| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Original Article
Volume 13, Number 9, September 2021, pages 474-478
Evaluation of Plasma Soluble Urokinase Plasminogen Activator Receptor Levels in Patients With COVID-19 and Non-COVID-19 Pneumonia: An Observational Cohort Study
Dimitrios Velissarisa, f, Maria Lagadinoua, Themistoklis Paraskevasa, Eleousa Oikonomoua, Vasileios Karamouzosb, Sofia Karteric, Dimitrios Bousisa, Nikolaos Pantzarisd, Konstantinos Tsiotsiose, Markos Marangosa
aDepartment of Internal Medicine, University Hospital of Patras, Rion, Greece
bIntensive Care Unit, University Hospital of Patras, Rion, Greece
cDivision of Oncology, Department of Medicine, University Hospital of Patras, Rion, Greece
dYork Teaching Hospital NHS Foundation Trust, York, UK
eDepartment of Nephrology, University Hospital of Patras, Rion, Greece
fCorresponding Author: Dimitrios Velissaris, Department of Internal Medicine, University Hospital of Patras, Rion, Greece
Manuscript submitted August 17, 2021, accepted August 30, 2021, published online September 30, 2021
Short title: Plasma suPAR in COVID-19 Patients
doi: https://doi.org/10.14740/jocmr4579
| Abstract | ▴Top |
Background: The respiratory system is the main system affected by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and a great number of infected people need hospitalization. Soluble urokinase plasminogen activator receptor (suPAR) is a biomarker indicative of acute and chronic inflammation. Current literature supports that suPAR has great predictive ability for mortality in patients with coronavirus disease 2019 (COVID-19). The aim of this study was to compare the value of suPAR and other laboratory biomarkers in patients with chest infection and suspected COVID-19.
Methods: A total of 41 consecutive patients with chest infection were enrolled in the study and were assigned into two groups according to the real-time polymerase chain reaction (PCR) result for SARS-CoV-2. The two groups had no significant difference in baseline data (age, sex), arterial oxygen partial pressure (PO2)/fraction of inspired oxygen (FiO2) ratio and mortality.
Results: Among patients with chest infection who required hospitalization, suPAR was significantly higher on admission in those with COVID-19 when compared to patients with non-COVID-19. suPAR had a great prognostic ability for in-hospital mortality in the COVID-19 subgroup.
Conclusions: A single measurement of suPAR on admission can provide prognostic information for patients with suspected COVID-19 pneumonia. In the subgroup of patients with positive real-time PCR result for SARS-CoV2, suPAR was significantly higher and had an excellent prognostic value for the in-hospital mortality.
Keywords: suPAR; Chest infection; COVID-19; Prognosis; Biomarker
| Introduction | ▴Top |
The novel coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has rapidly become a pandemic, exerting enormous pressures on the global healthcare systems. Although the majority of the SARS-CoV-2 infected cases are presented with mild symptoms, a significant number of patients worldwide need hospitalization. Especially for sepsis cases, including COVID-19, it is essential to find out more accurate biomarkers in order to triage patients and identify those who may safely be discharged home for self- isolation, those who should be hospitalized, and those at risk of deterioration who may require intensive care unit (ICU) admission.
The soluble urokinase plasminogen activator receptor (suPAR) is an easily measured biomarker related to inflammation; it has been suggested to mirror the degree of the underlying immune status, as it can be generated from activated immune cells. suPAR levels may be associated with the progression, severity and mortality risk in several inflammatory diseases and critically ill patients of various etiology including sepsis [1]. Moreover, the role of suPAR has been evaluated in the diagnosis, assessment, and prognosis of several infectious, autoimmune, and neoplastic disease processes [2]. Currently, there are no reports comparing the values of suPAR between COVID-19 and non-COVID-19 patients.
