| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Short Communication
Volume 14, Number 10, October 2022, pages 432-435
Cost-Effectiveness Analysis of Transplantation-Ineligible Elderly Patients With Acute Leukemia Harboring a Molecular Target: Ph-Positive Acute Leukemia and FLT3-Mutated Acute Myeloid Leukemia
Osamu Imatakia, b , Tomoya Ishidaa, Jun-ichiro Kidaa, Makiko Uemuraa, Haruyuki Fujitaa, Norimitsu Kadowakia
aDivision of Hematology, Department of Internal Medicine, Faculty of Medicine, Kagawa University, Kagawa, Japan
bCorresponding Author: Osamu Imataki, Division of Hematology, Department of Internal Medicine, Faculty of Medicine, Kagawa University, 1750-1 Ikenobe, Miki-town, Kita-county, Kagawa 761-0793, Japan
Manuscript submitted July 19, 2022, accepted September 6, 2022, published online October 28, 2022
Short title: TKIs and FLT3 in Treating Elderly Patients
doi: https://doi.org/10.14740/jocmr4799
| Abstract | ▴Top |
Background: Tyrosine kinase inhibitors (TKIs) and FMS-like tyrosine kinase 3 (FLT3) inhibitors are promising agents for Ph-positive acute leukemia (Ph+ AL) and FLT3 mutated acute myeloid leukemia (FLT3-AML), respectively.
Methods: We examined the cost-effectiveness ratio (CER) of dasatinib and ponatinib for Ph+ AL and the cost-effectiveness of gilteritinib and quizartinib for FLT3-AML in elderly patients. Molecular therapy can fit the elderly population better than chemotherapy (CT).
Results: The daily drug cost of dasatinib, ponatinib, gilteritinib, and quizartinib was $240, $170, $524, and $479 in terms of treatment maintenance dose, respectively. Treatment of Ph+ AL with stem cell transplantation (SCT), CT, dasatinib, and ponatinib yielded CERs of $322,375, $34,928, $61,104, and $46,234, respectively. The CERs for FLT3-AML treated with SCT, CT, gilteritinib, and quizartinib were $355,270, $42,717, $94,987, and $90,080, respectively. Treatment of elderly patients with TKIs and FLT3 inhibitors remained expensive and inferior to conventional CT.
Conclusion: Although TKIs and FLT3 inhibitors have an inferior CER than does conventional CT, their promising survival benefit with better QOL can offer a profound advantage. TKI or FLT3 inhibitor monotherapy is recommended as an alternative treatment option for unfit (vulnerable) elderly patients with Ph+ AL or FLT3-AML.
Keywords: Transplantation-ineligible elderly; Ph-positive acute leukemia; FLT3-mutated acute myeloid leukemia; Health economic evaluation; Cost-effectiveness analysis
| Introduction | ▴Top |
Various therapeutic target molecules have been discovered in hematological malignancies. The most representative case is BCR-ABL1 translocation (Philadelphia chromosome)-positive acute leukemia (Ph+ AL), which can be managed using tyrosine kinase inhibitors (TKIs) [1]. We are now in an era where long-term prognosis is expected after the administration of TKIs, even among the elderly [2, 3]. Similarly, a specific molecular-targeted therapeutic agent for FMS-like tyrosine kinase 3 (FLT3)-mutated acute myeloid leukemia (FLT3-AML), an FLT3 inhibitor, is used to induce remission before hematopoietic stem cell transplantation (SCT) for relapsed/refractory cases of AML [4, 5]. It has been developed as an effective therapeutic drug and is expected to improve prognosis, even among elderly patients who are not indicated for SCT. Furthermore, for the vulnerable elderly population, molecular-targeted therapy using TKIs or an FLT3 inhibitor affords a longer survival compared with cytotoxic chemotherapy (CT).
