| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Review
Volume 14, Number 1, January 2022, pages 8-21
Current State and Principles of Basal Insulin Therapy in Type 2 Diabetes
Metabolic Diseases Study Group, Department of Internal Medicine, Universidad del Cauca, Popayan, Colombia
Manuscript submitted January 13, 2022, accepted January 20, 2022, published online January 29, 2022
Short title: Basal Insulin in Type 2 Diabetes
doi: https://doi.org/10.14740/jocmr4660
- Abstract
- Introduction
- Methods
- Normal Glucose Homeostasis
- Normal Insulin Physiology
- Why, When, and How to Initiate?
- Basic Concepts About BIs
- Efficacy and Safety of BI
- Discussion
- Conclusion
- References
| Abstract | ▴Top |
Treatment with basal insulins is a fundamental part of management in many patients with type 2 diabetes mellitus. Multiple management schemes may be indicated in these individuals, for example, the use of oral antihyperglycemic agents with basal insulins (basal-supported oral therapy) or the combinations of basal insulins with glucagon-like peptide-1 receptor agonists; each of these strategies makes it easier to achieve glycemic control goals. A basic knowledge of the physiology, pharmacodynamic and pharmacokinetic aspects of the different basal insulins is essential to achieve treatment goals and compliance. This review addresses the principles of management with basal insulins.
Keywords: Diabetes; Insulin; NPH; Detemir; Glargine; Degludec
| Introduction | ▴Top |
Type 2 diabetes mellitus (T2DM) is a condition of a polygenic nature and variable penetrance, in which a series of alterations in the metabolism of macronutrients (lipids, carbohydrates, and proteins) coexist. This leads to a state of chronic hyperglycemia and a significant increase in the risk of micro- and macrovascular outcomes [1-3].
Multiple genetic, environmental, and epigenetic interactions have been described in T2DM along with various pathophysiological mechanisms, predominantly insulin resistance and the progressive decrease in insulin secretory capacity by pancreatic β cells. This last mechanism is the one that ultimately determines the need for insulin management [4, 5]. One of the strategies to achieve metabolic control in T2DM when management with oral antihyperglycemic drugs (OADs) and other parenteral treatments, such as glucagon-like peptide-1 receptor agonists (GLP-1RAs), is not sufficient is the use of insulin, especially basal insulins (BIs) [6].
This review describes the basic principles of management with BIs, from the most common pathophysiological aspects to the current evidence from randomized clinical trials (RCTs) with next-generation insulins.
| Methods | ▴Top |
A detailed search was carried out in the following databases: PubMed/MEDLINE, EMBASE, Scopus, BIOSIS, ClinicalTrials.gov, Web of Science, and Cochrane Library. This search was conducted for articles published from January 1 (2001) to August 31 (2021), using the terms “insulin”, “NPH”, “Glargine-100”, “Glargine-300”, “Degludec-100”, “Degludec-200”, “Detemir”, and “type 2 diabetes”. Other definitions of diabetes or other associated conditions (chronic kidney disease, elderly, intercurrent illnesses, or situations requiring temporary insulin management) were not taken into account in this review. Only RCTs that evaluated the efficacy and safety of BIs (and were exclusively in the English language) were taken into account.
| Normal Glucose Homeostasis | ▴Top |
Typically, a person is considered to be fasting when 8 - 12 h has elapsed without food intake. Metabolic changes occur after the absorption of a meal (generally after 3 - 5 h); this state is also known as the “postabsorptive state”, as opposed to the “postprandial” (or digestion in progress) state [7].
During the fasting state, the oxidation of free fatty acids (FFAs) contributes to a greater energy expenditure than the oxidation of some macronutrients (such as carbohydrates). This aspect is considered to be caused by a greater availability of lipids and carbohydrates since the concentrations of unesterified FFA at the plasma level increase in response to a lower concentration of insulin and high levels of counter-regulatory hormones (such as glucagon) [8].
After its absorption, the concentration of plasma glucose (PG) from the physiological point of view is established to be between 70 and 110 mg/dL. This value remains relatively stable thanks to the regulatory effects of glucagon and insulin. Under normal conditions, of the total glucose that can potentially be used, 50% use occurs in the brain, 25% at the splanchnic level, and 25% in insulin-dependent tissues (such as muscle and adipose tissue) [9].
Likewise, in the postprandial state, an increase in both PG concentration and insulin secretion is documented. The latter aspect is translated into a suppression of gluconeogenesis and an increase in the rate of glucose elimination (at the hepatic and posthepatic level) (Fig. 1) [10].
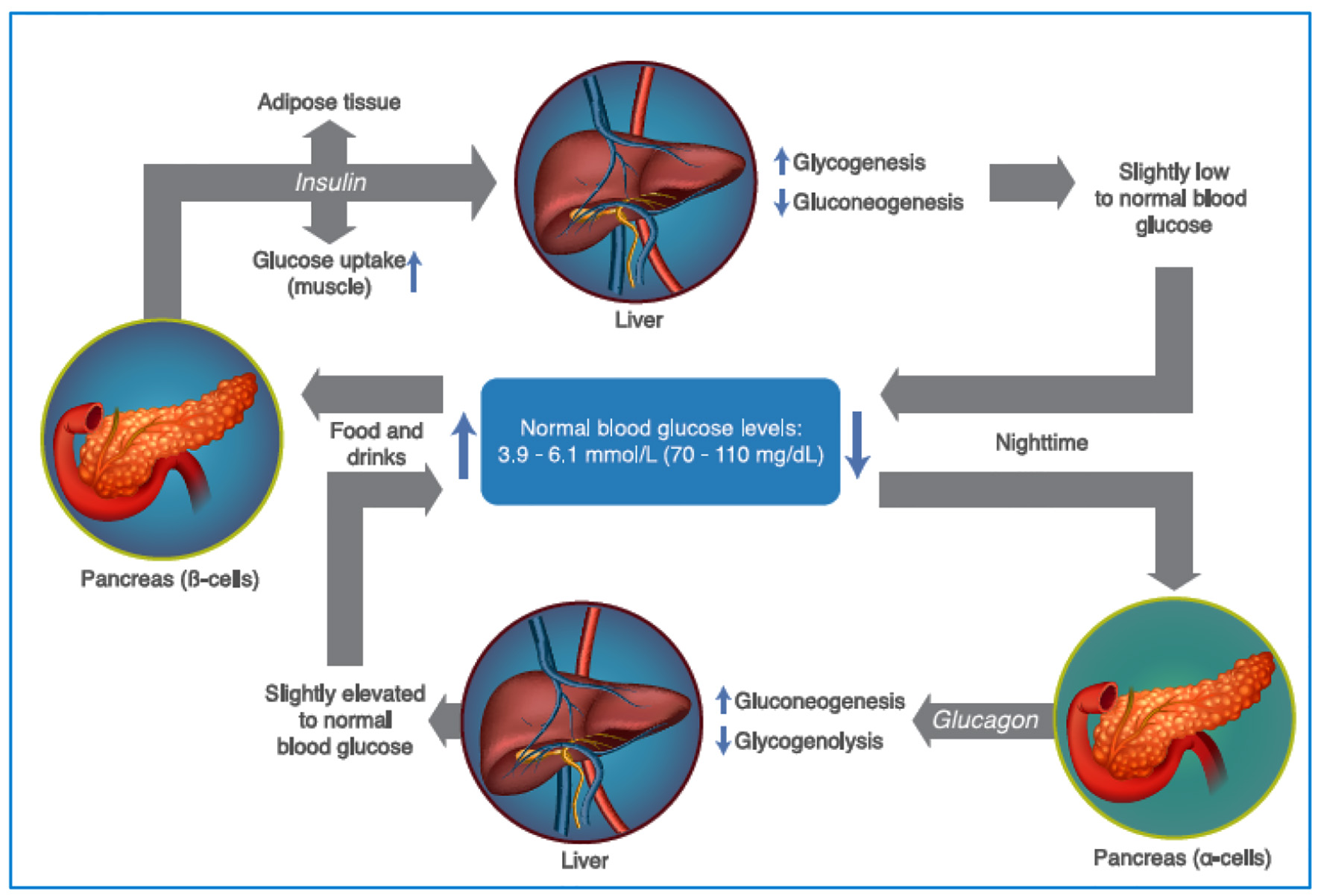 Click for large image | Figure 1. Maintenance of plasma glucose levels, through the regulation of insulin and glucagon. As glycemic levels drop, the pancreatic α cells secrete glucagon, increasing gluconeogenesis and glycogenolysis, increasing the levels of glycemia. After food intake, the levels of glycemia rise promoting insulin release (by the pancreatic β cells) which increase the glucose uptake in the muscle and adipose tissue, in addition to promoting glycogenogenesis and reducing gluconeogenesis (see text for additional details). Source: author’s elaboration. |
| Normal Insulin Physiology | ▴Top |
Insulin is synthesized and secreted from β cells and consists of two polypeptide chains, chain A (with 21 amino acids) and chain B (with 30 amino acids), linked by disulfide bridges [11].
Insulin synthesis depends on a precursor (preproinsulin), cleaved off and converted to proinsulin. Next, proinsulin is packaged in the Golgi apparatus (of the β cell) to be later converted (enzymatically) into insulin and connecting peptide (C-peptide) [12, 13].