The aim of the study is to compare the value of suPAR and other laboratory biomarkers in patients with chest infection and suspected COVID-19. Additionally, in this study we evaluate the predictive value of suPAR for in-hospital mortality and investigate any potential correlation between suPAR plasma levels and the commonly used inflammatory biomarkers of sepsis.
| Materials and Methods | ▴Top |
Patients aged 18 years or above, with symptoms of chest infection, who were admitted to the Department of Internal Medicine of the University Hospital of Patras, Greece, were consecutively enrolled into the study. The study period was from May 25, 2021 until June 15, 2021. All patients were initially treated in an isolated sub-ward of the Department, modified according to all the necessary precautions, as results from the SARS-CoV-2 test were available after some hours. Patients were divided into two groups: group A (patients whose real-time polymerase chain reaction (PCR) for SARS-CoV-2 infection confirmed positive (n = 21)) and group B (patients whose real-time PCR for SARS-CoV-2 infection was negative (n = 20)). Written informed consent was obtained from all the enrolled patients or a relative when necessary. The Ethics Committee of the Hospital approved the study protocol. The procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional or regional) and with the Helsinki Declaration of the World Medical Association.
Demographic characteristics (age, gender, body mass index (BMI)), routine laboratory testing including white blood cells (WBC), absolute lymphocyte count (ALC), coagulation parameters (platelets, fibrinogen, D-dimers), C-reactive protein (CRP), ferritin, lactate dehydrogenase (LDH), creatinine kinase (CPK), length of hospital stay, and final outcome of all patients were recorded. The arterial oxygen partial pressure (PO2)/fraction of inspired oxygen (FiO2) ratio was calculated at the same time with blood analysis for all patients. For the calculation of the plasma suPAR levels, the collected blood samples were centrifuged for 5 min and suPAR levels were measured with an enzyme immunoassay technique, alF QT suPARnostic (ViroGates, Birkerod, Denmark). All the above are presented in Table 1. The procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional or regional) and with the Helsinki Declaration of the World Medical Association.
 Click to view | Table 1. Demographic Characteristics and Laboratory Findings of the Two Groups of Patients |
Statistical analysis
Statistical analysis of data was performed using the SPSS-25 statistical software. The minimum value of the level of statistical significance (P value) was set at 0.05. All data are presented as mean ± standard deviation for continuous variables with a normal distribution, as median and interquartile range for continuous variables with a non-normal distribution, or as frequency and percentage for categorical variables. The Mann-Whitney U test was used to compare non-parametric continuous data, the unpaired Student’s t-test was used to compare data with normal distribution and the Fisher’s exact test was used for categorical data.
| Results | ▴Top |
Forty-one patients were enrolled in the study. The mean age of the participants was 61.6 ± 16.8 years; 29.3% (n = 12) were female and 70.7% (n = 29) were male. The overall mortality of our cohort was 19%. Age, sex, PaO2/FiO2, length of hospital stay and mortality did not significantly differ between the two groups (Table 1). Patients with a positive SARS-CoV-2 PCR test were assigned to group A and patients with a negative SARS-CoV-2 PCR test were assigned to group B.
A total of 21 patients were included in group A, five of which died. The mean age was 61.57 ± 17.08 years. Four out of five patients who died (80%) had extreme plasma suPAR values (> 14.8 ng/mL). A total of 20 patients were included in group B, three of which died. The mean age was 63.2 ± 16 years.
Comparison between the two groups revealed statistically significant difference in the values of suPAR (P = 0.021), white blood cells (P < 0.001), neutrophils (P = 0.02) and monocytes (P < 0.02, Fig. 1). Specifically, median suPAR was 12 ng/mL in the COVID-19-positive (group A) vs. 7.3 ng/mL in the COVID-19-negative group (group B). suPAR did not have great prognostic ability for in-hospital mortality in our entire cohort (area under the ROC curve (AUC) = 0.611), but it had excellent in the COVID positive subgroup (AUC = 0.792). In group A, suPAR values greater than 14.6 ng/mL had a sensitivity of 0.8 and specificity of 0.92 for predicting in-hospital mortality. Although in group A, suPAR values differed numerically between non-survivors and survivors (median values 15 ng/mL and 11.43 ng/mL, respectively), due to the small sample number, the difference was considered non-statistically significant (P = 0.063).
