| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Case Report
Volume 13, Number 10-11, November 2021, pages 510-514
Combination Therapy With Ustekinumab Plus Intensive Granulocyte and Monocyte Adsorptive Apheresis in Patients With Refractory Ulcerative Colitis
Satoshi Tanidaa, b, Keiji Ozekia, Takuya Kannoa, Takahito Katanoa, Naomi Sugimuraa, Hirotada Nishiea, Hiroyasu Iwasakia, Mamoru Tanakaa, Takaya Shimuraa, Eiji Kubotaa, Hiromi Kataokaa
aDepartment of Gastroenterology and Metabolism, Nagoya City University Graduate School of Medical Sciences, Nagoya, Japan
bCorresponding Author: Satoshi Tanida, Department of Gastroenterology and Metabolism, Nagoya City University Graduate School of Medical Sciences, 1 Kawasumi, Mizuho-cho, Mizuho-ku, Nagoya 467-8601, Japan
Manuscript submitted October 25, 2021, accepted October 30, 2021, published online November 20, 2021
Short title: UST Plus Intensive GMA in Refractory UC
doi: https://doi.org/10.14740/jocmr4625
| Abstract | ▴Top |
There are currently no reports on the efficacy and safety of combination therapy with ustekinumab (UST) plus intensive granulocyte and monocyte adsorptive apheresis (GMA) for the treatment of refractory ulcerative colitis (UC). We retrospectively evaluated the 10-week effectiveness of combination therapy with UST plus intensive GMA on refractory UC patients including two corticosteroid (CS)-dependent patients, two CS-refractory patients and one patient with loss of response to tacrolimus. Four patients were administered initial combination therapy of UST (6 mg/kg UST followed by subcutaneous injections of 90 mg UST every 8 weeks) plus intensive GMA. Of the four patients who received this combination therapy, two (50%) achieved clinical remission at 10 weeks. The rate of patients achieving endoscopic improvement (endoscopy subscore ≤ 1) at 10 weeks was 50%. In all cases, CSs were discontinued within 10 weeks. No adverse events were observed. Combination therapy with UST plus intensive GMA is helpful to reduce clinical disease activities in refractory UC patients and appears well tolerated.
Keywords: Ulcerative colitis; Induction therapy; Clinical remission; Endoscopic improvement
| Introduction | ▴Top |
Ulcerative colitis (UC) is characterized by mucosal ulceration, rectal bleeding, diarrhea, and abdominal pain. Studies have demonstrated the efficacy and safety of ustekinumab (UST) for patients with moderate-to-severe UC who failed to achieve clinical remission (CR) or to respond to conventional (i.e., non-biologic) therapy, anti-tumor necrotic factor (TNF)-α antagonists, or vedolizumab [1]. However, the efficacy of UST monotherapy at 6 mg/kg for inducing CR in randomized patients who had had an inadequate response, loss of response or intolerance to corticosteroids (CSs), immunosuppressives, and/or biologic therapies was reportedly 15.5% at 8 weeks [1]. The UST monotherapy has been limited when higher induction remission rates are the goal. On the other hand, intensive granulocyte and monocyte adsorptive apheresis (GMA) with Adacolumn® (JIMRO, Takasaki, Japan) has been superior to routine weekly GMA in terms of both remission rate and time to remission in patients with refractory UC [2]. This is also limited because the effectiveness of intensive GMA for inducing CR in severe UC patients is unsatisfactory [3]. There are currently no reports showing the efficacy of combination induction therapy with UST plus intensive GMA on refractory UC patients.
We conducted a retrospective assessment and report here on the 10-week outcomes of combination therapy comprising UST plus intensive GMA for patients with refractory UC.
| Case Reports | ▴Top |
Patients
This study retrospectively investigated four patients with moderate-to-severe UC treated in Nagoya City University Hospital between August 2020 and July 2021. Consecutive patients receiving combination therapy with UST plus intensive GMA for CS-refractory UC, CS-dependent UC or refractory UC who showed loss of response to tacrolimus (TAC) were enrolled. All procedures in the present study were conducted in compliance with the Declaration of Helsinki. All study protocols were approved by the Nagoya City University ethics committee (#60-21-0043) and informed consent was obtained in the form of an opt-out available on the hospital website during enrollment.
