| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Original Article
Volume 13, Number 8, August 2021, pages 413-419
Effects of Age on Esophageal Motility: A High-Resolution Manometry Study
Roupen Djinbachiana, b, Etienne Marchanda, Weixiang Yana, Mickael Bouina
aDivision of Gastroenterology, Montreal University Hospital Center (CHUM) and Montreal University Research Center (CRCHUM), Montreal, Canada
bCorresponding Author: Roupen Djinbachian, Department of Medicine, Division of Internal Medicine, Montreal University Hospital (CHUM) and Montreal University Hospital Research Center (CRCHUM), 900 Rue Saint-Denis, Montreal, QC H2X 0A9, Canada
Manuscript submitted August 6, 2021, accepted August 20, 2021, published online August 30, 2021
Short title: Effects of Age on Esophageal Motility
doi: https://doi.org/10.14740/jocmr4576
| Abstract | ▴Top |
Background: Studies have found possible physiologic changes to esophageal motility with aging currently not taken into account in routine high-resolution manometry (HRM) interpretation. We aimed to quantify the relationship between these physiologic changes and aging to improve HRM interpretation.
Methods: We conducted a retrospective analysis of patients who underwent HRM at a tertiary hospital center between 2015 and 2019. Inclusion criteria were patients aged ≥18 years with normal HRM. Exclusion criteria were abnormal HRM, abnormal upper digestive endoscopy or imagery. Outcomes were median integrated relaxation pressure (IRP), lower esophageal sphincter (LES) pressure, distal contractal integral (DCI), distal latency (DL), and peristaltic break (PB) according to the v4.0 Chicago classification criteria. Effect of age was examined through univariate and multivariate linear regression analysis.
Results: We identified 1,917 patients with HRM and included 722 patients with normal exams (median age 56 years (interquartile range (IQR) 46 - 66), 63.8% female). Indications for HRM included dysphagia (39.6%), gastroesophageal reflux disease (29.5%), and chest pain (11.5%). There was statistically significant relationship between age and IRP (r = 0.20, P < 0.0001) as well as DCI (r = 0.12, P = 0.001) and DL (r = -0.09, P = 0.02). No statistically significant relationship was found between age and LES pressure or PB.
Conclusion: We found that IRP, DCI, and to a lesser extent, DL, are significantly correlated with the normal aging process in symptomatic patients. These findings should be taken into consideration when interpreting esophageal HRM.
Keywords: Manometry; Esophagus; Aging; Esophageal motility disorders
| Introduction | ▴Top |
High-resolution manometry (HRM) has become the gold standard used for the diagnosis of esophageal motility disorders. It uses multiple barometric sensors that are packed with high density to provide high-definition spatiotemporal data for the entire length of the esophagus with higher accuracy than conventional manometry. Diagnostic criteria and thresholds for the diagnosis of esophageal disorders using HRM have been established and revised regularly within the Chicago classification system, currently in its fourth iteration [1]. These diagnostic thresholds for HRM parameters have been established in patients of varying ages and vary based on the manometry equipment used [2-6]. Studies have found possible conflicting physiologic changes to esophageal motility with aging that are not currently taken into account in routine HRM interpretation [7-11]. Many studies establishing normal HRM cutoffs do not include older patient populations, and age-related esophageal physiologic changes could explain symptoms encountered when HRM parameters are considered normal. Determining the age-related variations in HRM parameters in symptomatic patients is crucial for our understanding of esophageal motility disorders. Increasing awareness of the changes in esophageal motility with aging could also help in establishing appropriate diagnoses in both older and younger patient populations. We therefore aimed to quantify the relationship between these physiologic changes and aging to improve HRM interpretation in current clinical practice.
| Materials and Methods | ▴Top |
This study was reported according to the STROBE statement criteria [12].
Study design and ethics approval
We conducted a retrospective analysis of medical records for all patients who underwent high-resolution esophageal manometry at the Montreal University Hospital Center (CHUM) between January 2015 and December 2019. Study design was submitted and approved by the Montreal University Hospital Research Center (CRCHUM) ethics committee (CER: 19.267). This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
Inclusion criteria were patients 18 years and older who had normal high-resolution esophageal manometry results regardless of indication for manometry exam. Exclusion criteria were abnormal HRM results, abnormal upper endoscopy exam and/or biopsy, esophageal and gastric abnormalities on chest and abdominal imaging (including barium swallow studies), and previous esophageal surgery.