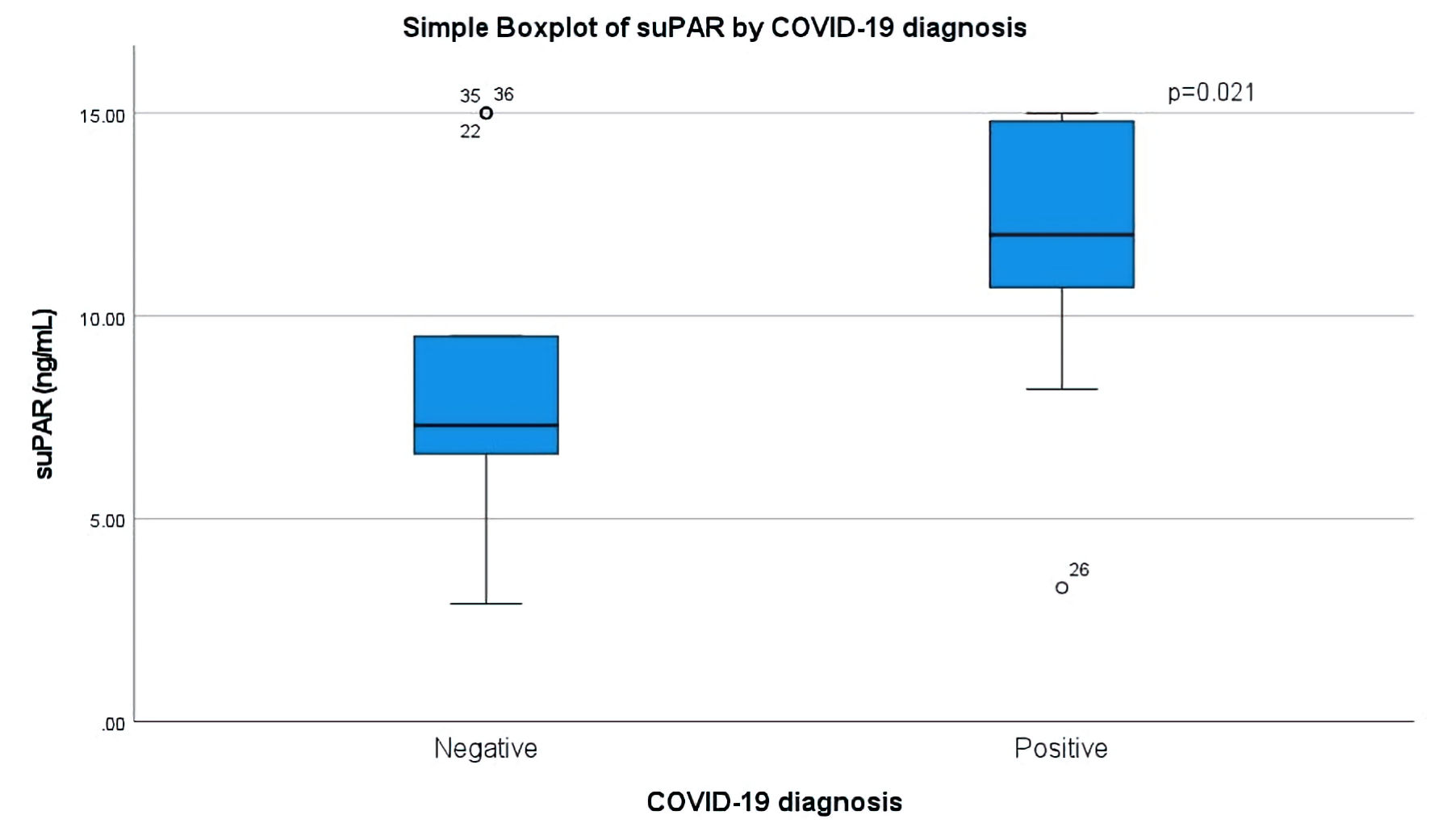 Click for large image | Figure 1. Boxplot of suPAR values in ng/mL (vertical) in patients with negative (left) and positive (right) real-time PCR (polymerase chain reaction) results for COVID-19. The Mann-Whitney U test was used to calculated statistical differences. COVID-19: coronavirus disease 2019; suPAR: soluble urokinase plasminogen activator receptor. |
Subsequently, in the subgroup analysis, we investigated potential correlations between suPAR and other laboratory values. In group A, there was an inverse correlation between the values of suPAR and monocytes with borderline statistical significance (P = 0.041). In group B, we found no significant correlations.
| Discussion | ▴Top |
The major finding in our study was that among patients with chest infection, suPAR is statistically significant higher on admission in patients requiring hospitalization with COVID-19 when compared to those with non-COVID-19. The two groups did not differ in mortality, length of hospital stay, age, gender, and disease severity as measured by the PaO2/FiO2 ratio.
