| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Case Report
Volume 14, Number 7, July 2022, pages 282-286
Negative Outcome Following Systemic Alteplase Administration Prior to Extracorporeal Membrane Oxygenation in a Kidney Transplant Patient With Cardiac Arrest: A Case Report
Kathryn A. Connora, b, d , Jennifer Falveyc, Stephen Rappaportb
aDepartment of Pharmacy Practice and Administration, St. John Fisher University, Wegmans School of Pharmacy, Rochester, NY 14618, USA
bSurgical Critical Care, University of Rochester Medical Center-Strong Memorial Hospital, Rochester, NY 14642, USA
cCardiovascular Pharmacy, University of Rochester Medical Center, Rochester, NY 14642, USA
dCorresponding Author: Kathryn A. Connor, Department of Pharmacy Practice and Administration, St. John Fisher University, Wegmans School of Pharmacy, Rochester, NY 14618, USA
Manuscript submitted May 18, 2022, accepted June 27, 2022, published online July 29, 2022
Short title: Alteplase Prior to ECMO: A Case Report
doi: https://doi.org/10.14740/jocmr4744
| Abstract | ▴Top |
A case of a negative outcome following systemic alteplase administration prior to extracorporeal membrane oxygenation (ECMO) in a kidney transplant patient with cardiac arrest is reported. A patient status-post kidney transplantation was admitted to the surgical intensive care unit (ICU) and experienced cardiac arrest after developing sudden-onset chest pain and shortness of breath. During cardiopulmonary resuscitation, alteplase 50 mg was administered intravenous push for suspected pulmonary embolism (PE) before the patient was evaluated for and started on veno-arterial ECMO. Within several hours, cardiopulmonary resuscitation needed to be reinitiated. Ultimately, the decision was made to cede further resuscitation efforts due to futility. A post-mortem examination included an immediate cause of death of acute myocardial infarction with extensive retroperitoneal hemorrhage. The role of ECMO is emerging in cardiac arrest, and should be considered as a management option before the administration of systemic thrombolysis in patients with increased bleeding risk.
Keywords: Critical care; ECMO; Surgical intensive care unit; Transplants
| Introduction | ▴Top |
Pulmonary embolism (PE)-associated cardiac arrest is associated with a high mortality rate, and treatment options include systemic thrombolysis, mechanical embolectomy, and extracorporeal membrane oxygenation (ECMO) when available, usually in addition to systemic anticoagulation. There is a paucity of literature addressing patient outcomes after the administration of a systemic thrombolytic during cardiac arrest secondary to confirmed massive PE with subsequent need for ECMO, with even fewer data in undifferentiated cardiac arrest [1-15]. Furthermore, there is a high likelihood of publication bias, with positive outcomes more likely to be reported.
Herein we present a patient case and report a negative outcome after an intravenous (IV) alteplase bolus dose was administered during undifferentiated cardiac arrest with subsequent need for ECMO. This case took place in the surgical intensive care unit (SICU) and subsequently the cardiac catheterization lab (cath lab) and cardiovascular intensive care unit (CICU) of a large urban academic medical center with robust abdominal and cardiac solid organ transplant programs.