Abnormal manometry results were defined as exams that had any diagnosis other than “normal” in the impression and diagnosis section of manometry reports, or abnormal median integrated relaxation pressure (IRP), mean distal contractal integral (DCI), and mean distal latency (DL).
Outcomes of interest and data measurements
Outcomes were median IRP, mean lower esophageal sphincter (LES) pressure, mean DCI, mean distal DL, and mean peristaltic break (PB).
All patients undergoing HRM at our hospital center have electronic manometry reports created post-procedure. We used these reports to extract data for outcomes. We used electronic medical records to extract patient age at the time of the procedure; sex; body mass index (BMI); reason for undergoing manometry exam; smoking, alcohol and drug habits; comorbidities (such as diabetes, neurocognitive disorders); neurologic diseases (such as Parkinson’s, multiple sclerosis, previous stroke, myasthenia gravis, myopathy); connective tissue disease (such as Sjogren, scleroderma, systemic lupus, myositis); medications (such as non-steroidal anti-inflammatory drugs (NSAIDs), calcium channel blockers (CCB), opioids); upper endoscopy results and biopsies; barium swallow results; chest and abdominal imaging reports. To ensure completeness of data, all available patient records outside of HRM data were also accessed to complete missing information on patient comorbidities and medication history.
HRM
HRM was performed using UNI-ESO-WG1A1 model esophageal manometry catheters (Sandhill Scientific, Colorado, USA). Patients were fasting for at least 4 h before the start of the procedure. A manometry catheter was inserted trans-nasally with patients sitting in a 90° position. Patients then performed 10 consecutive swallows with 5 mL of normal saline per swallow. Chicago classification parameters were then recorded and subsequently analyzed. Standardized upper limit of normal for IRP was 20 mm Hg using our equipment.
Statistical analysis and sample size
As our study included 13 predetermined predictor variables, we needed to include at least 130 patients to perform multivariate analysis. We selected 5 years of manometry data as those were most readily available in our center. Assuming 50% normal HRM rate, 1,000 patients were expected to be screened for exclusion criteria. We performed our analyses using Stata software 16.0 (StataCorp, College Station, TX). Continuous variables were presented as medians with interquartile range (IQR); categorical variables were presented as percentages. Univariate testing was performed through Pearson’s correlation and a two-tailed P < 0.05 was considered as statistically significant. All missing information for covariates were assumed to be missing at random as patients with missing information were those only referred to our center for manometry and not followed by a physician at our center. For multivariate analysis we performed multivariate linear regression using full information maximum likelihood estimation by performing backward stepwise regression, eliminating the least significant variable with each iteration [13, 14]. Variables used for the stepwise regression were sex; BMI; alcohol use; gastroesophageal reflux disease (GERD); diabetes; neurocognitive disorders; neurologic diseases; connective tissue disease; NSAID use; proton pump inhibitor (PPI) use; beta-blocker (BB) use; CCB use. A two-tailed P < 0.05 was used as threshold in the multivariate model. Results for the multivariate model were reported as coefficients with two-tailed 95% confidence intervals (CIs). For binomial variables, coefficients represented mean change in dependent variable if the independent variables were true.
| Results | ▴Top |
Patient characteristics
In total, 1,917 patients with HRM were evaluated for exclusion criteria and 722 patients were included in our analysis (Fig. 1). Of the included patients, 64.3% were female, median age was 56 years (IQR 46 - 66), and median BMI was 25.8 kg/m2 (IQR 22.7 - 29.4). Indications for HRM included dysphagia (39.6%); GERD (29.5%); chest pain (11.5%); other (12.6%). Of the patients, 16.6% were using NSAIDs, 9.1% CCB, 55.6% PPIs, 9.8% BBs, and 4.7% opioids. Of the patients, 0.7% had a past medical history of scleroderma, 3.8% of neurological disease, and 2.6% of connective tissue disease (Table 1).