Another finding was that almost all the patients diagnosed with COVID-19 who died had elevated plasma levels of suPAR on admission. This finding is in agreement with studies conducted prior to the COVID-19 pandemic, where elevated suPAR was found to be a strong marker of disease severity, readmission, and mortality in patients admitted to the emergency department [3, 4].
Many biomarkers have been used as prognostic indexes of sepsis, but in the cases of COVID-19 this issue remains a matter of debate due to the unpredictable course of the SARS-CoV-2 infection. In the DISCOVER study, prospectively recruited COVID-19 patients admitted to a UK hospital were analyzed in an attempt to identify on admission biomarkers indicative of poor outcome. Among the evaluated new and commonly used biomarkers, it was showed that interleukin-6 (IL-6) and suPAR had the best performance for the prediction of COVID-19 outcomes [5]. A prospective study evaluated the association between suPAR and the incidence of severe complications of COVID-19. The primary endpoint of the study was the time from admission until the development of a composite outcome, such as acute respiratory distress syndrome (ARDS), ICU admission, or death. In a total of 403 COVID-19 patients, suPAR had an excellent prognostic utility in predicting severe complications in hospitalized COVID-19 patients [2]. In the study by Rovina et al, plasma suPAR levels were found increased in COVID-19 patients and it was finally suggested that suPAR can be an early predictor of severe respiratory failure [6].
suPAR, a marker of immune activation, has been shown to predict deterioration in several infectious and inflammatory disorders, also its role has been investigated in many viral diseases and has been well correlated to disease severity [7, 8]. Data from literature suggest that plasma suPAR levels of patients with various pathologies, such as obesity, diabetes, cardiac diseases, and cancer are elevated (> 4 ng/mL), with good statistical significance, when compared to healthy individuals. Many of these pathologies are corresponding to comorbidities of patients with SARS-CoV-2 infection. It is also well documented that elevated plasma suPAR levels are indicative of an underlying prolonged inflammation. As a result, elevated suPAR levels and prolonged background inflammation mirror a dysregulated uPA/uPAR system, raising the question whether the uPA/uPAR system could be a field for investigation in SARS-CoV-2 infection [9]. In daily clinical practice, suPAR is an easily measured inflammatory biomarker. Results can quickly be obtained on-site in many hospital settings, even in the emergency department, avoiding the need for samples to be passed through the clinical laboratory, or can even be obtained prior to hospitalization (time to result 20 min) guiding clinicians’ decisions [10].
We identified three additional studies that report the differences of suPAR values between viral and bacterial causes of infection, with ambiguous results. According to a study by Lin et al [11], suPAR values were lower in blood stream infections when caused by viral infections compared to bacterial infections. Moreover, no differences were found between the two groups, in lower respiratory tract infections in a pediatric population and in meningitis [12, 13].
In our study, we also found statistically significant correlation between suPAR and monocytes in the group of patients with SARS-CoV-2 infection. In the studied cohort, all COVID-19 patients with plasma suPAR levels > 10 ng/mL had monocytes and lymphocytes absolute counts below normal levels, elevated CRP, ferritin and LDH. It has been already observed that lymphocyte and monocyte populations decrease in COVID-19, and recent research showed a link between neutrophil-to-monocyte ratio and COVID-19 in hospital mortality [2].
The main limitation of our study is its small sample size, however, it was a homogeneous patients’ sample. Secondly it was performed in a single tertiary center and included patients with moderate to severe disease requiring hospitalization. Third, there was no separation between viral and bacterial causes of pneumonia in non-COVID patients.
Conclusions
A single measurement of suPAR on admission can provide prognostic information for patients with suspected COVID-19 pneumonia. In the subgroup of patients with positive real-time PCR result for SARS-CoV2, suPAR was significantly higher and had an excellent prognostic value for the in-hospital mortality.
Acknowledgments
The authors have none to declare.
Financial Disclosure
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
The authors have no conflict of interest to declare.
Informed Consent
Written informed consent was obtained from all the enrolled patients or a relative when necessary.