In the Golgi apparatus, proinsulin enters the secretory and storage vesicles (rich in zinc and calcium (Ca2+)). Once inside the vesicles, hexamer structures develop from proinsulin, with two zinc atoms per proinsulin hexamer, which subsequently become the insulin hexamer (producing C-peptide as well), leaving the C-terminal and N-terminal free. Insulin is then synthetized and stored as a hexamer, but its active form is that of a monomeric hormone [13].
In healthy individuals, the concentration of PG fluctuates and is affected by factors such as nutritional intake, physical activity, hormone interactions, and macromolecules; however, the PG remains within a narrow range of values (63 - 126 mg/dL) [14]. In a normal physiological setting, circulating insulin levels are closely related to tissue insulin sensitivity (which is defined as the ability to remove glucose and suppress hepatic gluconeogenesis in response to insulin) [14, 15]. The insulin secretion rate is regulated according to the concentration of PG; this secretion increases as PG levels rise above 60 mg/dL. About 50% of the total daily insulin secretion occurs during the basal periods [16].
Circulating PG is taken up by the facilitating glucose transporter isoform 2 (GLUT2) (solute carrier family 2 member 2 (SLC2A2)). When glucose enters the cell (through GLUT2), it undergoes a phosphorylation process (mediated by glucokinase), and glucose-6-phosphate is generated, which, through glycolysis, forms pyruvate. Pyruvate then enters the Krebs cycle to produce nicotinamide adenine dinucleotide and flavin adenine dinucleotide, which are oxidatively metabolized to produce adenosine triphosphate (ATP). This production induces an increase in the ATP/adenosine diphosphate ratio, which leads to the closure of potassium channels (K+) sensitive to ATP (KATP) [17, 18].
After the closing of the channels, there is a decrease in the magnitude of the K+ current (outward), inducing the depolarization of the cell membrane, with the respective opening of the voltage-dependent Ca2+ channels. This cytosolic increase of the Ca2+ levels generates exocytosis of insulin granules [19].
The fact that KATP channels explain glucose’s metabolic and electrophysiological effects also allowed us to identify that these channels are the cellular target of sulfonylureas (SUs). The ability of these drugs to induce the closure of KATP channels explains their usefulness and efficacy in the oral management of individuals with T2DM. The β-cell KATP channel is a heterotetramer that is made up of four K+ channel subunits (Kir6.2) and four SU receptor subunits (SUR1) (Fig. 2) [20, 21].
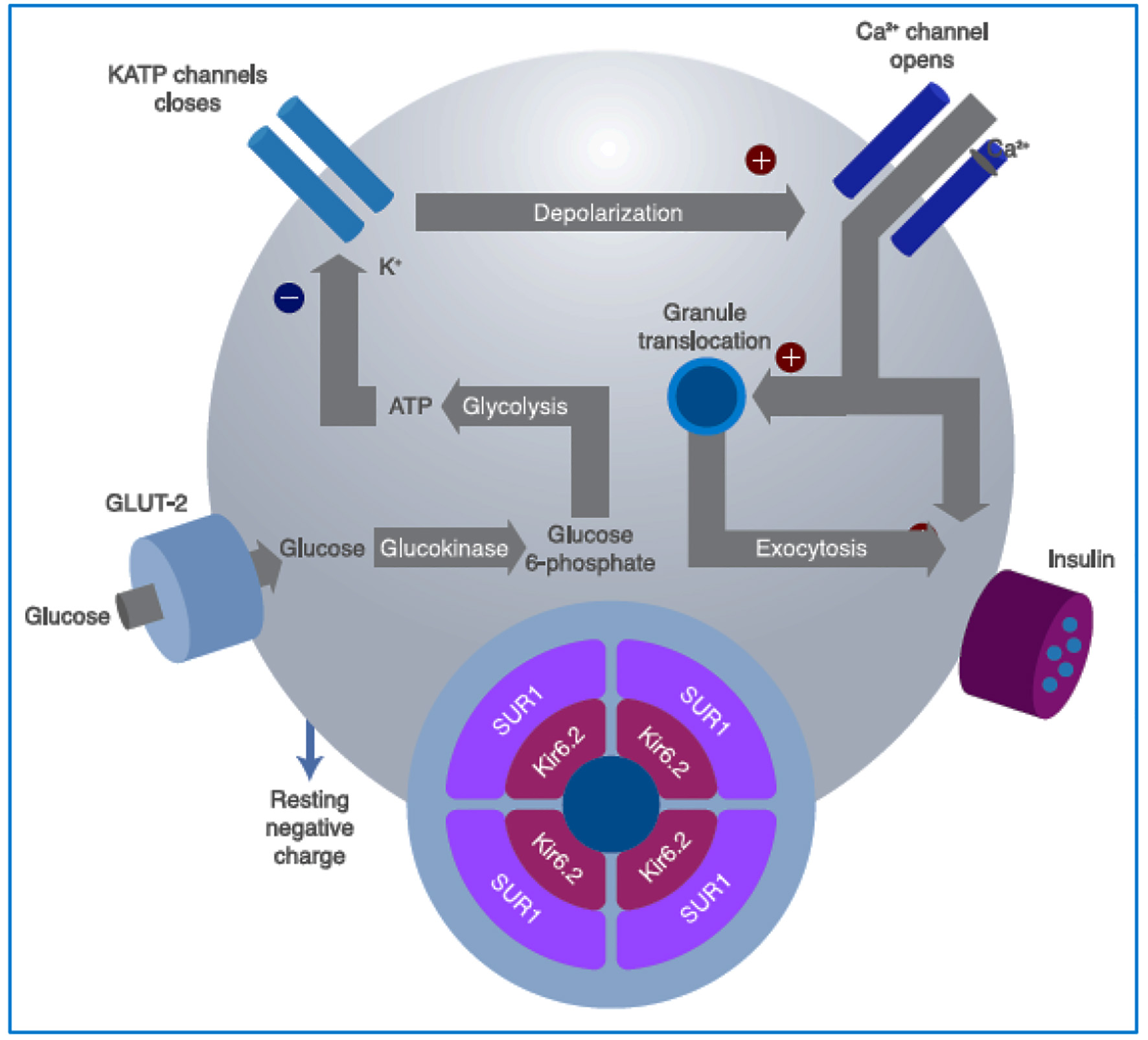 Click for large image | Figure 2. Intracellular mechanisms through which glucose stimulates insulin secretion. Glucose is metabolized inside the β cell for ATP production, closing the ATP-sensitive K+ channels on the cell membrane. This prevents the K+ ions from exiting the cell, causing membrane depolarization, which also leads to opening of the voltage-dependent Ca2+ channels on the membrane, allowing for the entrance of Ca2+ into the cell, increasing its cytosol concentration, and finally inducing granule exocytosis. Sulfonylureas bind to the SUR1 component of the KATP channel (see text for further details). GLUT: glucose transporter; SU: sulfonylureas; SUR1: sulfonylurea receptor subunits; ATP: adenosine triphosphate. Source: author’s elaboration. |
After eating a meal, there is a rapid and large release of insulin from the β cells (known as the first phase of insulin secretion). In this phase, the peripheral uptake of other nutrients is promoted (in addition to the suppression of hepatic gluconeogenesis). Finally, the possibility of significant increases in postprandial blood glucose levels is decreased [22].
Meanwhile, the second phase of insulin secretion (after eating a meal) is established more slowly and continuously and is sustained until the blood glucose level returns to normal (unlike the first phase, wherein insulin secretion occurs quickly and in the short term). Additionally, the second phase has the characteristic of being independent of the extracellular glucose level (Fig. 3) [23].