| Materials and Methods | ▴Top |
We examined the cost-effectiveness of dasatinib (D) [2] and ponatinib (P) [3] for Ph+ ALL and the cost-effectiveness of gilteritinib (G) [4] and quizartinib (Q) [5] for FLT3-AML compared with SCT [6-8] or CT [9]. The target population of this research was defined as elderly patients who were not eligible for SCT. We analyzed two patterns of acute leukemia that harbor targeted molecules, i.e., BCR-ABL1 translocation and FLT3 mutation. In this setting, the research population could be treated with either CT or molecular therapy. We adopted CT for fit elderly patients and molecular therapy for unfit vulnerable elderly patients. Therefore, fit patients were treated with chemotherapy in a hospitalized setting, whereas vulnerable patients were treated in an outpatient clinic. This is the current Japanese practical situation. The duration of the CT period was defined in the protocol of standard therapy established by the Japanese Adult Leukemia Study Group (JALSG). This practical protocol consists of induction and consolidation therapy (for AML) [10], and an additive maintenance phase (only for acute lymphoblastic leukemia (ALL)) [11]. Conversely, oral molecular therapy was used until progressive disease or death, whichever occurred earlier. SCT is the standard treatment modality for younger eligible populations who have an HLA-matched donor. However, elderly patients are generally ineligible for SCT, and this approach is adopted only in a small group of elderly cases who maintain intact organ function and a good performance status, for whom a physician might consider an optional indication for SCT. Therefore, we additively performed cost analysis of SCT as a standard reference, although the survival data were adopted from the clinical trial of younger recipients.
For medical cost analysis, the cost-effectiveness ratio (CER) was evaluated according to estimated overall survival obtained from each clinical evidence [2-9]. For the estimation of costs, we obtained data from payer’s perspective in Japan. The inpatient treatment cost was estimated based on the Japanese nationwide gross-costing system, i.e., the Diagnosis Procedure Combination/Per-Diem Payment System (DPC/PDPS), which includes TKI treatment costs as a practical protocol. Conversely, the outpatient cost was estimated based on the performed payment (fee-for-service payment). Considering the annual discount rate of 3%, a Markov decision model was adopted and the CER was calculated for treatments in four branches (SCT, CT, drug 1, and drug 2), based on overall survival from the decision point of each branch of the disease. The transplantation cost was calculated based on previous reports [12]. The medical cost of each drug was calculated according to the dosage stated in the package insert, and the domestic drug price in 2020 was used. Survival time after SCT and survival time after treatment with each drug were fitted with the results of updated and consensus comparative studies. For convenience, the cost-effectiveness was calculated using the survival time up to 1 year after the treatment, and the results are reported as CER per patient per year. The currency conversion was as follows: 1 US dollar to 113 Japanese yen. No sensitivity analysis or Monte Carlo simulation was performed.
The study was approved by the Internal Review Committee of the Kagawa University Hospital (H-023). This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
| Results | ▴Top |
The daily drug cost of the D, P, G, and Q drugs was $240, $170, $524, and $479 in terms of treatment maintenance dose, respectively. Treatment of Ph+ AL with SCT, CT, D, and P yielded CERs of $322,375, $34,928, $61,104, and $46,234, respectively (Fig. 1a). The CERs for FLT3-AML treated with SCT, CT, G, and Q were $355,270, $42,717, $94,987, and $90,080, respectively (Fig. 1b). The CER of treatment with an FLT3 inhibitor for FLT3-AML was higher than that of CT treatment for the same disease because of the high daily cost of the drug; however, the median survival time when using the G and Q was 9.3 and 6.2 months, respectively. In addition, the CERs associated with molecular therapies (TKIs and FLT3 inhibitors) for each disease were comparable. Treatment with FLT3 inhibitors in the elderly remained expensive in terms of proper health economy, as defined by the World Health Organization (WHO) and the National Institute for Health and Care Excellence (NICE). The total cost of molecular therapies was not much higher than that of SCT. The use of TKIs for Ph+ AL is cost effective [13-15], whereas the application of FLT3 inhibitors for FLT3-AML is still rare [16]. TKIs, including imatinib and second [13] to third [14] generation TKI agents, exhibit acceptable cost-effectiveness for Ph+ AL. Our results showed that molecular target therapy was effective compared with conventional CT with TKIs and FLT3 inhibitors. Thus, patients with acute leukemia, especially the elderly, harboring a target molecule should receive less-intensive molecular target therapy if they are ineligible for intensive CT. Currently, TKI availability has improved the prognosis of Ph+ ALL compared with Ph- ALL in older patients [15]. This benefit must be theoretically compared among AML cases with/without FLT3 mutation.