| Case Report | ▴Top |
Investigations and diagnosis
A 52-year-old African American man with end-stage renal disease (ESRD) secondary to hypertensive nephrosclerosis underwent a deceased donor kidney transplant and was admitted to the SICU for hypotension and hyperkalemia requiring continuous renal replacement therapy (CRRT). This case occurred at the beginning of the pandemic, before the first cases of coronavirus disease 2019 (COVID-19) were documented in our region, and this patient was not suspected to have and was not tested for COVID-19. The patient weighed 122.2 kg with a body mass index (BMI) of 38 kg/m2. He had been receiving chemical and mechanical venous thromboembolism (VTE) prophylaxis with heparin subcutaneously and intermittent pneumatic compression (IPC) devices. Other pertinent past medical history included intermittent hemodialysis (IHD) for 8 years, right upper extremity fistula thrombus on apixaban, which was not re-started after his transplant surgery, acute mesenteric ischemia, hypertension, prior myocardial infarction (MI), heart failure with reduced ejection fraction, obstructive sleep apnea, type 2 diabetes, nicotine abuse, and depression. He had stopped smoking marijuana and cigarettes 5 years ago. His code status was full code, and he had no known allergies. On the morning of the cardiac arrest, which was post-operative day 3 from kidney transplant, he was stable and conversant, with a persistent tachycardia to 115 beats/min, potassium of 5 - 5.2 mEq/L (reference range 3.3 - 5.1 mEq/L), improving oliguria, resolving lactic acidosis, and planned IHD that day. His hospital medication list included acetaminophen, acyclovir, baby aspirin, bupropion, docusate, fluconazole, subcutaneous heparin, metoprolol, mycophenolate, as-needed oxycodone, pantoprazole, patiromer, polyethylene glycol, trimethoprim-sulfamethoxazole, and tacrolimus. At 09:22, he had witnessed sudden-onset chest pain and shortness of breath during morning rounds. He quickly became unresponsive and advanced cardiac life support (ACLS) was initiated by the SICU team. The original rhythm was ventricular fibrillation, and amiodarone 300 mg IV, lidocaine 100 mg IV and multiple rounds of epinephrine and bicarbonate IV were administered, in addition to defibrillation five times. The rhythm progressed to pulseless electrical activity (PEA), and the patient was intubated and labs were sent, including one troponin level that did not guide treatment. Twenty-two minutes after the start of the code, alteplase 50 mg intravenous push (IVP) was administered for presumed massive PE; the patient progressed to asystole, and the Pulmonary Embolism Response Team (PERT) arrived. At 27 min, the Cardiac Surgery team was called to the bedside to evaluate the patient for veno-arterial extracorporeal membrane oxygenation (VA-ECMO) for prolonged asystole, and successfully cannulated the left femoral vein and artery at the bedside. The end-tidal carbon dioxide (ETCO2) was low at 13 mm Hg at 35 min, and at 45 min ECMO was started. The patient was stable but acidotic with a pH < 7.
Treatment
The patient was transferred to the cath lab for insertion of a reperfusion catheter, then went to the computed tomography (CT) scanner to evaluate for PE prior to CICU admission. In the cath lab, there was concern for bleeding peri-reperfusion procedure, and the patient received three units of packed red blood cells (PRBCs) and 2 L of fluids. The patient then stabilized with a hemoglobin of 10 g/dL (reference range 13.7 - 17.5 g/dL) and a hematocrit of 29% (reference range 40-51%). Minimal pulsatility with ECMO was noted, and the circuit began chugging. Pulsatility and hemodynamics improved after blood products and fluid were administered. No anticoagulation was used for the ECMO circuit.
In the CICU, the patient was mostly unresponsive, except for posturing, shivering, and coughing. He was not on any inotropic or vasopressor support, but only had a flow rate of about 2 L/min on ECMO. Therapeutic hypothermia was administered, and he was cooled to 30 °C via the ECMO circuit. He initially had mean arterial pressures (MAPs) greater than 80 mm Hg with 20 - 30 points of pulsatility. After about an hour, the MAP dropped to less than 65 mm Hg and he developed 4 - 10 points of pulsatility. Epinephrine was initiated. Flow rates dropped below 2 L/min on ECMO despite aggressive fluid resuscitation and massive blood transfusion. His blood pressure continued to drop, and norepinephrine was initiated. There was concern for an acute abdominal bleed. Transesophageal echocardiography (TEE) revealed significantly underfilled right and left ventricles, with no evidence of tamponade. Arterial ECMO cannula repositioning was attempted, and vasoactive medications were intensified; however, during this event, the patient became more hypotensive, unresponsive, and at 16:55, CPR was initiated again. ACLS was performed for 1 h without return of spontaneous circulation (ROSC). A new arterial cannula was placed in the right femoral artery during this code; however, ECMO flow remained poor at less than 1 L/min. The patient’s rhythm during this arrest was PEA; however, he occasionally had fine ventricular fibrillation requiring defibrillation, then asystole. Amid significant discussion with the attending surgeons, care team, and the patient’s family, it was decided to cede further resuscitation efforts due to futility.
Follow-up and outcomes
The patient died at 18:04, and autopsy findings included an immediate cause of death of acute MI with an underlying cause of death of extensive retroperitoneal hemorrhage. His chest CT was ultimately found to be negative for PE. An intrabdominal CT taken at about the same time as the chest CT showed prominent acute right retroperitoneal acute bilobed hematoma. Figure 1 presents a graphical representation of hemoglobin results and transfusion requirements, when alteplase was administered, and when VA-ECMO cannulation occurred.