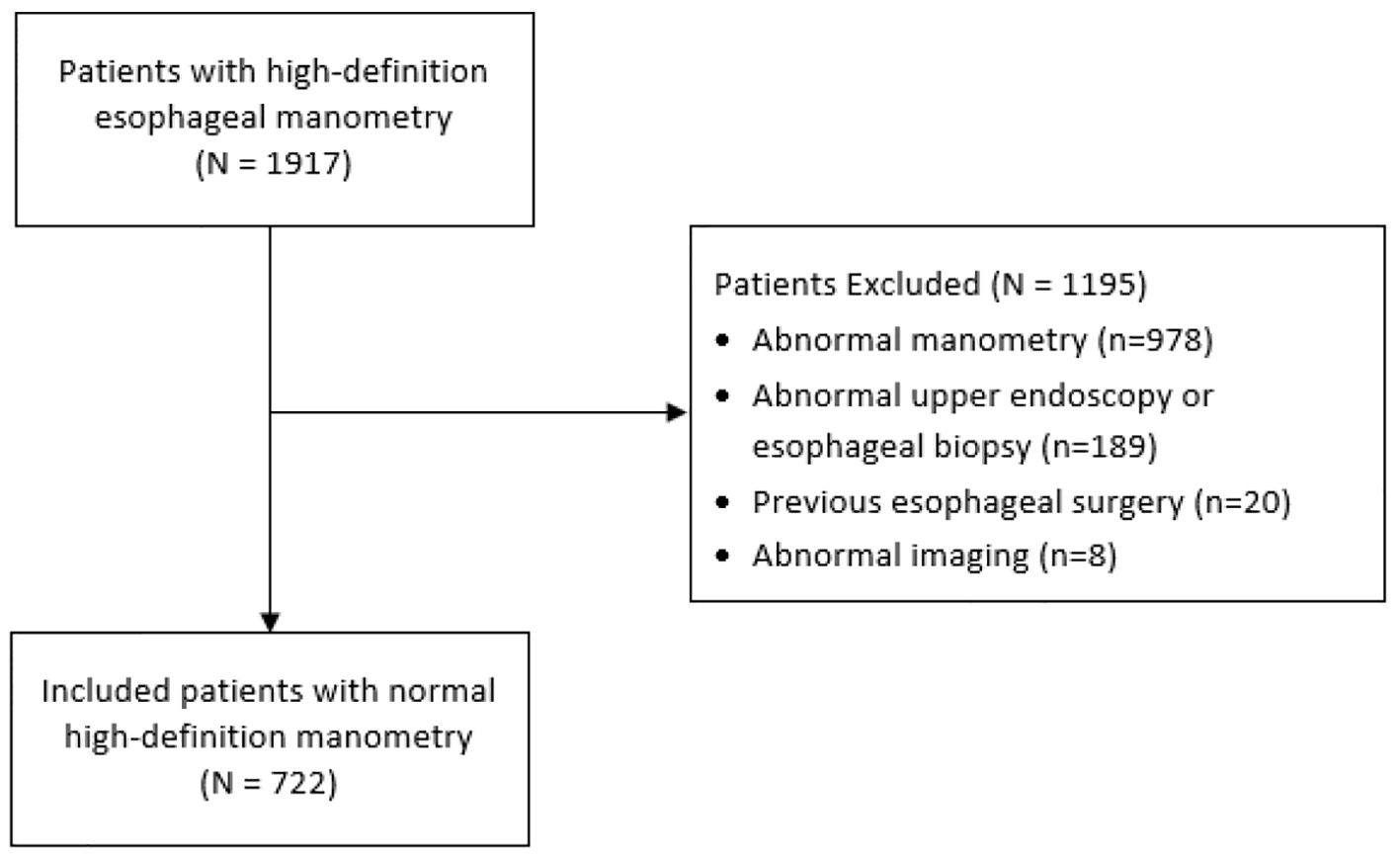 Click for large image | Figure 1. Study flow diagram. |
 Click to view | Table 1. Patient Characteristics |
IRP
IRP values were available in 716 patients. There was a statistically significant relationship between age and IRP with a positive coefficient of 0.20 (P < 0.0001) (Fig. 2). Multivariate analysis showed statistically significant relationships between IRP and age (P < 0.001), BMI (r = -0.14 (95% CI -0.24 to -0.05), P = 0.004), and NSAID use (P = 0.048). There was a trend towards increased IRP with female sex (P = 0.058). NSAID use was associated with a 1.21 mm Hg (95% CI -2.4 to -0.01) decrease in IRP (Table 2).