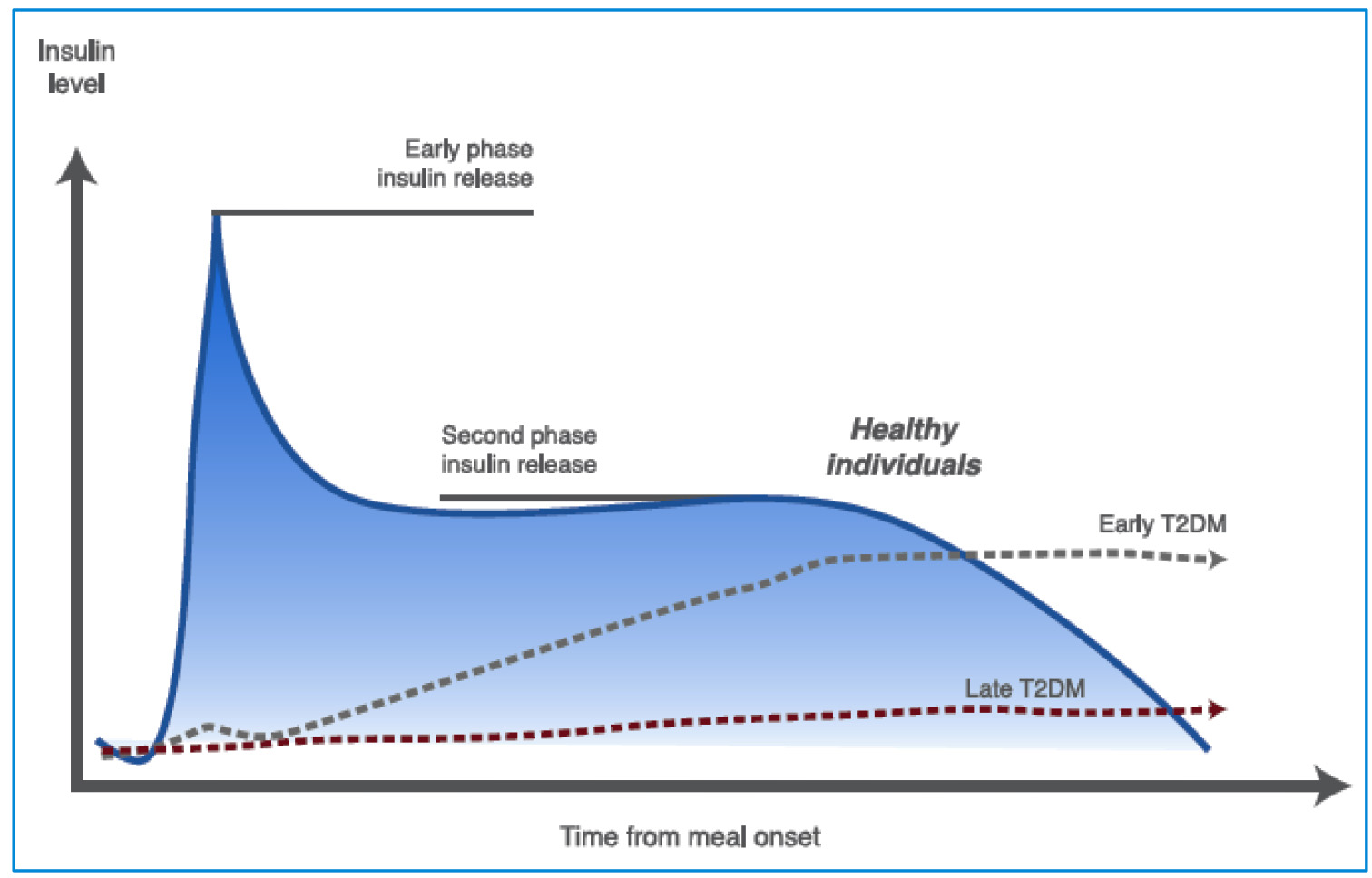 Click for large image | Figure 3. Insulin secretion phases in healthy and T2DM individuals. In early phase insulin release the β cells produce insulin in response to rising glucose levels. Proinsulin (the precursor molecule) is cleaved into C-peptide and insulin. The “early phase insulin release” occurs within 2 min of glucose arriving in the blood stream and continues for about 10 - 15 min. This phase prevents post-prandial hyperglycemia. A second phase of insulin release continues until blood glucose levels return to normal (see text for further details). T2DM: type 2 diabetes mellitus. Source: author’s elaboration. |
Paradoxically, a significant number of individuals with prediabetes (impaired fasting glucose, impaired glucose tolerance, or both) may be hyperinsulinemic. However, insulin secretion has been shown to decrease significantly before the diagnosis of diabetes is established. In fact, a PG value ≥ 198 mg/dL (after an oral glucose tolerance test) indicates that the insulin secretory capacity is reduced in relation to insulin resistance and hyperglycemia concomitant. Such “insulinopenia” is one of the defining characteristics of the onset of T2DM [23, 24].
| Why, When, and How to Initiate? | ▴Top |
Why initiate
The development of injectable insulin therapies for T2DM presents unique challenges not evident in other classes of antidiabetic therapies. Insulin is the only antidiabetic therapy available for which there is no maximal dose for efficacy. However, achieving the therapeutic goals is countered by the risk and the fear of hypoglycemia, and both serve as barriers to tight glycemic control with the most effective antidiabetic agent available. This risk, alongside weight gain and the subcutaneous route of administration, often leads to insulin being relegated to a last-option therapy [25].
When to initiate
In the clinical setting, it is sometimes difficult to define the right moment when BIs management should be considered in individuals with T2DM (who do not achieve metabolic control); different international organizations make different recommendations in this regard (Table 1) [26-28]. Likewise, it is clear that the higher the A1c, the stronger the requirement for BIs management. Hence, it has been shown that when the A1c is < 7.5%, the primary determinant of this value is postprandial glycemia, and when the A1c is > 8.5%, the primary determinant is fasting glucose levels (Fig. 4) [29]. This concept is the foundation to considering the use of BI in patients with very high A1c levels. Therefore, the higher the A1c, the stronger the indication for BI use since its use is intended to reduce hepatic glucose production (the primary source of increased fasting blood sugar) and limit nocturnal and interprandial hyperglycemia [29, 30].
 Click to view | Table 1. Recommendations for Considering the Initiation of BI Therapy in T2DM |
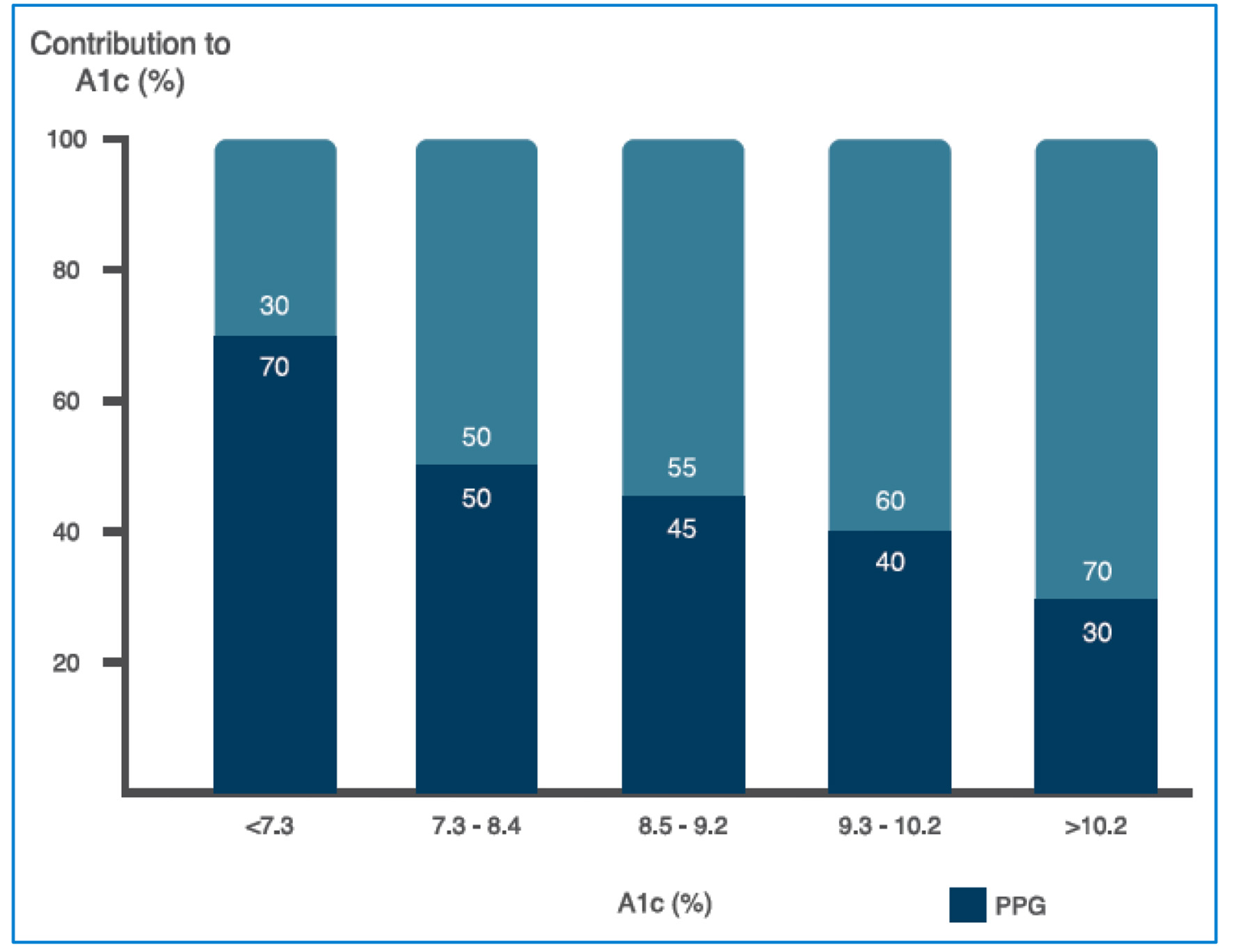 Click for large image | Figure 4. Relative contribution of FPG and PPG across a broad range of A1c levels (see text for further details). FPG: fasting plasma glucosa, PPG: postprandial plasma glucose. Source: adapted from Ref. [29]. |
This “balance” between fasting plasma glucose (FPG), postprandial glycemia (PPG), and A1c level is essential when considering the use or the requirement for BI and the use of drugs with a postprandial effect (GLP-1RA).
How to initiate
There are several ways to calculate the starting dose of the BIs. One such method can be dividing the average value of the PG by 18; another way is dividing an individual’s body weight by 10. However, in RCTs, it is usually started with a dose of 0.1 - 0.2 U/kg/day (or with a dose of 10 U/day). Regardless of the initial insulinization strategy, what is truly important is to guarantee an adequate and permanent titration (Table 2) [30-32].
 Click to view | Table 2. General Recommendations for Starting the BI Dose and for Adjusting When Switching From One Insulin to Another [30-32] |
Moreover, there has always been a debate around establishing a limit or “ceiling” of the BI dose. It has been said that when a patient has reached the “ceiling” dose for BI of 0.5 U/kg/day and has not been able to reach the target metabolic control, insisting on increasing the BI dose increases the risk of hypoglycemia, with a significant body weight increase (and little impact on A1c) [31, 32].