Author Contributions
DV and MM conceived and designed the study. ML, TP, EO, DB and KT acquired the data. VK, SK and NP analyzed and interpreted the data. DV, ML and TP drafted the paper. EO, VK, SK, DB, NP, KT and MM critically reviewed the manuscript. All authors read and approved the final manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
COVID-19: coronavirus disease 2019; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; suPAR: soluble urokinase plasminogen activator receptor; PCR: polymerase chain reaction; WBC: white blood cells; ALC: absolute lymphocyte count; CRP: C-reactive protein; LDH: lactate dehydrogenase; CPK: creatinine kinase
| References | ▴Top |
- Velissaris D, Pierrakos C, Karamouzos V, Pantzaris ND, Gogos C. The use of soluble urokinase plasminogen activator receptor (suPAR) as a marker of sepsis in the emergency department setting. A current review. Acta Clin Belg. 2021;76(1):79-84.
doi pubmed - Oulhaj A, Alsuwaidi AR, Suliman A, Gasmelseed H, Khan S, Alawi S, Hukan Y, et al. Admission levels of Soluble Urokinase Plasminogen Activator Receptor (suPAR) are Associated with the development of severe complications in hospitalised COVID-19 patients: a prospective cohort study. Int J Infect Dis. 2021;107:188-194.
doi pubmed - Rasmussen LJ, Ladelund S, Haupt TH, Ellekilde G, Poulsen JH, Iversen K, Eugen-Olsen J, et al. Soluble urokinase plasminogen activator receptor (suPAR) in acute care: a strong marker of disease presence and severity, readmission and mortality. A retrospective cohort study. Emerg Med J. 2016;33(11):769-775.
doi pubmed - Rasmussen LJH, Ladelund S, Haupt TH, Ellekilde GE, Eugen-Olsen J, Andersen O. Combining national early warning score with soluble urokinase plasminogen activator receptor (suPAR) improves risk prediction in acute medical patients: a registry-based cohort study. Crit Care Med. 2018;46(12):1961-1968.
doi pubmed - Arnold DT, Attwood M, Barratt S, Morley A, Elvers KT, McKernon J, Donald C, et al. Predicting outcomes of COVID-19 from admission biomarkers: a prospective UK cohort study. Emerg Med J. 2021;38(7):543-548.
doi pubmed - Rovina N, Akinosoglou K, Eugen-Olsen J, Hayek S, Reiser J, Giamarellos-Bourboulis EJ. Soluble urokinase plasminogen activator receptor (suPAR) as an early predictor of severe respiratory failure in patients with COVID-19 pneumonia. Crit Care. 2020;24(1):187.
doi pubmed - Velissaris D, Dimopoulos G, Parissis J, Alexiou Z, Antonakos N, Babalis D, Gerakari S, et al. Prognostic role of soluble urokinase plasminogen activator receptor at the emergency department: a position paper by the Hellenic sepsis study group. Infect Dis Ther. 2020;9(3):407-416.
doi pubmed - Stauning MA, Altintas I, Kallemose T, Eugen-Olsen J, Lindstrom MB, Rasmussen LJH, Gamst-Jensen H, et al. Soluble urokinase plasminogen activator receptor as a decision marker for early discharge of patients with COVID-19 symptoms in the emergency department. J Emerg Med. 2021.
doi pubmed - D'Alonzo D, De Fenza M, Pavone V. COVID-19 and pneumonia: a role for the uPA/uPAR system. Drug Discov Today. 2020;25(8):1528-1534.
doi pubmed - Chalkias A, Mouzarou A, Samara E, Xanthos T, Ischaki E, Pantazopoulos I. Soluble urokinase plasminogen activator receptor: a biomarker for predicting complications and critical care admission of COVID-19 patients. Mol Diagn Ther. 2020;24(5):517-521.
doi pubmed - Lin MF, Sun B, Liu ZY, Tang P, Zhang LJ, Wang YY. Evaluation of the clinical diagnostic value of traditional inflammatory markers and novel biomarkers in intracellular bacterial bloodstream infections. Cytokine. 2020;136:155238.
doi pubmed - Garcia-Monco JC, Coleman JL, Benach JL. Soluble urokinase receptor (uPAR, CD 87) is present in serum and cerebrospinal fluid in patients with neurologic diseases. J Neuroimmunol. 2002;129(1-2):216-223.
doi - Citlenbik H, Ulusoy E, Er A, Caglar A, Akgul F, Kume T, Yilmaz D, et al. Levels of soluble urokinase plasminogen activator receptor in pediatric lower respiratory tract infections. Pediatr Allergy Immunol Pulmonol. 2019;32(3):121-127.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