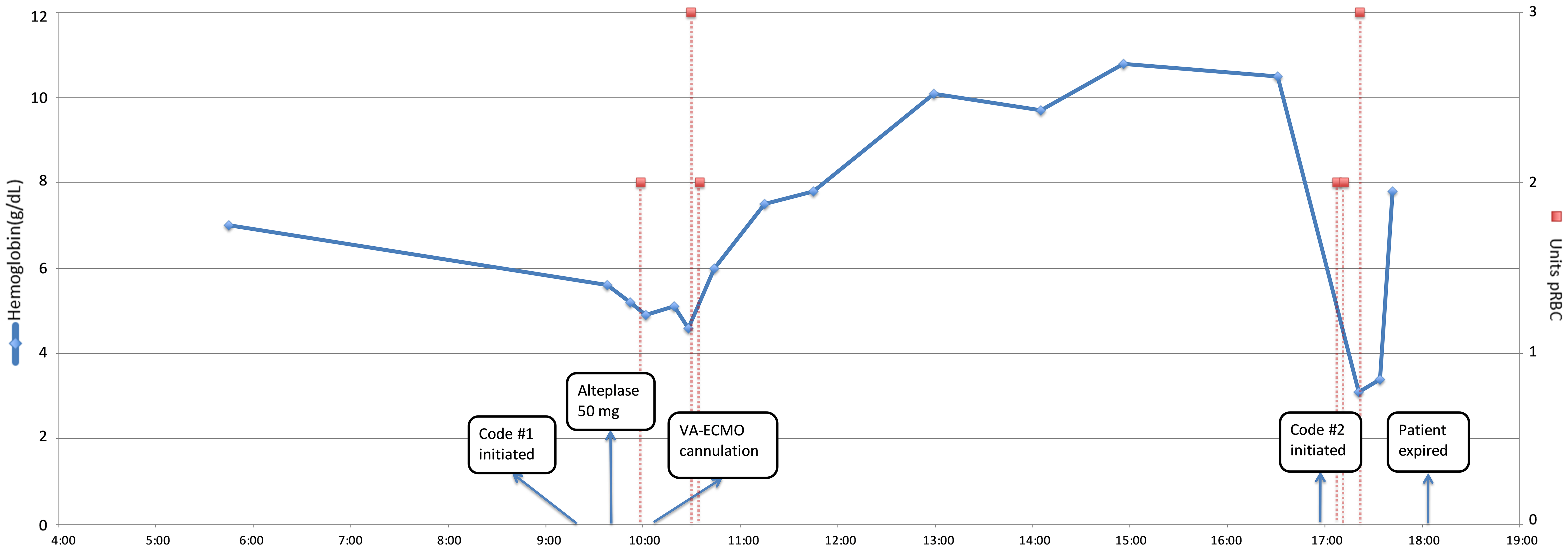 Click for large image | Figure 1. Time course of hemoglobin results and transfusion requirements. VA-ECMO: veno-arterial extracorporeal membrane oxygenation; pRBC: packed red blood cells. |
| Discussion | ▴Top |
The role of ECMO during cardiac arrest is emerging, therefore little is known about the safety of ECMO initiation post-systemic thrombolysis. Although not administered routinely in the setting of cardiac arrest given cost and lack of prospective data indicating benefit, there are no absolute contraindications for administering systemic thrombolysis in a cardiac arrest situation [16]. Our patient had recent major abdominal surgery, but had no other known factors that would increase the risk of bleeding. The clinicians involved decided that the risks of systemic thrombolysis were justifiable, given the patient’s severity of illness, high surgical risk of and clinical suspicion for PE, refractoriness to current management, and poor prognosis.
Although there is no established dosing of thrombolytics to be used peri-arrest, recommended doses are stated in clinical practice guidelines [16-18]. Fengler and Brady recommend treating patients in cardiac arrest from a suspected PE with a 50 mg IV bolus of alteplase, which should be repeated if the patient does not have ROSC in 15 min. Alternatively, a 20-unit IV bolus of reteplase or a 0.5-mg/kg IV bolus (max. 50 mg) of tenecteplase may be given.