Treatment and assessments
The primary outcome was the CR rate at 10 weeks for patients receiving combination therapy with UST plus intensive GMA. The secondary outcome was endoscopic improvement (defined as a Mayo endoscopic subscore ≤ 1) and changes in C-reactive protein (CRP) values. Disease activities and severities were assessed using the full Mayo score [4] at baseline and 10 weeks. Patients were enrolled to this study according to the following criteria: 1) CS-refractory or CS-dependent UC; or 2) loss of response to TAC; and 3) moderate-to-severe UC (Mayo score 6 - 12 points at baseline), including an endoscopic subscore of 2 - 3 despite concurrent treatment with CSs, and/or TAC. Only CS dosages were tapered off as appropriate. Any adverse events, including date of onset, severity, outcome, and relationships of events to these therapies were recorded. The data are presented as mean ± standard error of the mean, and comparisons were made using Wilcoxon signed-rank tests. A significance level of 0.05 was used for all statistical tests, and two-tailed tests were applied as appropriate.
The demographic and baseline data of the four patients (two men and two women) are shown in Table 1. Mean age was 44.8 years, and mean disease duration was 9.0 years. The colonic involvement was extensive in two cases and left-sided in two cases. Concurrent medications included 5-aminosalicylates, CSs, and TAC. Of the four patients, two were CS-refractory, two were CS-dependent, and one experienced loss of response to TAC. No patients had any history of prior use of anti-TNF-α antagonists. Mean dose of CSs was 12.5 mg/day (Tables 1 and 2).
 Click to view | Table 1. Baseline Demographic Variables of the Four Cases With UC Refractory to Medications Including TAC Who Were Selected for This Combination Therapy With UST Plus Intensive GMA |
 Click to view | Table 2. Clinical Courses Within 10 Weeks for the Four Cases |
The four patients were administered combination therapy of comprising UST (6 mg/kg UST followed by subcutaneous injections of 90 mg UST every 8 weeks) plus intensive GMA. Mean full Mayo scores at baseline and 10 weeks were 11.0 ± 0.41 points and 2.5 ± 0.96 points, respectively, and mean Mayo endoscopic subscores at baseline and 10 weeks were 2.75 ± 0.25 and 1.5 ± 0.29, respectively (Figs. 1a and 2). Both full Mayo scores (P = 0.066) and endoscopic subscores (P = 0.059) showed no significant differences. Mean CRP levels of the four patients were 1.70 ± 0.89 mg/dL at baseline and 0.08 ± 0.03 mg/dL at 10 weeks, also showing no significant difference (P = 0.068) (Fig. 1b). This combination therapy tended to reduce disease activities including full Mayo scores, endoscopic scores, and CRP values.