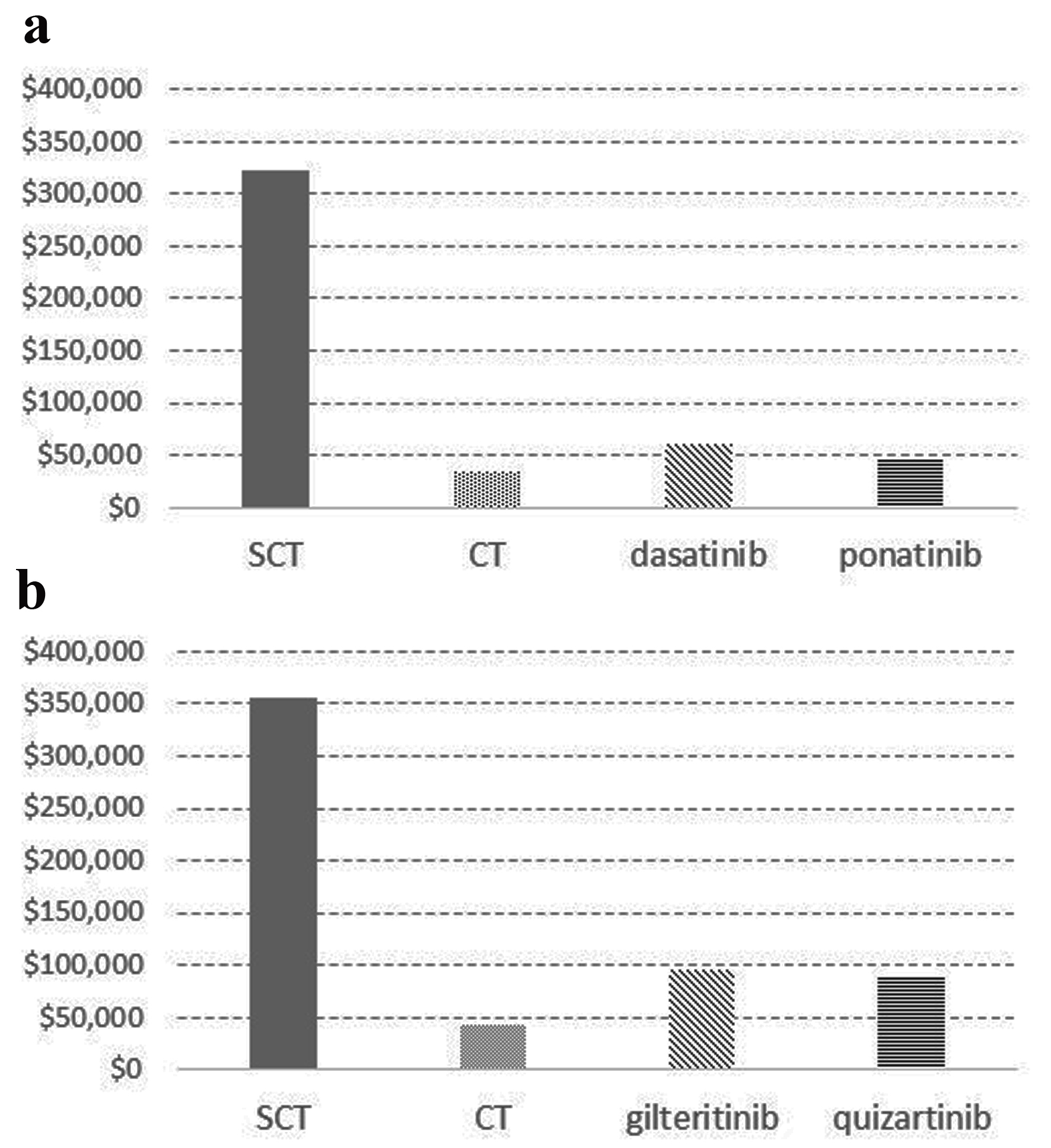 Click for large image | Figure 1. Cost-effectiveness ratio for patients with Philadelphia chromosome-positive acute leukemia and FLT3-mutated AML treated with stem cell transplantation, chemotherapy, and molecular targeting agents. (a) The CERs for Ph+ AL treated with SCT, CT, dasatinib (D), and ponatinib (P) were $322,375, $34,928, $61,104, and $46,234, respectively. (b) The CERs for FLT3-AML treated with SCT, CT, gilteritinib (G), and quizartinib (Q) were $355,270, $42,717, $94,987, and $90,080, respectively. CER: cost-effectiveness ratio; CR: cytotoxic chemotherapy; FLT3: FMS-like tyrosine kinase 3; FLT3-AML: FLT3-mutated acute myeloid leukemia; Ph+ AL: Philadelphia chromosome-positive acute leukemia; SCT: stem cell transplantation |
| Discussion | ▴Top |
Pandya et al reported the cost-effectiveness of gilteritinib for relapsed or refractory FLT3-AML [16]. This was the first and only report on the CER of gilteritinib. In this study, the total incremental cost compared with salvage CT was $148,106 [16], and the incremental cost per QALY gained was $115,192. This incremental cost was similar to the best supportive care ($107,435). Our results indicated that the incremental CER compared with CT was $52,270 for gilteritinib and $47,363 for quizartinib. According to the Institute for Clinical and Economic Review [17], because these incremental costs are less than $100,000 - $150,000, they are eligible for medical cost policy [17]. Although there is no current research on the outcomes of quizartinib (as of January 1, 2022), our results suggest that they are similar to those of gilteritinib. Because FLT3-AML fulfills the unmet medical needs [4], FLT3 inhibitor monotherapy is recommended for elderly patients with this disease, such as situationally unfit elderly and relapse and refractory cases with post-SCT recurrence. Although FLT3 inhibitors have a higher drug cost than conventional CT, their promising survival benefit with better QOL can offset the cost.
Conclusion
In conclusion, our study was the first cost-effective cross-sectoral analysis of FLT3 inhibitor therapy for AML and TKI therapy for Ph+ AL. This study provided a platform for the cost evaluation of the management of elderly patients with leukemia in cases of monotherapy using a molecular targeting drug. Moreover, we advocate the potential benefit of the clinical application of molecular-targeted therapy in patients who are unfit for intensive CT. Furthermore, this treatment modality can be used even for relapse and refractory cases after SCT. In the future, additional drugs can be innovated, and we should prepare for the reasonable usage of those novel agents.