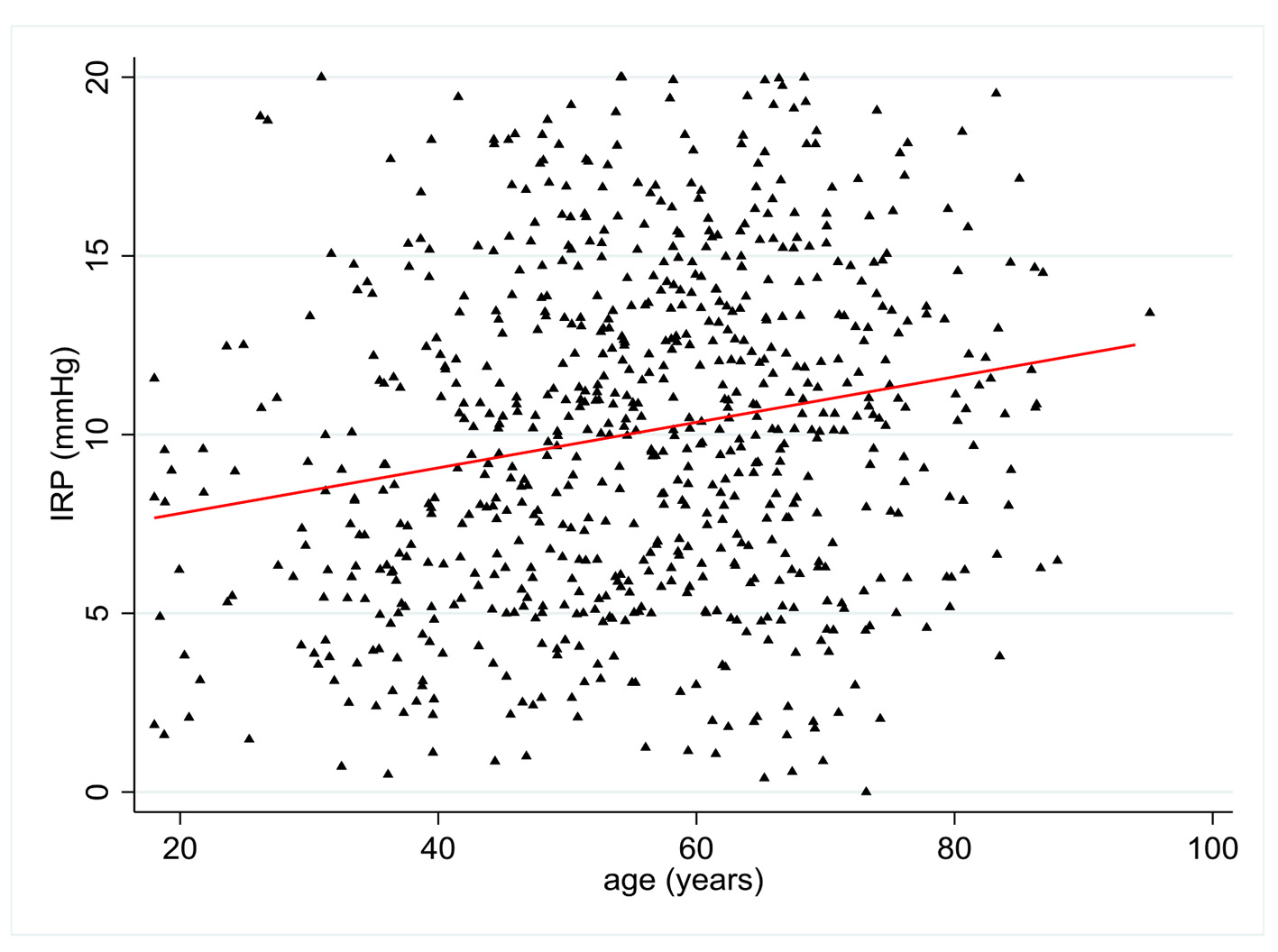 Click for large image | Figure 2. Change in integrated relaxation pressure (IRP) (mm Hg) according to patient age (years). |
 Click to view | Table 2. Multiple Linear Regression Model for IRP |
DCI
DCI values were available in 704 patients. There was a statistically significant relationship between age and DCI with a positive coefficient of 0.12 (P = 0.001) (Fig. 3). Multivariate analysis showed no statistically significant relationship between DCI and all pre-established covariates except age.