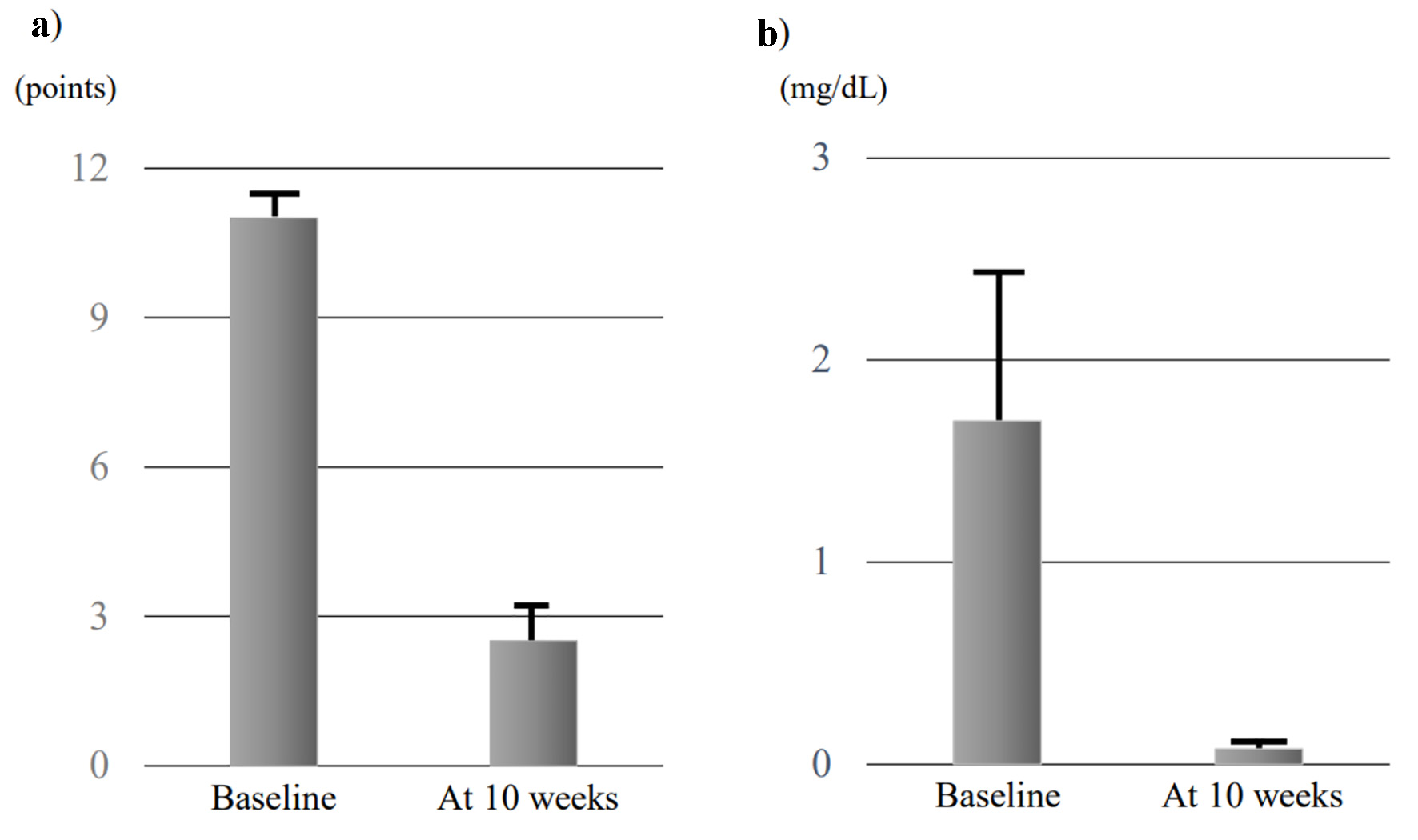 Click for large image | Figure 1. Evolution of mean full Mayo scores (a) and CRP (b) at baseline and 10 weeks in the four cases. Data are presented as mean ± standard error of the mean, and comparisons were made using Wilcoxon signed-rank tests. A significant level of 0.05 was used for all statistic tests, and two-tailed tests were applied as appropriate. CRP: C-reactive protein. |
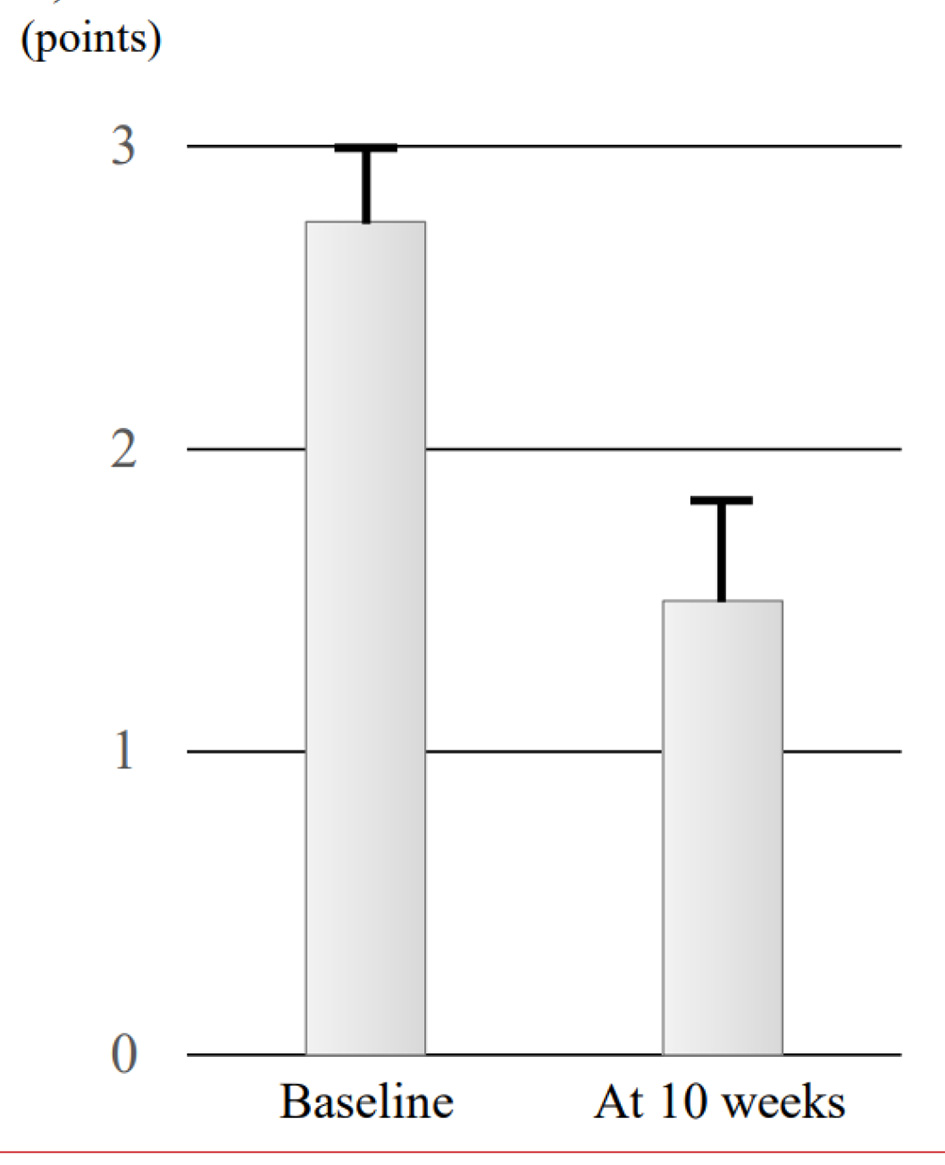 Click for large image | Figure 2. Evolution of mean endoscopic subscore at baseline and 10 weeks in the four cases. Data are presented as mean ± standard error of the mean, and comparisons were made using Wilcoxon signed-rank tests. A significant level of 0.05 was used for all statistic tests, and two-tailed tests were applied as appropriate. |
The rate of CR at 10 weeks was 50%. The rate of patients showing endoscopic improvement (defined as endoscopy subscore ≤ 1) [5] at 10 weeks was 50%. In addition, all four patients were successfully withdrawn from CSs by 10 weeks (Table 2).
Regarding safety, no cases of lymphoma, non-melanoma skin cancer, leukemia, or tuberculosis as adverse event of special interests were identified in the present study. No adverse events were observed. Combination therapies with UST and intensive GMA appeared safe and well tolerated in all patients.
| Discussion | ▴Top |
We have reported here the effectiveness of combining UST induction therapy with intensive GMA in four consecutive cases of refractory UC.