By providing circulatory and respiratory support, ECMO can increase survival as rescue therapy or a bridge to further management in unstable acute massive PE. ECMO has historically been considered to be contraindicated following the administration of systemic thrombolytics due to the increased risk of fatal hemorrhage with cannulation [4, 10]. The use of thrombolytic agents is not listed as a specific contraindication in our institutional ECMO guideline inclusion and exclusion criteria. Despite the paucity of data, clinical practice guidelines recommend considering bolus-dose thrombolytics for suspected PE in the setting of cardiac arrest, but do not discuss the role of ECMO [16-18]. In our patient case, we decided to administer systemic thrombolysis 5 min before there was any discussion of using ECMO, and 23 min before ECMO was started. While this may have been an inevitable outcome in a complex patient with multiple comorbidities, we cannot rule out a possible adverse drug reaction (significant bleeding) from administering alteplase, which may have been related to the patient’s recent abdominal surgery. We believe the possibility of using ECMO should have been considered before the provision of thrombolysis as an earlier decision may have negated the decision for thrombolytic therapy to be deployed in this undifferentiated cardiac arrest. This conclusion is independent of the specific cause of bleeding, whether it be the thrombolysis, recent surgery, ECMO, or a combination of these factors.
Although there have been numerous successful cases of ECMO provision after failed systemic thrombolysis, publication bias should be considered as well as the potential catastrophic bleeding risk that is inherent with systemic thrombolysis and subsequent provision of ECMO, particularly after major surgery. It is difficult to make recommendations or draw parallels to our patient based on published case reports due to the likelihood of publication bias, and the heterogeneous nature of the cases in the literature. In addition, most of these reports were specific to a known PE, and we did not diagnose our patient with a PE. Scott and colleagues recently published a systematic review that indicated that VA-ECMO may improve survival in massive PE-related cardiac arrest in patients who remain unstable, even after the administration of bolus-dose alteplase [15]. The authors report 51 patients receiving systemic thrombolysis prior to ECMO, with 34 surviving. Additionally, they report six patients with major bleed in the thrombolytic cohort with all surviving. Comparing patients who received systemic thrombolysis to those who did not, the odds of death were not increased (odds ratio (OR): 0.75, 95% confidence interval (CI): 0.39 - 1.54). Unfortunately, due to small numbers, they were unable to rule out a clinically important difference. This analysis highlights the importance of reporting negative outcomes, either as a rare case of fatal bleeding in this clinical situation, or simply as a rare published report.
Importantly, our patient was treated without confirming the diagnosis of PE (e.g., undifferentiated cardiac arrest). In subsequent patients in similar clinical situations in our institution, ECMO provision is discussed before preparing systemic thrombolytics in undifferentiated cardiac arrest/suspected PE.
Learning points
Given the emerging role of ECMO during cardiac arrest, there is a paucity of information about the safety of ECMO initiation post-systemic thrombolysis. Although there have been successful cases of ECMO provision after failed systemic thrombolysis, publication bias should be considered. Institutions should design an evidence-based management guideline for acute massive PE in cardiac arrest/imminent cardiac arrest that delineates the place in therapy of thrombolytic bolus regimens and/or ECMO. The healthcare team should discuss the possibility of ECMO during cardiac arrest before administering systemic thrombolysis, especially in patients with high bleeding risk.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
In writing this case report, the authors did not attempt to obtain permission from the family of the decreased. No patient-identifying information or images were included, and all attempts to de-identify this patient were made.
Author Contributions
KC, SR, and JF were the clinical pharmacists on the ICU teams caring for this patient. KC researched and wrote the case report. SR created the figure and provided expert knowledge to revise the case report critically. JF also provided expert knowledge to revise the case report critically.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
PE: pulmonary embolism; ECMO: extracorporeal membrane oxygenation; IV: intravenous; SICU: surgical intensive care unit; cath lab: cardiac catheterization lab; CICU: cardiovascular intensive care unit; ESRD: end-stage renal disease; CRRT: continuous renal replacement therapy; COVID-19: coronavirus disease 2019; VTE: venous thromboembolism; IPC: intermittent pneumatic compression; MI: myocardial infarction; IHD: intermittent hemodialysis; ACLS: advanced cardiac life support; PEA: pulseless electrical activity; IVP: intravenous push; PERT: Pulmonary Embolism Response Team; VA-ECMO: veno-arterial extracorporeal membrane oxygenation; ETCO2: end-tidal carbon dioxide; CT: computed tomography; PRBCs: packed red blood cells; MAPs: mean arterial pressures; TEE: transesophageal echocardiography; ROSC: return of spontaneous circulation; OR: odds ratio
| References | ▴Top |
- Akoluk A, Mazahir U, Douedi S, Aziz A, Obagi A, Kiss D, Flynn D, et al. Pulmonary embolism in COVID-19 treated with VA-ECLS and catheter tPA. Clin Med Insights Circ Respir Pulm Med. 2020;14:1179548420957451.