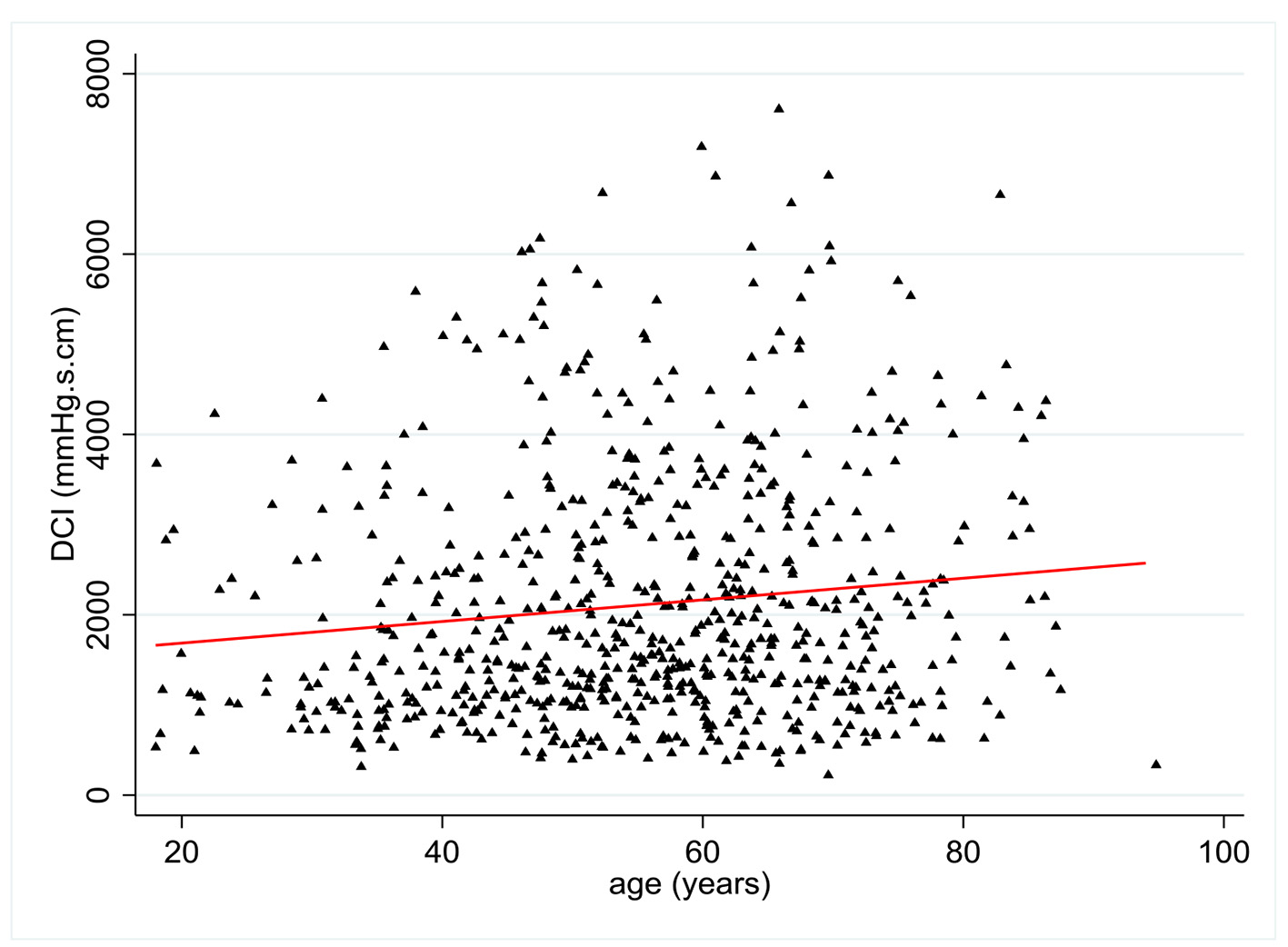 Click for large image | Figure 3. Change in distal contractal integral (DCI) (mm Hg.s.cm) according to patient age (years). |
DL
DL values were available in 676 patients. There was a statistically significant relationship between age and DL with a negative coefficient of 0.09 (P = 0.02) (Fig. 4). Multivariate analysis showed no statistically significant relationship between DL and all pre-established covariates except age.