In clinical settings, UST is an efficacious therapy to induce and maintain CR in patients with moderately-to-severely active UC. The UNIFI phase 3 trial of UST involved patients with moderate-to-severe UC and revealed that overall rates of CR at week 8 were 15.5% in patients randomized to receive UST at 6 mg/kg and 5.3% in patients allocated placebo (P < 0.001) [1]. Rates of CR for biologic-naive patients who had received CSs and/or immunosuppressive agents other than biologics were 18.4% at week 8 in patients receiving UST and 9.9% at week 8 in patients receiving placebo. In addition, the rate of 8-week endoscopic improvement in the total cohort including patients with biologics failure and non-failure who received UST at 6 mg/kg (27.0 %) was significantly higher compared to those receiving placebo (13.8 %) (P < 0.001) [1]. Moreover, the rate of 8-week endoscopic improvement for biologic-naive patients receiving UST at 6 mg/kg was 33.3%, compared to 21.2% in patients receiving placebo [1]. Therapeutic treatment with UST monotherapy for refractory UC has limitations, suggesting that treatment in addition to UST is necessary for biologic-naive patients with active UC and loss of response to TAC. In the present study, the rates of CR and endoscopic improvement at 10 weeks in four patients receiving combination therapy with UST plus intensive GMA were both 50.0%. Based on these outcomes, adding intensive GMA to UST monotherapy likely appears effective as a combination therapy for inducing CR and endoscopic improvements.
A retrospective, multicenter study showed effectiveness of the combination of GMA after loss of response to TNF agents in UC [6]. GMA is currently available for the treatment of active UC that has had loss of response to standard pharmacotherapies, including biologics. Activated granulocytes and monocytes (GMs) adsorption to cellulose acetate beads induces downregulation of inflammatory cytokines, including interleukin (IL)-1β, IL-6, IL-8, and TNF-α and release of anti-inflammatory cytokine [7-10]. Therefore, levels of these cytokines in the intestinal tissue were significantly lowered in inflammatory bowel disease (IBD) patients who had achieved clinical or endoscopic remission, compared to patients who clinically remained unchanged after GMA therapy [11].
Furthermore, an in vitro study observed that the concentration of soluble cell adhesion molecule-1 and soluble vascular cell adhesion molecule-1 in blood samples was significantly reduced after GMs adsorption with acetate beads [9]. Such findings suggest that the present combination therapy suppressed leukocyte migration by drastically downregulating circulating inflammatory cytokines and expression of leukocyte-related adhesion molecules as an effect of GMA, and by downregulating local inflammatory cytokines at microenvironmental sites in the gut mucosa, as an effect of UST, thereby inducing rapid CR [12, 13]. In addition, serious adverse side effects appear rare in patients receiving GMA [3, 14]. Additional studies on larger cohorts of patients are warranted to verify these findings.
Regarding safety, some risks for systemic disease, cancer development and opportunistic infections, including leukocytoclastic vasculitis, non-melanoma skin cancers, prostate, colon, renal papillary, and rectal cancers, and cytomegalovirus colitis, legionella pneumonia, and ophthalmic and oral herpes simplex infections have been reported with the use of UST [1, 15]. No adverse events occurred within 10 weeks of administration in the present study. The present study showed limitations in terms of the sample size and insufficient time to fully characterize the safety of combination therapy with UST and intensive GMA.
In conclusions, combination therapy with UST plus intensive GMA is helpful to ameliorate UC inflammation and clinically to reduce disease activities in refractory UC patients, and appears well tolerated.
Acknowledgments
None to declare.
Financial Disclosure
This work was supported by a Grant-in Aid for scientific research C (KAKENHI 19K08399) from the Japan Society for the Promotion of Science (JSPS).
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
Informed consent was obtained in the form of opt-out on the website during enrollment.
Author Contributions
ST has designed and performed the study. ST, KO, and TK have drafted the manuscript and TK, NS, HN, HI, MT, and TS performed critical editing. ST, KO, TK, and TK assisted with and supported sample collection and subsequent analysis with statistics. ST prepared and wrote manuscript. EK and HK carefully supervised this study.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
UC: ulcerative colitis; UST: ustekinumab; GMA: granulocyte and monocyte adsorptive apheresis; TAC: tacrolimus; CRP: C-reactive protein; CR: clinical remission
| References | ▴Top |
- Sands BE, Sandborn WJ, Panaccione R, O'Brien CD, Zhang H, Johanns J, Adedokun OJ, et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2019;381(13):1201-1214.