doi pubmed - Algahtani H, Azzam M, Albanna AS, Shirah B. Neurological recovery from multiple cardiac arrests due to acute massive pulmonary embolism managed by cardiopulmonary resuscitation and extracorporeal membrane oxygenation. Cardiovasc Revasc Med. 2018;19(1 Pt B):120-122.
doi pubmed - Fernandes P, Allen P, Valdis M, Guo L. Successful use of extracorporeal membrane oxygenation for pulmonary embolism, prolonged cardiac arrest, post-partum: a cannulation dilemma. Perfusion. 2015;30(2):106-110.
doi pubmed - Giraud R, Banfi C, Siegenthaler N, Bendjelid K. Massive pulmonary embolism leading to cardiac arrest: one pathology, two different ECMO modes to assist patients. J Clin Monit Comput. 2016;30(6):933-937.
doi pubmed - Li I, Filiberti A, Mokszycki R, Galletta G. Multiple boluses of alteplase followed by extracorporeal membrane oxygenation for massive pulmonary embolism. Am J Emerg Med. 2019;37(9):1808.1808.e5-e6.
doi pubmed - Lupei MI, Kloesel B, Trillos L, Apostolidou I. Survival of intraoperative massive pulmonary embolism using alteplase and VA-ECMO. J Clin Anesth. 2019;57:112.
doi pubmed - Maggio P, Hemmila M, Haft J, Bartlett R. Extracorporeal life support for massive pulmonary embolism. J Trauma. 2007;62(3):570-576.
doi pubmed - McDonald C, Laurie J, Janssens S, Zazulak C, Kotze P, Shekar K. Successful provision of inter-hospital extracorporeal cardiopulmonary resuscitation for acute post-partum pulmonary embolism. Int J Obstet Anesth. 2017;30:65-68.
doi pubmed - Miyazaki K, Hikone M, Kuwahara Y, Ishida T, Sugiyama K, Hamabe Y. Extracorporeal CPR for massive pulmonary embolism in a "hybrid 2136 emergency department". Am J Emerg Med. 2019;37(12):2132-2135.
doi pubmed - Newman J, Park D, Manetta F. Extracorporeal membrane oxygenation for failed tPA therapy of pulmonary embolism. Cardiovascular and Thoracic Open Volume. 2016;2:1-3.
doi - Northey LC, Shiraev T, Omari A. Salvage intraosseous thrombolysis and extracorporeal membrane oxygenation for massive pulmonary embolism. J Emerg Trauma Shock. 2015;8(1):55-57.
doi pubmed - Arlt M, Philipp A, Iesalnieks I, Kobuch R, Graf BM. Successful use of a new hand-held ECMO system in cardiopulmonary failure and bleeding shock after thrombolysis in massive post-partal pulmonary embolism. Perfusion. 2009;24(1):49-50.
doi pubmed - George B, Parazino M, Omar HR, Davis G, Guglin M, Gurley J, Smyth S. A retrospective comparison of survivors and non-survivors of massive pulmonary embolism receiving veno-arterial extracorporeal membrane oxygenation support. Resuscitation. 2018;122:1-5.
doi pubmed - Maj G, Melisurgo G, De Bonis M, Pappalardo F. ECLS management in pulmonary embolism with cardiac arrest: which strategy is better? Resuscitation. 2014;85(10):e175-176.
doi pubmed - Scott JH, Gordon M, Vender R, Pettigrew S, Desai P, Marchetti N, Mamary AJ, et al. Venoarterial extracorporeal membrane oxygenation in massive pulmonary embolism-related cardiac arrest: a systematic review. Crit Care Med. 2021;49(5):760-769.
doi pubmed - Fengler BT, Brady WJ. Fibrinolytic therapy in pulmonary embolism: an evidence-based treatment algorithm. Am J Emerg Med. 2009;27(1):84-95.
doi pubmed - Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, Nelson ME, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e419S-e496S.
doi pubmed - Lavonas EJ, Drennan IR, Gabrielli A, Heffner AC, Hoyte CO, Orkin AM, Sawyer KN, et al. Part 10: special circumstances of resuscitation: 2015 American Heart Association Guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132(18 Suppl 2):S501-518.
doi
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