Limitations
This study consisted in an analysis with limited settings, and re-analysis trials under various conditions are desired depending on the purpose. For instance, this study lacked the application of various analytic methods, such as a sensitivity analysis or Monte Carlo simulation. Moreover, this study did not compensate for the cost of adverse reactions, such as drug side effects. Finally, this study did not include direct non-medical costs (such as transport or food and health care) or indirect costs (such as the cost for social deficit). In the future, CER could be improved by optimizing the treatment indications and improving the therapeutic effects.
Acknowledgments
None to declare.
Financial Disclosure
The authors have no funding source to disclose concerning this report. This work was supported by JSPS KAKENHI Grant Numbers JP19K17927.
Conflict of Interest
The authors declare that they have no competing interests.
Informed Consent
Written informed consent was obtained from the patient for publication of this study.
Author Contributions
OI and TI managed the patient’s case, contributed to the literature search, and wrote the manuscript. JIK and MU made substantial contributions to the concept and design of this report. HF qualified the patient’s data, and suggested important intellectual content. MU took part in critical discussions. NK was involved in supervision of the manuscript and managed the research. All authors approved the final version of the manuscript.
Data Availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
CER: cost-effectiveness ratio; CT: chemotherapy; D: dasatinib; FLT3: FMS-like tyrosine kinase 3; FLT3-AML: FLT3-mutated acute myeloid leukemia; G: gilteritinib; NICE: National Institute for Health and Care Excellence; P: ponatinib; Ph+ AL: Philadelphia chromosome-positive acute leukemia; Q: quizartinib; SCT: stem cell transplantation; TKI: tyrosine kinase inhibitor; WHO: World Health Organization
| References | ▴Top |
- Baccarani M, Pileri S, Steegmann JL, Muller M, Soverini S, Dreyling M, ESMO Guidelines Working Group. Chronic myeloid leukemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;118(Suppl 7):vii72-vii77.