doi pubmed - Sakuraba A, Motoya S, Watanabe K, Nishishita M, Kanke K, Matsui T, Suzuki Y, et al. An open-label prospective randomized multicenter study shows very rapid remission of ulcerative colitis by intensive granulocyte and monocyte adsorptive apheresis as compared with routine weekly treatment. Am J Gastroenterol. 2009;104(12):2990-2995.
doi pubmed - Hanai H, Watanabe F, Yamada M, Sato Y, Takeuchi K, Iida T, Tozawa K, et al. Adsorptive granulocyte and monocyte apheresis versus prednisolone in patients with corticosteroid-dependent moderately severe ulcerative colitis. Digestion. 2004;70(1):36-44.
doi pubmed - Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317(26):1625-1629.
doi pubmed - Reinisch W, Sandborn WJ, Panaccione R, Huang B, Pollack PF, Lazar A, Thakkar RB. 52-week efficacy of adalimumab in patients with moderately to severely active ulcerative colitis who failed corticosteroids and/or immunosuppressants. Inflamm Bowel Dis. 2013;19(8):1700-1709.
doi pubmed - Rodriguez-Lago I, Sempere L, Gutierrez A, Nunez A, Leo Carnerero E, Hinojosa E, Mora M, et al. Granulocyte-monocyte apheresis: an alternative combination therapy after loss of response to anti-TNF agents in ulcerative colitis. Scand J Gastroenterol. 2019;54(4):459-464.
doi pubmed - Kashiwagi N, Hirata I, Kasukawa R. A role for granulocyte and monocyte apheresis in the treatment of rheumatoid arthritis. Ther Apher. 1998;2(2):134-141.
doi pubmed - Saniabadi AR, Hanai H, Takeuchi K, Umemura K, Nakashima M, Adachi T, Shima C, et al. Adacolumn, an adsorptive carrier based granulocyte and monocyte apheresis device for the treatment of inflammatory and refractory diseases associated with leukocytes. Ther Apher Dial. 2003;7(1):48-59.
doi pubmed - Nishise S, Abe Y, Nomura E, Sato T, Sasaki Y, Iwano D, Yoshizawa K, et al. Relationship between tumor necrosis factor-alpha release and granulocyte and monocyte adsorption to cellulose acetate beads. Ther Apher Dial. 2014;18(3):252-257.
doi pubmed - Contini P, Negrini S, Bodini G, Trucchi C, Ubezio G, Strada P, Savarino V, et al. Granulocytes and monocytes apheresis induces upregulation of TGFbeta1 in patients with active ulcerative colitis: A possible involvement of soluble HLA-I. J Clin Apher. 2017;32(1):49-55.
doi pubmed - Yamamoto T, Saniabadi AR, Umegae S, Matsumoto K. Impact of selective leukocytapheresis on mucosal inflammation and ulcerative colitis: cytokine profiles and endoscopic findings. Inflamm Bowel Dis. 2006;12(8):719-726.
doi pubmed - Chen XL, Mao JW, Wang YD. Selective granulocyte and monocyte apheresis in inflammatory bowel disease: Its past, present and future. World J Gastrointest Pathophysiol. 2020;11(3):43-56.
doi pubmed - Tanida S, Mizoshita T, Ozeki K, Katano T, Tanaka M, Nishie H, Shimura T, et al. Combination therapy with intensive granulocyte and monocyte adsorptive apheresis plus Ustekinumab in patients with refractory Crohn's disease. Ther Apher Dial. 2018;22(3):295-300.
doi pubmed - Thanaraj S, Hamlin PJ, Ford AC. Systematic review: granulocyte/monocyte adsorptive apheresis for ulcerative colitis. Aliment Pharmacol Ther. 2010;32(11-12):1297-1306.
doi pubmed - Hong SJ, Krugliak Cleveland N, Akiyama S, Zullow S, Yi Y, Shaffer SR, Malter LB, et al. Real-world effectiveness and safety of Ustekinumab for ulcerative colitis from 2 tertiary IBD centers in the United States. Crohn's & Colitis 360. 2021;3(1):otab002.
doi
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