Therefore, in the case of a patient who titrated the BI dose up to 0.5 U/kg/day, failing to accomplish the pre-established goals, the recommendation is to intensify treatment either with a basal-plus or basal-bolus regimen or with premixed insulins or a regimen with BI + GLP-1RA (though intensification is also possible with OADs; for example, inter alia, sodium glucose cotransporter-2 (SGLT2) inhibitors and dipeptidyl peptidase-4 (DPP-4) inhibitors) [26].
| Basic Concepts About BIs | ▴Top |
By definition, the duration of the effect over time of a BI should be prolonged, which is why, when classifying the different insulins with this characteristic, they are summarized as the following: neutral protamine Hagedorn (NPH), detemir (Det), and glargine-100 (Glar-100) and glargine-300 (Glar-300), respectively, and degludec-100 (Deg-100) and degludec-200 (Deg-200), respectively. Among these, NPH insulin is considered an intermediate action BI (in relation to its effect in the time). The initial objective of BIs management is to normalize the FPG and, according to evolution and metabolic control, to focus later on PPG control [33, 34].
In clinical practice, a formula that evaluates the difference between PPG and FPG (divided by the value of FPG) may be useful. The result of this calculation ((PPG - FPG)/FPG) helps to establish whether the predominant origin of hyperglycemia in an individual is due to the PPG or the FPG. Thus, the higher the value (of this calculation), the greater the influence of the PPG; on the contrary, with a lower said result, it can be concluded that the resulting hyperglycemia is predominantly due to FPG. The importance of making this calculation is that, in the first case, the use of rapid-acting insulin analogs (lispro, aspart, or glulisine), regular insulin, or insulin premixes may be the first management option. In the second case, the main option would be the use of BIs. It may also be useful to evaluate the relationship between FPG and A1c since, with a result ≥ 1.3, it would indicate that the main component of hyperglycemia is fasting glucose [35].
From the moment they are absorbed and reach the bloodstream, all BIs have the same mechanism of action on the tissues in which they act. The challenge in the generation of new BIs is to develop strategies that delay the absorption of the molecule, prolonging its half-life and causing a lower frequency of “peaks” and “valleys”. For example, available BIs have a longer half-life thanks to a variety of mechanisms such as, inter alia, the addition of protamine or albumin and pH-dependent precipitation, causing differences in their behavior once they are found in the subcutaneous tissue, which modifies its rate of absorption (thus called “insulin protraction”) [36, 37].
NPH
NPH insulin was discovered in 1936. However, it was in 1946 that the first “isophane NPH” insulin became commercially available, combining insulin and protamine in stoichiometric amounts at a neutral pH (when it was shown that its effect by the subcutaneous route could be prolonged to the additional protamine). In this way, the insulin remained in “suspension” in the subcutaneous tissue, delaying the absorption time, thus prolonging its half-life [38].
The onset of action of NPH is approximately 2 h, and its maximum effect and duration are 6 - 14 and 10 - 16 h, respectively. These characteristics have led to classifying NPH as an “intermediate” acting insulin. Therefore, if it is administered at bedtime, it can be considered a BI, while if it is administered in the morning, it can be considered an insulin basal/prandial. In general terms, the usual range of NPH doses is between 10 and 80 U, and it is considered that when it is used in low doses (i.e., 10 U/day), it behaves with a lower rate of “peaks” and with a shorter half-life, while in high doses a higher rate of “peaks” with a longer half-life is observed [39, 40].
Det
Det insulin is a long-acting analog, which differs from human insulin in that an amino acid has been omitted at position B30 and a fatty acid chain (C14) has been added to amino acid B29. Additionally, and to adjust the pH (Det has a pH of 7.4), substances such as sodium hydroxide or hydrochloric acid can be added. When used in low doses, the half-life of Det is close to 6 h, while in high doses, it can be as long as 24 h. Det has a slower and longer absorption than NPH, its maximum serum concentration (Cmax) is achieved 6 - 8 h after its application, and it has a bioavailability of close to 60%. Its main binding protein (once it reaches the circulation) is albumin [40-43].
Deg
Deg insulin is a long-acting analog and differs from human insulin in that the amino acid threonine has been omitted at position B30 and a side chain with glutamic acid and a C16 fatty acid has been added. This insulin forms soluble and stable dihexamers when phenol and zinc are added. Therefore, when phenol is injected into the subcutaneous tissue, it diffuses, causing the creation of a more soluble deposit (in the form of multihexamers). For its part, zinc undergoes a slower diffusion, allowing its release to be more gradual, continuous, and prolonged from the application site. These characteristics allow its half-life to be greater than 24 h, with a durability of around 40 h [44, 45].
Deg reaches its steady state after administration for 3 - 4 days. In this state, Deg-100 and Deg-200 behave similarly (when supplied in the same dose in U/kg). Therefore, concentrating the molecule (Deg-200) has not been shown to affect the formation of multihexamers or their absorption from the subcutaneous tissue (when compared to Deg-100) [46, 47].
In general, the elimination t½ is considered the time it takes for the amount of insulin in the blood to drop by half. Therefore, from the moment the maximum concentration of insulin in the circulation is reached, at least four half-lives of insulin elimination must elapse to achieve an almost complete elimination (which explains why it requires 4 - 5 half-lives to achieve its steady state). This concept is inherent to all BIs [48].
Glar-100 and Glar-300
Glar insulin is a long-acting analog in which two arginine molecules have been added to chain B (in the C-terminal region), and the amino acid arginine has been replaced by glycine in chain A (in position 21). These modifications cause a change in the isoelectric point of the pH, from 5.4 to 6.7, which causes the insulin to be less soluble in the subcutaneous tissue, thus producing microprecipitates. Additionally, zinc is added (so that it crystallizes in the subcutaneous tissue), and its absorption is further delayed [49].
The action of Glar begins 1 - 2 h after its application, maintaining a constant concentration profile in plasma (peakless), which is sustained for 20 - 24 h. Two presentations are commercially available: Glar-100 (100 U/mL) and Glar-300 (300 U/mL). Glar is metabolized into two active metabolites: M1 and M2 (21A-Gly-insulin and 21A-Gly-des-30B-Thr-insulin, respectively) [50].
The main circulating metabolite of Glar-100 is M1, which increases according to the dose supplied. At the pharmacokinetic (PK) and pharmacodynamic (PD) levels, it has been found that the effects after the application of Glar-100 are mainly based on the metabolite M1. For its part, the M2 metabolite is not detectable in most individuals exposed to Glar-100. However, when detected, its concentration is independent of the administered dose of insulin. With Glar-100, the steady state is reached 2 - 4 days after the first dose (once a day) [51, 52].
For its part, the Glar-300 is characterized by being more concentrated than the Glar-100, with a PK profile where its effect is more prolonged and “flat” (compared to Glar-100). This allows its steady state to be reached in over 5 days, with less “variability” among those who receive it [52, 53]. The PK and PD characteristics of the BI are summarized in Table 3 [34-37].
 Click to view | Table 3. PK and PD Characteristics of BIs [34-37] |
| Efficacy and Safety of BI | ▴Top |
In general, the PK and PD characteristics of the long-acting BIs (Det, Glar-100, Glar-300, Deg-100, and Deg-200) allow the widely known pattern of insulin secretion (from β cell) to be imitated. Clinically, it resulted in lower rates of hypoglycemia, with a similar efficacy (i.e., Det vs. NPH, and Glar-100 vs. NPH) (Fig. 5) [40, 43, 52, 54, 55]. Likewise, no RCTs have compared the efficacy of Deg-100, Deg-200, or Glar-300 with NPH. Whereas, head-to-head studies among long-acting BIs have found that Det and Glar-100 have similar efficacy and safety profiles (relative to the risk of hypoglycemia) (Table 4) [40-43, 56]. Similarly, Deg-100 and Glar-100 denote similar efficacy, with a lower risk of hypoglycemia (nocturnal) with Deg-100 [57]. Additionally, the RCTs that have compared Glar-300 with Glar-100 also show that the efficacy is similar, but the risk of overall (anytime) and nocturnal hypoglycemia is lower with Glar-300 (Fig. 6) [57-59]. Moreover, the studies evaluating the efficacy and safety of the more recently approved long-acting BIs (Deg-100, Deg-200, and Glar-300) found that the efficacy of Glar-300 vs. Deg-100 is similar, with a profile safety in favor of Glar-300 on the risk of hypoglycemia (demonstrated only in the insulin titration period, defined between week 0 and 12). Finally, no differences were found in the efficacy or the risk of hypoglycemia when comparing Glar-300 and Deg-200 (Fig. 7) [58, 60-62].