doi pubmed - Foa R, Vitale A, Vignetti M, Meloni G, Guarini A, De Propris MS, Elia L, et al. Dasatinib as first-line treatment for adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2011;118(25):6521-6528.
doi pubmed - Cortes JE, Kim DW, Pinilla-Ibarz J, le Coutre PD, Paquette R, Chuah C, Nicolini FE, et al. Ponatinib efficacy and safety in Philadelphia chromosome-positive leukemia: final 5-year results of the phase 2 PACE trial. Blood. 2018;132(4):393-404.
doi pubmed - Perl AE, Martinelli G, Cortes JE, Neubauer A, Berman E, Paolini S, Montesinos P, et al. Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML. N Engl J Med. 2019;381(18):1728-1740.
doi pubmed - Cortes JE, Khaled S, Martinelli G, Perl AE, Ganguly S, Russell N, Kramer A, et al. Quizartinib versus salvage chemotherapy in relapsed or refractory FLT3-ITD acute myeloid leukaemia (QuANTUM-R): a multicentre, randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2019;20(7):984-997.
doi - Terwilliger T, Abdul-Hay M. Acute lymphoblastic leukemia: a comprehensive review and 2017 update. Blood Cancer J. 2017;7(6):e577.
doi pubmed - Ravandi F. How I treat Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2019;133(2):130-136.
doi pubmed - De Kouchkovsky I, Abdul-Hay M. Acute myeloid leukemia: a comprehensive review and 2016 update. Blood Cancer J. 2016;6(7):e441.
doi pubmed - Ravandi F. Current management of Philadelphia chromosome positive ALL and the role of stem cell transplantation. Hematology Am Soc Hematol Educ Program. 2017;2017(1):22-27.
doi pubmed - Miyawaki S, Ohtake S, Fujisawa S, Kiyoi H, Shinagawa K, Usui N, Sakura T, et al. A randomized comparison of 4 courses of standard-dose multiagent chemotherapy versus 3 courses of high-dose cytarabine alone in postremission therapy for acute myeloid leukemia in adults: the JALSG AML201 Study. Blood. 2011;117(8):2366-2372.
doi pubmed - Mizuta S, Matsuo K, Yagasaki F, Yujiri T, Hatta Y, Kimura Y, Ueda Y, et al. Pre-transplant imatinib-based therapy improves the outcome of allogeneic hematopoietic stem cell transplantation for BCR-ABL-positive acute lymphoblastic leukemia. Leukemia. 2011;25(1):41-47.
doi pubmed - Saito AM, Cutler C, Zahrieh D, Soiffer RJ, Ho VT, Alyea EP, Koreth J, et al. Costs of allogeneic hematopoietic cell transplantation with high-dose regimens. Biol Blood Marrow Transplant. 2008;14(2):197-207.
doi pubmed - Carpiuc KT, Stephens JM, Botteman MF, Feng W, Hay JW. A review of the clinical and economic outcomes of imatinib in Philadelphia chromosome-positive acute lymphoblastic leukemia. Expert Opin Pharmacother. 2007;8(16):2775-2787.
doi pubmed - Stevenson M, Pandor A, Hamilton J, Stevens J, Rowntree C, Martyn-St James M, Rawdin A, et al. Ponatinib for treating acute lymphoblastic leukaemia: an evidence review group perspective of a NICE single technology appraisal. Pharmacoeconomics. 2018;36(7):759-768.
doi pubmed - Rousselot P, Delannoy A. Optimal pharmacotherapeutic management of acute lymphoblastic leukaemia in the elderly. Drugs Aging. 2011;28(9):749-764.
doi pubmed - Pandya BJ, Qi CZ, Garnham A, Yang H, Shah MV, Zeidan AM. Cost-effectiveness of gilteritinib for relapsed/refractory FLT3(mut+) acute myeloid leukemia. J Manag Care Spec Pharm. 2021;27(10):1469-1481.
doi pubmed - The Institute for Clinical and Econoimc Review. 2020-2023 value assessment framework. 2020. Accessed January 1, 2022. https://icer.org/wp-content/uploads/2020/10/ICER_2020_2023_VAF_102220.pdf.
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


