulmonary tumor thrombotic microangiopathy; SMs: second malignancies; TR: tricuspid regurgitation; UCRP: urothelial carcinoma of the renal pelvis
| References | ▴Top |
- Brice P, de Kerviler E, Friedberg JW. Classical Hodgkin lymphoma. Lancet. 2021;398(10310):1518-1527.
doi - Ahmadzadeh A, Yekaninejad MS, Jalili MH, Bahadoram M, Efazat M, Seghatoleslami M, Yazdi F, et al. Evaluating the survival rate and the secondary malignancies after treating Hodgkin's lymphoma patients with chemotherapy regimens. Int J Hematol Oncol Stem Cell Res. 2014;8(2):21-26.
- von Tresckow B, Kreissl S, Goergen H, Brockelmann PJ, Pabst T, Fridrik M, Rummel M, et al. Intensive treatment strategies in advanced-stage Hodgkin's lymphoma (HD9 and HD12): analysis of long-term survival in two randomised trials. Lancet Haematol. 2018;5(10):e462-e473.
doi - Foss Abrahamsen A, Andersen A, Nome O, Jacobsen AB, Holte H, Foss Abrahamsen J, Kvaloy S. Long-term risk of second malignancy after treatment of Hodgkin's disease: the influence of treatment, age and follow-up time. Ann Oncol. 2002;13(11):1786-1791.
doi pubmed - Green DM, Hyland A, Barcos MP, Reynolds JA, Lee RJ, Hall BC, Zevon MA. Second malignant neoplasms after treatment for Hodgkin's disease in childhood or adolescence. J Clin Oncol. 2000;18(7):1492-1499.
doi pubmed - Koontz MZ, Horning SJ, Balise R, Greenberg PL, Rosenberg SA, Hoppe RT, Advani RH. Risk of therapy-related secondary leukemia in Hodgkin lymphoma: the Stanford University experience over three generations of clinical trials. J Clin Oncol. 2013;31(5):592-598.
doi pubmed - Metayer C, Lynch CF, Clarke EA, Glimelius B, Storm H, Pukkala E, Joensuu T, et al. Second cancers among long-term survivors of Hodgkin's disease diagnosed in childhood and adolescence. J Clin Oncol. 2000;18(12):2435-2443.
doi pubmed - Ng AK, Bernardo MV, Weller E, Backstrand K, Silver B, Marcus KC, Tarbell NJ, et al. Second malignancy after Hodgkin disease treated with radiation therapy with or without chemotherapy: long-term risks and risk factors. Blood. 2002;100(6):1989-1996.
doi pubmed - Maraldo MV, Giusti F, Vogelius IR, Lundemann M, van der Kaaij MA, Ramadan S, Meulemans B, et al. Cardiovascular disease after treatment for Hodgkin's lymphoma: an analysis of nine collaborative EORTC-LYSA trials. Lancet Haematol. 2015;2(11):e492-502.
doi - Rizzo A, Mollica V, Massari F. Expression of programmed cell death ligand 1 as a predictive biomarker in metastatic urothelial carcinoma patients treated with first-line immune checkpoint inhibitors versus chemotherapy: a systematic review and meta-analysis. Eur Urol Focus. 2022;8(1):152-159.
doi pubmed - Rizzo A, Mollica V, Santoni M, Ricci AD, Gadaleta-Caldarola G, Montironi R, Massari F. Impact of clinicopathological features on immune-based combinations for advanced urothelial carcinoma: a meta-analysis. Future Oncol. 2022;18(6):739-748.
doi pubmed - Mollica V, Rizzo A, Montironi R, Cheng L, Giunchi F, Schiavina R, Santoni M, et al. Current strategies and novel therapeutic approaches for metastatic urothelial carcinoma. Cancers (Basel). 2020;12(6):1449.
doi pubmed - Bhatia S, Robison LL, Oberlin O, Greenberg M, Bunin G, Fossati-Bellani F, Meadows AT. Breast cancer and other second neoplasms after childhood Hodgkin's disease. N Engl J Med. 1996;334(12):745-751.
doi pubmed - Rueffer U, Josting A, Franklin J, May M, Sieber M, Breuer K, Engert A, et al. Non-Hodgkin's lymphoma after primary Hodgkin's disease in the German Hodgkin's Lymphoma Study Group: incidence, treatment, and prognosis. J Clin Oncol. 2001;19(7):2026-2032.
doi pubmed - Von Hoff DD, Layard MW, Basa P, Davis HL, Jr., Von Hoff AL, Rozencweig M, Muggia FM. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med. 1979;91(5):710-717.
doi pubmed - Cascales A, Pastor-Quirante F, Sanchez-Vega B, Luengo-Gil G, Corral J, Ortuno-Pacheco G, Vicente V, et al. Association of anthracycline-related cardiac histological lesions with NADPH oxidase functional polymorphisms. Oncologist. 2013;18(4):446-453.
doi pubmed - Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, 3rd, Dokainish H, Edvardsen T, Flachskampf FA, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the american society of echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29(4):277-314.
doi pubmed - von Herbay A, Illes A, Waldherr R, Otto HF. Pulmonary tumor thrombotic microangiopathy with pulmonary hypertension. Cancer. 1990;66(3):587-592.
doi - Chinen K, Tokuda Y, Fujiwara M, Fujioka Y. Pulmonary tumor thrombotic microangiopathy in patients with gastric carcinoma: an analysis of 6 autopsy cases and review of the literature. Pathol Res Pract. 2010;206(10):682-689.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