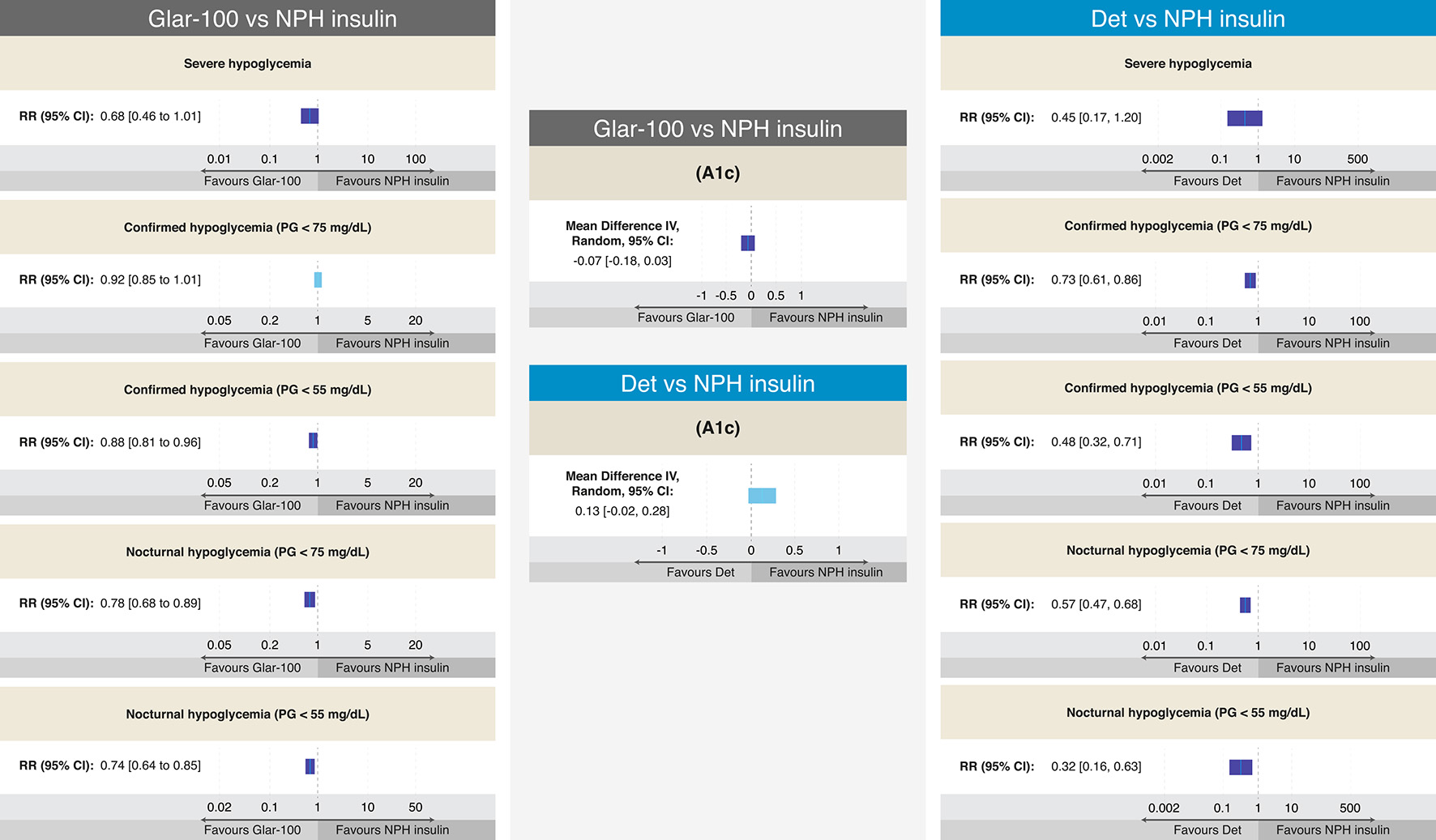 Click for large image | Figure 5. Efficacy and results on different definitions of hypoglycemia from RCTs comparing Glar-100 vs. NPH and Det vs. NPH in T2DM. The figure summarizes the different results of the RCTs that evaluated the safety and efficacy of Glar-100 vs. NPH (data extracted and adapted from Refs. [54] and [55]) and Det vs. NPH (data extracted and adapted from Refs. [40], [43], and [55]). Studies comparing Glar-100 vs. NPH showed differences favoring Glar-100 in three aspects: confirmed hypoglycemia (PG < 55 mg/dL), nocturnal hypoglycemia (PG < 75 mg/dL) and nocturnal hypoglycemia (PG < 55 mg/dL). On the other hand, the studies that compared Det vs. NPH showed differences, favoring Det in four aspects: confirmed hypoglycemia (PG < 75 mg/dL and < 55 mg/dL), nocturnal hypoglycemia (PG < 75 mg/dL and < 55 mg/dL). No differences were found in the A1c value between Glar-100 vs. NPH or between Det vs. NPH. CI: confidence interval; Det: detemir; Glar: glargine; NPH: neutral protamine Hagedorn; PG: plasma glucosa; RCTs: randomized clinical trials; RR: risk ratio; T2DM: type 2 diabetes mellitus. Source: author’s elaboration. |
 Click to view | Table 4. Efficacy and Risk of Hypoglycemia From RCTs Comparing Det vs. Glar-100 in T2DM [40-43] |
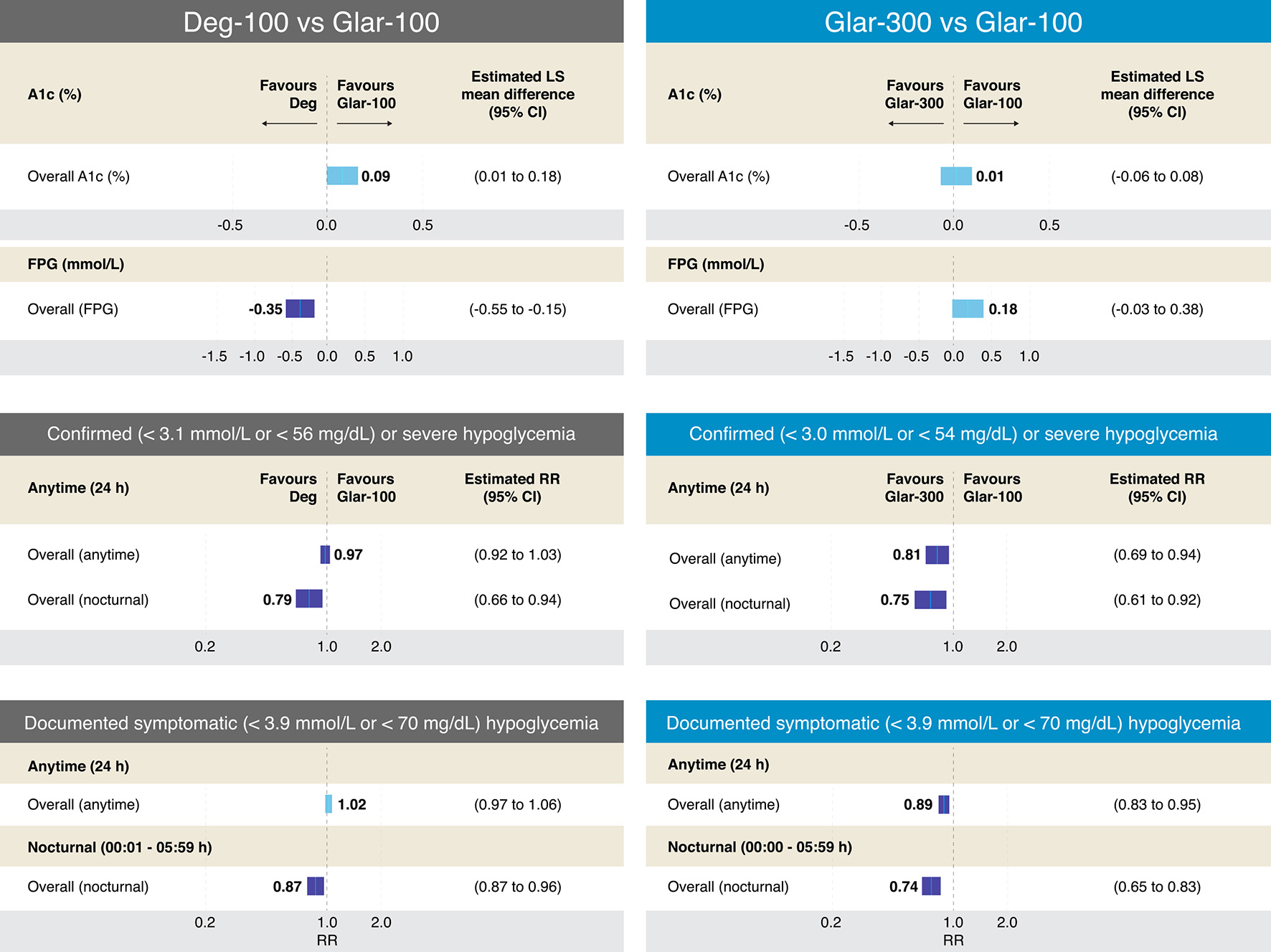 Click for large image | Figure 6. Efficacy and results on different definitions of hypoglycemia from RCTs comparing Deg-100 vs. Glar-100 and Glar-300 vs. Glar-100 in T2DM. The figure summarizes the different results of the RCTs that evaluated the safety and efficacy of Deg-100 vs. Glar-100 (data extracted and adapted from Refs. [57] and [58]) and Glar-300 vs. Glar-100 (data extracted and adapted from Refs. [58] and [59]). The studies that compared Deg-100 vs. Glar-100, showed differences in favor of Glar-100 on the A1c value, while the FPG value was significantly reduced with Deg-100. Differences in favor of Deg-100 were also found in two aspects: confirmed or severe hypoglycemia and documented symptomatic (overall and nocturnal (PG < 56 mg/dL and < 70 mg/dL, respectively). On the other hand, the studies that compared Glar-300 vs. Glar-100 found no differences in the A1c value; however, differences were found in favor of Glar-300 in four aspects: confirmed severe hypoglycemia (< 54 mg/dL, overall (anytime) and overall (nocturnal)), and in documented symptomatic hypoglycemia (< 70 mg/dL, overall (anytime) and overall (nocturnal)). CI: confidence interval; Deg: degludec; FPG: fasting plasma glucosa; Glar: glargine; PG: plasma glucosa; RCTs: randomized clinical trials; RR: risk ratio; T2DM: type 2 diabetes mellitus. Source: author’s elaboration. |
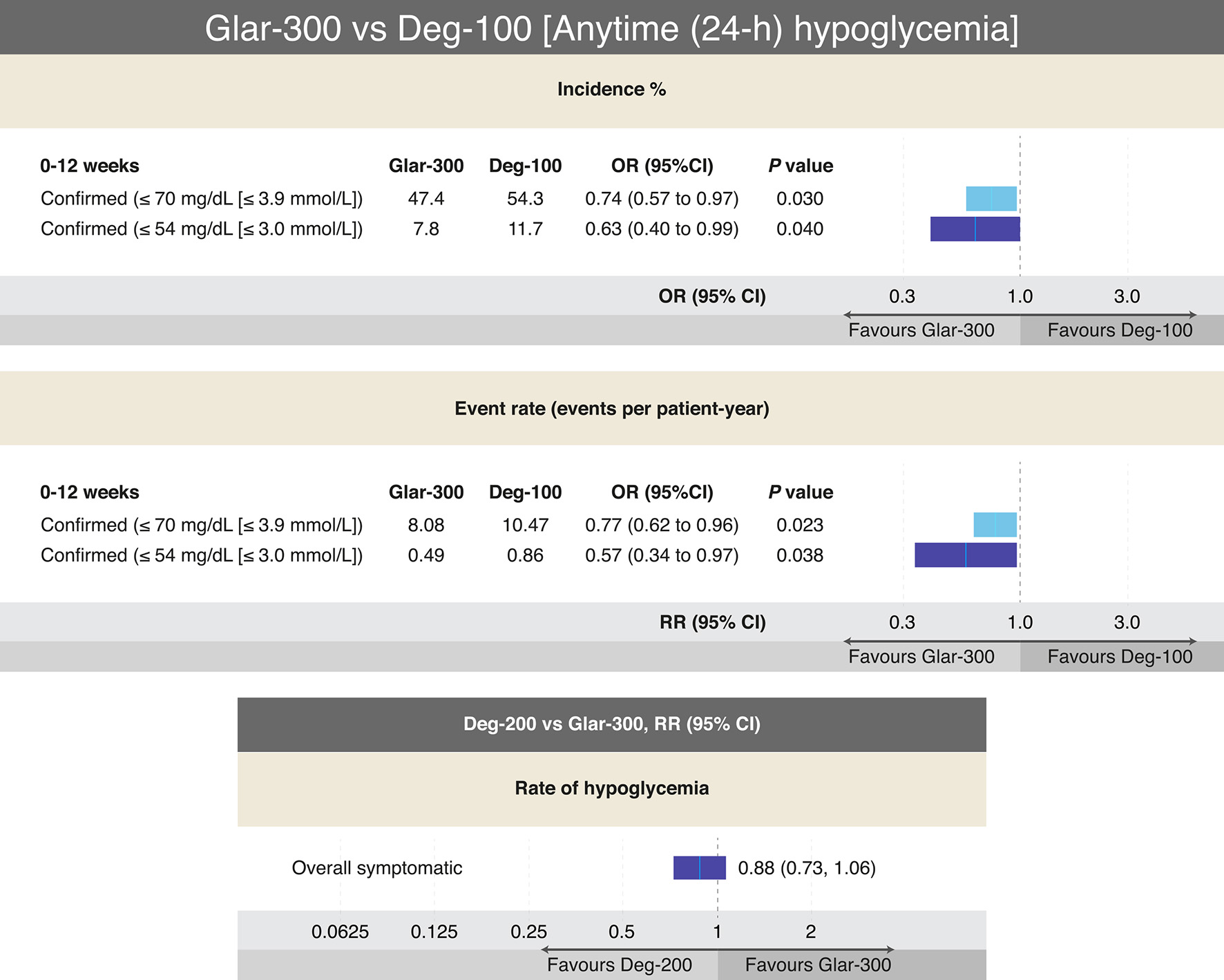 Click for large image | Figure 7. Outcomes on different definitions of hypoglycemia from RCTs comparing Glar-300 to Deg-100 and Deg-200 to Glar-300 in T2DM. The only head-to-head study that has compared Glar-300 vs. Deg-100, showed differences favoring Glar-300 on the risk of confirmed hypoglycemia (≤ 70 mg/dL and ≤ 54 mg/dL) only in the insulin titration period (0 - 12 weeks); data extracted and adapted from Refs. [58] and [60]. On the other hand, the only head-to-head study that compared Deg-200 vs. Glar-300 found no difference in the risk of overall hypoglycemia (data extracted and modified from Refs. [61] and [62]). In both studies no differences were found on the A1c value. CI: confidence interval; Deg: degludec; Glar: glargine; OR: odds ratio; RR: risk ratio; T2DM: type 2 diabetes mellitus. Source: author’s elaboration. |
On the other hand, studies have shown that, in relation to weight, Det showed less weight gain than NPH and Glar-100. Likewise, a lower weight gain was documented with Glar-300 than Glar-100 (Table 5) [38-40, 56-63]. Moreover, when evaluating the average doses of BI, it was found that with the use of Deg-100, a lower dose was required (compared to Glar-100), while with Glar-300, a higher average dose was required (compared to Glar-100) [58, 59].
 Click to view | Table 5. Effects on Body Weight and Differences in the Average Dose of the Different BIs in T2DM |
| Discussion | ▴Top |
Historically, insulin treatment has been considered a third or fourth management option in individuals with T2DM by a significant number of clinicians (and most patients). The concept is that the use of insulin should only be considered when all other nonparenteral options have been exhausted, despite the different recommendations of numerous international organizations, management guides, and expert committees [62].