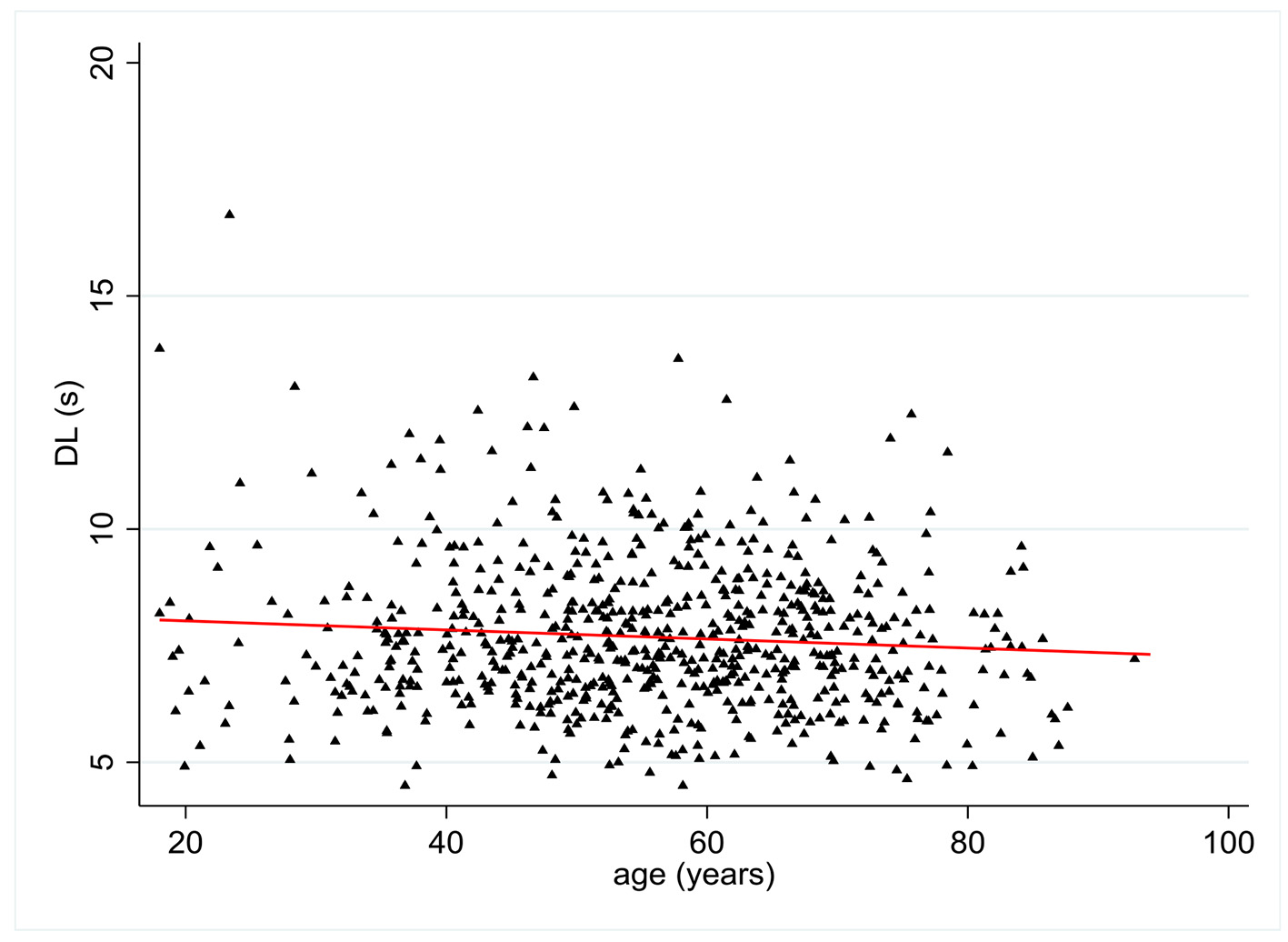 Click for large image | Figure 4. Change in distal latency (DL) (s) according to patient age (years). |
PB
PB values were available in 700 patients. No statistically significant relationship was found between age and PB (r = 0.04, P = 0.3) (Supplementary Figure 1, www.jocmr.org). Multivariate analysis showed statistically significant relationships between PB and NSAID use (P = 0.028).