However, it must be taken into account that many patients with T2DM can be managed with OADs together with BIs (basal-supported oral therapy (BOT)); the availability of drugs with different mechanisms of action (DPP-4 inhibitors, metformin, SGLT2 inhibitors, SU, thiazolidinediones (TZDs), inter alia) does not contraindicate the use of BOTs; however, it must be taken into account that the combination of SU with BIs increases the risk of weight gain and hypoglycemia; moreover, the use of TZDs with BIs can also induce significant weight gain. Therefore, any BOT scheme must be individualized, always establishing the risks (weight gain and a higher rate of hypoglycemia) and the potential benefits, since management with BIs could help to suspend OADs in compromised individuals of renal and/or hepatic function, or in those receiving multiple OADs or in situations where these drugs are considered to have progressively lost their efficacy (such as SUs) or where it is considered that there is a risk of other outcomes, for example, heart failure (for TZDs) [26-28].
On the other hand, the use of BIs with GLP-1RAs is a useful and effective management strategy. Potentially, all GLP-1RAs can be combined with BIs, this combination has shown greater efficacy in the control of A1c, FPG and PPG (in relation to the exclusive use with BI or with GLP-1RAs, in individuals with T2DM receiving management with OADs); it is currently considered that any individual with T2DM in treatment with OADs with A1c levels > 10.0% (or with levels that exceed the value of the A1c two percentage points above the “individual” goal) can benefit from the combined treatment BIs + GLP-1RA [26, 64].
Two titratable, fixed-ratio combination (FRC) therapies are currently available, Glar-100/lixisenatide (GlarLixi) and Deg-100/liraglutide (DegLira); both combinations have shown greater efficacy in metabolic control (A1c, FPG, and GPP) with a lower risk of hypoglycemia, less weight gain, and a lower dose of insulin (when compared to either of the two BIs in monotherapy). A lower risk of gastrointestinal adverse events (when compared to either of the two GLP-1RAs) has also been documented. It should be taken into account that, when an individual has the indication for the combination BI + GLP-1RAs, if one of the objectives of management is to reduce the risk of cardiovascular events, then it is recommended to use each one of the components separately, since for the available presentation (DegLira) the only way for the patient to receive the dose of Lira that has been shown to reduce the risk of cardiovascular outcomes (1.8 mg), is supplying at the same time a dose of Deg of 50 U [64, 65].
Despite the benefits of early initiation of insulin on metabolic control, the preservation of β cells and the potential benefit of reducing vascular complications are clear. There are still doubts and misconceptions surrounding the initiation and intensification of insulin management, and among these are the patient’s fear of using needles, the time required for the application of insulin, social stigmatization, risk of hypoglycemia, impact on weight gain, and cost of modern insulins [66].
Although each of these conditions could eventually be understood and justified from the perception of the patient, the treating physician may not believe that to be the case. Ultimately, all of these factors translate into therapeutic inertia, poor metabolic control, and an unacceptably high risk of microvascular, macrovascular, and mortality outcomes [67, 68].
In this review, it has been described that the efficacy of all of the insulins available and considered as BI have a similar efficacy. The differences found between long-acting analog insulins and NPH insulin are based on the former showing a profile of improved safety compared to NPH. With a lower risk of hypoglycemia (as well as the advantage of being able to be applied once a day), these aspects clearly explain why long-acting BIs are preferred in the clinical setting. However, in places where the cost and access to long-acting analog insulins make their use difficult, NPH continues to be valid and useful [69]. In these cases, the benefit outweighs the potential risk of hypoglycemia from NPH.
In those clinical scenarios where access to all BIs is not an inconvenience (as is the cost), the decision to choose one or the other insulin should be based on aspects such as the lower risk of hypoglycemia, lower increase in weight, and lower frequency of applications throughout the day. In accordance with the above, BIs such as Glar-100, Glar-300, Deg-100, and Deg-200 should be preferred in relation to Det.
Likewise, the lower risk of hypoglycemia found with insulins such as Deg-100 and Glar-300 makes their use more likely to be preferred (in relation to Glar-100). Moreover, the lower increase in weight documented with Glar-300 is an additional benefit. In this sense, it can be argued that in cases where an individual under treatment with Glar-100 has an unacceptably high frequency of hypoglycemia or where a therapeutic objective is weight reduction (or at least no weight gain), it is reasonable to consider a switch to insulins such as Deg-100 or Glar-300 (with greater evidence of benefit for body weight than Glar-100) [70, 71].
On the other hand, in insulin-naive individuals, who require the initiation of BIs, the use of Glar-300, Deg-100, and Deg-200 is perfectly valid. However, when there is a scenario in which the patient has a higher risk of hypoglycemia a priori (mainly in the insulin titration period), the use of Glar-300 could be preferred over Deg-100. However, this has not been shown to result in higher adherence, lower risk of hypoglycemia, or better long-term metabolic control.
Finally, the improvement and advances in the development and generation of long-acting BIs should provide greater peace of mind and safety for both the treating physician and the patient, and this should be reflected (at least a priori) in less therapeutic inertia, in fewer doubts and fears in insulin management in individuals with T2DM.
| Conclusion | ▴Top |
In the clinic, it is not always easy to decide the ideal moment to start BIs management in individuals with T2DM. However, when making this decision, some key aspects must always be considered, such as, inter alia, metabolic control (FPG, PPG, and A1c), risk of hypoglycemia, effect on body weight, and frequency of application. In this sense, long-acting BIs have some advantages over NPH and Det insulin. Likewise, along with inducing a lower risk of hypoglycemia and less weight gain than Glar-100, Glar-300 also has an advantage over Deg-100 in that it produces lower risk of hypoglycemia (specifically in the insulin titration period). Finally, between Glar-300 and Deg-200, there are more similarities in efficacy and safety than differences between both.
Acknowledgments
None to declare.
Financial Disclosure
The author declares that he has not received any economic or financial incentive when making this manuscript.
Conflict of Interest
Hernando Vargas-Uricoechea has received honoraria from Sanofi and Novo Nordisk as speaker and on advisory boards.
Author Contributions
The author carried out the literature search, the design of the tables and figures and wrote the final manuscript.
Data Availability
The author declares that data supporting the findings of this study are available within the article. However, any required additional information can be provided by the corresponding author.
| References | ▴Top |
- Petersmann A, Muller-Wieland D, Muller UA, Landgraf R, Nauck M, Freckmann G, Heinemann L, et al. Definition, classification and diagnosis of diabetes mellitus. Exp Clin Endocrinol Diabetes. 2019;127(S 01):S1-S7.
doi pubmed - Reed J, Bain S, Kanamarlapudi V. A review of current trends with type 2 diabetes epidemiology, aetiology, pathogenesis, treatments and future perspectives. Diabetes Metab Syndr Obes. 2021;14:3567-3602.
doi pubmed - Galicia-Garcia U, Benito-Vicente A, Jebari S, Larrea-Sebal A, Siddiqi H, Uribe KB, Ostolaza H, et al. Pathophysiology of Type 2 Diabetes Mellitus. Int J Mol Sci. 2020;21(17):6275.
doi pubmed - Dhawan S, Natarajan R. Epigenetics and type 2 diabetes risk. Curr Diab Rep. 2019;19(8):47.
doi pubmed - Weir GC, Gaglia J, Bonner-Weir S. Inadequate beta-cell mass is essential for the pathogenesis of type 2 diabetes. Lancet Diabetes Endocrinol. 2020;8(3):249-256.
doi - Priya G, Kalra S, Bahendeka S, Jawad F, Aye TT, Shahjada S, Sehgal A, et al. Initiation of basal bolus insulin therapy. J Pak Med Assoc. 2020;70(8):1462-1467.
- Dimitriadis GD, Maratou E, Kountouri A, Board M, Lambadiari V. Regulation of postabsorptive and postprandial glucose metabolism by insulin-dependent and insulin-independent mechanisms: an integrative approach. Nutrients. 2021;13(1):159.
doi pubmed - Kruger DF, Martin CL, Sadler CE. New insights into glucose regulation. Diabetes Educ. 2006;32(2):221-228.
doi pubmed - Lefebvre PJ, Scheen AJ. Glucose metabolism and the postprandial state. Eur J Clin Invest. 1999;29(Suppl 2):1-6.
doi pubmed - Hatting M, Tavares CDJ, Sharabi K, Rines AK, Puigserver P. Insulin regulation of gluconeogenesis. Ann N Y Acad Sci. 2018;1411(1):21-35.
doi pubmed - Ostergaard M, Mishra NK, Jensen KJ. The ABC of insulin: the organic chemistry of a small protein. Chemistry. 2020;26(38):8341-8357.
doi pubmed - Liu M, Weiss MA, Arunagiri A, Yong J, Rege N, Sun J, Haataja L, et al. Biosynthesis, structure, and folding of the insulin precursor protein. Diabetes Obes Metab. 2018;20(Suppl 2):28-50.
doi pubmed - Marques RG, Fontaine MJ, Rogers J. C-peptide: much more than a byproduct of insulin biosynthesis. Pancreas. 2004;29(3):231-238.
doi pubmed - Fu Z, Gilbert ER, Liu D. Regulation of insulin synthesis and secretion and pancreatic Beta-cell dysfunction in diabetes. Curr Diabetes Rev. 2013;9(1):25-53.
doi pubmed - Antoszewski A, Feng CJ, Vani BP, Thiede EH, Hong L, Weare J, Tokmakoff A, et al. Insulin dissociates by diverse mechanisms of coupled unfolding and unbinding. J Phys Chem B. 2020;124(27):5571-5587.
doi pubmed - Rorsman P, Braun M. Regulation of insulin secretion in human pancreatic islets. Annu Rev Physiol. 2013;75:155-179.
doi pubmed - Olson AL, Humphries K. Recent advances in understanding glucose transport and glucose disposal. F1000Res. 2020;9(F1000 Faculty Rev):639.
doi pubmed - Thorens B. GLUT2, glucose sensing: and glucose homeostasis. Diabetologia. 2015;58(2):221-232.
doi pubmed - Jacobson DA, Shyng SL. Ion channels of the islets in type 2 diabetes. J Mol Biol. 2020;432(5):1326-1346.
doi pubmed - Bonfanti DH, Alcazar LP, Arakaki PA, Martins LT, Agustini BC, de Moraes Rego FG, Frigeri HR. ATP-dependent potassium channels and type 2 diabetes mellitus. Clin Biochem. 2015;48(7-8):476-482.
doi pubmed - Tinker A, Aziz Q, Li Y, Specterman M. ATP-sensitive potassium channels and their physiological and pathophysiological roles. Compr Physiol. 2018;8(4):1463-1511.
doi pubmed - Caumo A, Luzi L. First-phase insulin secretion: does it exist in real life? Considerations on shape and function. Am J Physiol Endocrinol Metab. 2004;287(3):E371-385.
doi pubmed - Campbell JE, Newgard CB. Mechanisms controlling pancreatic islet cell function in insulin secretion. Nat Rev Mol Cell Biol. 2021;22(2):142-158.
doi pubmed - Wysham C, Shubrook J. Beta-cell failure in type 2 diabetes: mechanisms, markers, and clinical implications. Postgrad Med. 2020;132(8):676-686.
doi pubmed - Russell-Jones D, Pouwer F, Khunti K. Identification of barriers to insulin therapy and approaches to overcoming them. Diabetes Obes Metab. 2018;20(3):488-496.
doi pubmed - American Diabetes Association Professional Practice Committee; American Diabetes Association Professional Practice Committee, Draznin B, Aroda VR, Bakris G, Benson G, Brown FM, et al. Summary of revisions: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Supplement_1):S4-S7.
doi pubmed - Garber AJ, Handelsman Y, Grunberger G, Einhorn D, Abrahamson MJ, Barzilay JI, Blonde L, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm - 2020 executive summary. Endocr Pract. 2020;26(1):107-139.
doi pubmed - Diabetes Canada Clinical Practice Guidelines Expert Committee. Diabetes Canada 2018 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes. 2018;42(Suppl 1):S1-S325.
- Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c). Diabetes Care. 2003;26(3):881-885.
doi pubmed - Schernthaner G, Guerci B, Gallwitz B, Rose L, Nicolay C, Kraus P, Kazda C. Impact of postprandial and fasting glucose concentrations on HbA1c in patients with type 2 diabetes. Diabetes Metab. 2010;36(5):389-394.
doi pubmed - Khunti K, Giorgino F, Berard L, Mauricio D, Harris SB. The importance of the initial period of basal insulin titration in people with diabetes. Diabetes Obes Metab. 2020;22(5):722-733.
doi pubmed - Perreault L, Vincent L, Neumiller JJ, Santos-Cavaiola T. Initiation and titration of basal insulin in primary care: barriers and practical solutions. J Am Board Fam Med. 2019;32(3):431-447.
doi pubmed - Gonzalvo JD. Introduction to basal insulin therapy: clinical management of diabetes. Am J Manag Care. 2018;24(6 Suppl):S87-S92.