LES pressure
LES pressure values were available in 718 patients. No statistically significant relationship was found between age and LES pressure (r = 0.01, P = 0.79) (Supplementary Figure 2, www.jocmr.org). Multivariate analysis showed statistically significant relationship between LES pressure and BB use (P = 0.02).
| Discussion | ▴Top |
Our study shows a significant positive correlation between older age and IRP (P < 0.0001) as well as DCI to a lesser extent (P = 0.001). There was also a weaker significant negative correlation between older age and DL (P = 0.01). Analysis using linear regression showed that the expected differences observed between 20- and 80-year-old patients are close to 4 mm Hg for median IRP, 720 mm Hg.s.cm for mean DCI, and 0.6 s for mean DL. This represents a clinically significant variation in HRM parameters when comparing both age extremes.
To our knowledge, this is the largest study to date studying the effect of age on HRM parameters according to the Chicago classification. The current medical literature suffers from low number of included patients, poor quantification of the extent of change in HRM parameters with every year of aging, and conflicting results on parameters affected by aging. One prospective study found higher IRP, lower LES pressure and higher DCI in asymptomatic elderly patients when compared with younger patients [7]. Another study found a lower DCI in elderly patients but no change in LES pressure [8]. A third found higher LES pressure in the elderly [9]. Two studies only found significant differences in patients suffering from GERD [10, 11]. All studies grouped patients in specific age categories and did not perform regression analysis, which could have reduced observed differences between age categories. In our multivariate model, IRP, DCI, and DL were not significantly correlated with GERD as an HRM indication. IRP was however correlated with BMI and NSAID use. There remained a strong statistically significant correlation between age and IRP in our multivariate model regardless of covariate status. Our findings are consistent with a smaller (n = 54) previously published study showing a negative correlation for DL and a positive correlation for IRP and DCI with aging [15]. However, patients were divided into three age groups with few patients per age group and no patients > 67 years old were included, leading the authors to conclude that larger studies including older subjects were needed. In our study, 184 (25.5%) of patients were 65 years and older and 105 (14.5%) were 40 years or younger.
One study performed on patients with non-cardiac chest pain showed that older age was predictive of esophageal motility disorder diagnosis [11]. As our study found that IRP and DCI increased and DL decreased with age, it is possible that the increase in motility disorder diagnoses could be secondary to these observed changes in esophageal physiology with aging. This could also partially explain the symptoms frequently encountered in the older age group when no formal esophageal motility disorder is diagnosed. Increasing awareness of the changes in esophageal motility with aging could help in establishing appropriate diagnoses for both age extremes and can help when interpreting manometry results in the elderly population.
The strength in our study lies in its large sample size, allowing us to perform single and multivariate analyses with high effect size precision. The inclusion of a substantial number of older (≥ 65 years) and younger (≤ 40 years) patients also represents a strength, as many studies lack data on older population groups. The increased representation of all patient age groups improves the external validity of our study and lends credibility to our results. The larger sample size also allowed for granular estimation of changes of HRM parameters with each year of aging. There are however several limitations to our study. The retrospective nature of our study could limit interpretation as we relied on previously completed HRM forms and electronic records for patient data. Missing data on patient comorbidities, BMI, and medication somewhat limit the interpretation of our multivariate analysis. Full information maximum likelihood estimation was used to limit the impact of missing variables as it performs similarly or better than techniques such as multiple imputation [13, 14]. As data were most likely missing completely at random or at a minimum, missing at random, it is unlikely to introduce significant bias to our analysis with the current statistical techniques used. The high variability of HRM parameters within our included population could limit the interpretation of effect size on a patient per patient basis. The single centered nature of our study, performed at a tertiary referral center with only one manufacturer for HRM equipment could also limit the generalizability of our results to other population groups and with other HRM equipment used.
In conclusion, we found that IRP, DCI, and to a lesser extent, DL, are significantly correlated with the normal aging process in symptomatic patients. These changes occurring with aging may explain the increased prevalence of symptoms in the elderly. These results are applicable in clinical practice and should be taken into consideration when interpreting HRM in the elderly. Larger prospective studies need to be performed to validate these results.
| Supplementary Material | ▴Top |
Suppl 1. Change in peristaltic break (PB) according to patient age (years).
Suppl 2. Change in lower esophageal sphincter (LES) pressure according to patient age (years).
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Not applicable.