- Smyth T. Understanding the principles of insulin use in type 1 and type 2 diabetes management. Nurs Stand. 2021;36(1):61-66.
doi pubmed - Silver B, Ramaiya K, Andrew SB, Fredrick O, Bajaj S, Kalra S, Charlotte BM, et al. EADSG guidelines: insulin therapy in diabetes. Diabetes Ther. 2018;9(2):449-492.
doi pubmed - Pettus J, Santos Cavaiola T, Tamborlane WV, Edelman S. The past, present, and future of basal insulins. Diabetes Metab Res Rev. 2016;32(6):478-496.
doi pubmed - Cheng AYY, Patel DK, Reid TS, Wyne K. Differentiating basal insulin preparations: understanding how they work explains why they are different. Adv Ther. 2019;36(5):1018-1030.
doi pubmed - Saleem F, Sharma A. NPH Insulin. [Updated Jun 25, 2021]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021. Available from: https://www.ncbi.nlm.nih.gov/books/NBK549860/.
- Brunner GA, Sendhofer G, Wutte A, Ellmerer M, Sogaard B, Siebenhofer A, Hirschberger S, et al. Pharmacokinetic and pharmacodynamic properties of long-acting insulin analogue NN304 in comparison to NPH insulin in humans. Exp Clin Endocrinol Diabetes. 2000;108(2):100-105.
doi pubmed - Peterson GE. Intermediate and long-acting insulins: a review of NPH insulin, insulin glargine and insulin detemir. Curr Med Res Opin. 2006;22(12):2613-2619.
doi pubmed - Philips JC, Scheen A. Insulin detemir in the treatment of type 1 and type 2 diabetes. Vasc Health Risk Manag. 2006;2(3):277-283.
doi pubmed - Jones MC, Patel M. Insulin detemir: a long-acting insulin product. Am J Health Syst Pharm. 2006;63(24):2466-2472.
doi pubmed - Keating GM. Insulin detemir: a review of its use in the management of diabetes mellitus. Drugs. 2012;72(17):2255-2287.
doi pubmed - King A. Advances in insulin therapy: a review of insulin degludec. J Fam Pract. 2012;61(5 Suppl):S28-31.
- Rendell M. Insulin degludec: a long-acting modern insulin analogue with a predictable pharmacokinetic/pharmacodynamic profile. Drugs Today (Barc). 2013;49(6):387-397.
- Heinemann L, Beals JM, Malone J, Anderson J, Jacobson JG, Sinha V, Corrigan SM. Concentrated insulins: History and critical reappraisal. J Diabetes. 2019;11(4):292-300.
doi pubmed - Woo V, Berard L, Roscoe R. Understanding the clinical profile of insulin degludec, the latest basal insulin approved for use in Canada: a narrative review. Diabetes Ther. 2020;11(11):2539-2553.
doi pubmed - Heise T, Korsatko S, Nosek L, Coester HV, Deller S, Roepstorff C, Segel S, et al. Steady state is reached within 2-3 days of once-daily administration of degludec, a basal insulin with an ultralong duration of action. J Diabetes. 2016;8(1):132-138.
doi pubmed - Lamb YN, Syed YY. LY2963016 Insulin Glargine: A Review in Type 1 and 2 Diabetes. BioDrugs. 2018;32(1):91-98.
doi pubmed - Bolli GB, Hahn AD, Schmidt R, Eisenblaetter T, Dahmen R, Heise T, Becker RH. Plasma exposure to insulin glargine and its metabolites M1 and M2 after subcutaneous injection of therapeutic and supratherapeutic doses of glargine in subjects with type 1 diabetes. Diabetes Care. 2012;35(12):2626-2630.
doi pubmed - Lucidi P, Porcellati F, Candeloro P, Cioli P, Andreoli AM, Marzotti S, Schmidt R, et al. Glargine metabolism over 24 h following its subcutaneous injection in patients with type 2 diabetes mellitus: a dose-response study. Nutr Metab Cardiovasc Dis. 2014;24(7):709-716.
doi pubmed - Clements JN, Threatt T, Ward E, Shealy KM. Clinical Pharmacokinetics and Pharmacodynamics of Insulin Glargine 300 U/mL. Clin Pharmacokinet. 2017;56(5):449-458.
doi pubmed - Owens DR. Pharmacokinetics and pharmacodynamics of insulin glargine 300 U/mL in the treatment of diabetes and their clinical relevance. Expert Opin Drug Metab Toxicol. 2016;12(8):977-987.
doi pubmed - Owens DR, Traylor L, Mullins P, Landgraf W. Patient-level meta-analysis of efficacy and hypoglycaemia in people with type 2 diabetes initiating insulin glargine 100U/mL or neutral protamine Hagedorn insulin analysed according to concomitant oral antidiabetes therapy. Diabetes Res Clin Pract. 2017;124:57-65.
doi pubmed - Semlitsch T, Engler J, Siebenhofer A, Jeitler K, Berghold A, Horvath K. Ultra-)long-acting insulin analogues versus NPH insulin (human isophane insulin) for adults with type 2 diabetes mellitus. Cochrane Database Syst Rev. 2020;11:CD005613.
doi pubmed - Zhuang YG, Peng H, Huang F. A meta-analysis of clinical therapeutic effect of insulin glargine and insulin detemir for patients with type 2 diabetes mellitus. Eur Rev Med Pharmacol Sci. 2013;17(19):2566-2570.
- Zhou W, Tao J, Zhou X, Chen H. Insulin Degludec, a Novel Ultra-Long-Acting Basal Insulin versus Insulin Glargine for the Management of Type 2 Diabetes: A Systematic Review and Meta-Analysis. Diabetes Ther. 2019;10(3):835-852.
doi pubmed - Vargas-Uricoechea H, Frias JP. Efficacy and safety of the second generation basal insulin analogs in type 2 diabetes mellitus: A critical appraisal. Diabetes Metab Syndr. 2019;13(3):1975-1989.
doi pubmed - Vargas-Uricoechea H. Efficacy and safety of insulin glargine 300 U/mL versus 100 U/mL in diabetes mellitus: a comprehensive review of the literature. J Diabetes Res. 2018;2018:2052101.
doi pubmed - Rosenstock J, Cheng A, Ritzel R, Bosnyak Z, Devisme C, Cali AMG, Sieber J, et al. More similarities than differences testing insulin glargine 300 Units/mL versus insulin degludec 100 units/ml in insulin-naive type 2 diabetes: the randomized head-to-head BRIGHT trial. Diabetes Care. 2018;41(10):2147-2154.
doi pubmed - Philis-Tsimikas A, Klonoff DC, Khunti K, Bajaj HS, Leiter LA, Hansen MV, Troelsen LN, et al. Risk of hypoglycaemia with insulin degludec versus insulin glargine U300 in insulin-treated patients with type 2 diabetes: the randomised, head-to-head CONCLUDE trial. Diabetologia. 2020;63(4):698-710.
doi pubmed - Cernea S, Raz I. Insulin Therapy: Future Perspectives. Am J Ther. 2020;27(1):e121-e132.
doi pubmed - Hong T, Lu J, Zhang P, Zhang Z, Xu Q, Li Y, Cui N, et al. Efficacy and Safety of Basal Analog Regimens in Type 2 Diabetes Mellitus: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Diabetes Ther. 2019;10(3):1051-1066.
doi pubmed - Lisco G, De Tullio A, Guastamacchia E, Triggiani V. Fixed-Ratio Combinations of Basal Insulin and GLP-1RA in the Management of Type 2 Diabetes Mellitus: Highlights from the Literature. Endocr Metab Immune Disord Drug Targets. 2021;21(4):626-646.
doi pubmed - Blonde L, Aschner P, Bailey C, Ji L, Leiter LA, Matthaei S, Global Partnership for Effective Diabetes M. Gaps and barriers in the control of blood glucose in people with type 2 diabetes. Diab Vasc Dis Res. 2017;14(3):172-183.
doi pubmed - Reach G, Pechtner V, Gentilella R, Corcos A, Ceriello A. Clinical inertia and its impact on treatment intensification in people with type 2 diabetes mellitus. Diabetes Metab. 2017;43(6):501-511.
doi pubmed - Khunti K, Gomes MB, Pocock S, Shestakova MV, Pintat S, Fenici P, Hammar N, et al. Therapeutic inertia in the treatment of hyperglycaemia in patients with type 2 diabetes: A systematic review. Diabetes Obes Metab. 2018;20(2):427-437.
doi pubmed - Aschner P, Gagliardino JJ, Ilkova H, Lavalle F, Ramachandran A, Mbanya JC, Shestakova M, et al. Persistent poor glycaemic control in individuals with type 2 diabetes in developing countries: 12 years of real-world evidence of the International Diabetes Management Practices Study (IDMPS). Diabetologia. 2020;63(4):711-721.
doi pubmed - Arthur B, Smith A, Bennett N, Bury D, Marshall B. PURL: NPH insulin: It remains a good option. J Fam Pract. 2020;69(2):94-95.
- Wilson LM, Castle JR. Recent Advances in Insulin Therapy. Diabetes Technol Ther. 2020;22(12):929-936.
doi pubmed - Cheng A, Bailey TS, Mauricio D, Roussel R. Insulin glargine 300 U/mL and insulin degludec: A review of the current evidence comparing these two second-generation basal insulin analogues. Diabetes Metab Res Rev. 2020;36(7):e3329.
doi
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