Author Contributions
Roupen Djinbachian: acquisition of data; analysis and interpretation of data; statistical support; drafting of the manuscript; critical revision of the manuscript for important intellectual content. Etienne Marchand: acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content. Weixiang Yan: analysis and interpretation of data; critical revision of the manuscript for important intellectual content. Mickael Bouin: study concept and design; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
BMI: body mass index; BB: beta-blocker; CCB: calcium channel blocker; DCI: distal contractal integral; DL: distal latency; GERD: gastroesophageal reflux disease; HRM: high-resolution manometry; IRP: integrated relaxation pressure; LES: lower esophageal sphincter; NSAID: non-steroidal anti-inflammatory drug; PB: peristaltic break; PPI: proton pump inhibitor
| References | ▴Top |
- Yadlapati R, Kahrilas PJ, Fox MR, Bredenoord AJ, Prakash Gyawali C, Roman S, Babaei A, et al. Esophageal motility disorders on high-resolution manometry: Chicago classification version 4.0((c)). Neurogastroenterol Motil. 2021;33(1):e14058.
doi pubmed - Bogte A, Bredenoord AJ, Oors J, Siersema PD, Smout AJ. Normal values for esophageal high-resolution manometry. Neurogastroenterol Motil. 2013;25(9):762-e579.
doi pubmed - Sweis R, Anggiansah A, Wong T, Kaufman E, Obrecht S, Fox M. Normative values and inter-observer agreement for liquid and solid bolus swallows in upright and supine positions as assessed by esophageal high-resolution manometry. Neurogastroenterol Motil. 2011;23(6):509-e198.
doi pubmed - Ghosh SK, Pandolfino JE, Rice J, Clarke JO, Kwiatek M, Kahrilas PJ. Impaired deglutitive EGJ relaxation in clinical esophageal manometry: a quantitative analysis of 400 patients and 75 controls. Am J Physiol Gastrointest Liver Physiol. 2007;293(4):G878-885.
doi pubmed - Niebisch S, Wilshire CL, Peters JH. Systematic analysis of esophageal pressure topography in high-resolution manometry of 68 normal volunteers. Dis Esophagus. 2013;26(7):651-660.
doi pubmed - Weijenborg PW, Kessing BF, Smout AJ, Bredenoord AJ. Normal values for solid-state esophageal high-resolution manometry in a European population; an overview of all current metrics. Neurogastroenterol Motil. 2014;26(5):654-659.
doi pubmed - Besanko LK, Burgstad CM, Cock C, Heddle R, Fraser A, Fraser RJ. Changes in esophageal and lower esophageal sphincter motility with healthy aging. J Gastrointestin Liver Dis. 2014;23(3):243-248.
doi pubmed - Shim YK, Kim N, Park YH, Lee JC, Sung J, Choi YJ, Yoon H, et al. Effects of age on esophageal motility: use of high-resolution esophageal impedance manometry. J Neurogastroenterol Motil. 2017;23(2):229-236.
doi pubmed - Besanko LK, Burgstad CM, Mountifield R, Andrews JM, Heddle R, Checklin H, Fraser RJ. Lower esophageal sphincter relaxation is impaired in older patients with dysphagia. World J Gastroenterol. 2011;17(10):1326-1331.
doi pubmed - Kawami N, Iwakiri K, Sano H, Tanaka Y, Sakamoto C. Effects of aging and acid reflux on esophageal motility. Digestion. 2015;91(3):181-186.
doi pubmed - Gomez Cifuentes J, Lopez R, Thota PN. Factors predictive of gastroesophageal reflux disease and esophageal motility disorders in patients with non-cardiac chest pain. Scand J Gastroenterol. 2018;53(6):643-649.
doi pubmed - von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Initiative S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453-1457.
doi pubmed - von Hippel PT. New confidence intervals and bias comparisons show that maximum likelihood can beat multiple imputation in small samples. Structural Equation Modeling: A Multidisciplinary Journal. 2016;23:422-437.
- Larsen R. Missing data imputation versus full information maximum likelihood with second-level dependencies. Structural Equation Modeling: A Multidisciplinary Journal. 2011;18:649-662.
- Jung KW, Jung HY, Myung SJ, Kim SO, Lee J, Yoon IJ, Seo SY, et al. The effect of age on the key parameters in the Chicago classification: a study using high-resolution esophageal manometry in asymptomatic normal individuals. Neurogastroenterol Motil. 2015;27(2):246-257.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


