| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Original Article
Volume 13, Number 5, May 2021, pages 258-267
Low Serum Albumin Predicts Severe Outcomes in COVID-19 Infection: A Single-Center Retrospective Case-Control Study
Roshan Acharyaa, f , Dilli Poudelb, Riley Bowersc, Aakash Patela, Evan Schultza, Michael Bourgeoisa, Rishi Paswana, Scott Stockholma, Macelyn Battena, Smita Kafled, Kriti Loniale, Irlene Lockleare
aDepartment of Internal Medicine, Cape Fear Valley Medical Center, Fayetteville, NC 28304, USA
bDepartment of Rheumatology, Indiana Regional Medical Center, Indiana, PA 15701, USA
cDepartment of Pharmacy, Cape Fear Valley Medical Center, Fayetteville, NC 28304, USA
dRN-BSN Program, Fayetteville State University, Fayetteville, NC 28301, USA
eDepartment of Pulmonology and Critical Care, Cape Fear Valley Medical Center, Fayetteville, NC 28304, USA
fCorresponding Author: Roshan Acharya, Department of Internal Medicine, Cape Fear Valley Hospital, Campbell University Jerry M. Wallace School of Osteopathic Medicine, 1638 Owen Drive, Fayetteville, NC 28304, USA
Manuscript submitted April 18, 2021, accepted May 3, 2021, published online May 25, 2021
Short title: Hypoalbuminemia Has Higher Risk in COVID-19
doi: https://doi.org/10.14740/jocmr4507
| Abstract | ▴Top |
Background: Coronavirus disease 2019 (COVID-19) can cause serious complications such as multiorgan failure and death which are difficult to predict. We conducted this retrospective case-control observational study with the hypothesis that low serum albumin at presentation can predict serious outcomes in COVID-19 infection.
Methods: We included severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) reverse transcriptase-polymerase chain reaction (RT-PCR) confirmed, hospitalized patients from March to July 2020 in a tertiary care hospital in the USA. Patients were followed for 21 days for the development of the primary endpoint defined as the composite outcome which included acute encephalopathy, acute kidney injury, the requirement of new renal replacement therapy, acute hypercoagulability, acute circulatory failure, new-onset heart failure, acute cardiac injury, acute arrhythmia, acute respiratory distress syndrome (ARDS), high flow oxygen support, intensive care unit (ICU) stay, mechanical ventilation or death; and the secondary endpoint of death only. Univariate and multivariate logistic regression analyses were performed to study the effect of albumin level and outcomes.
Results: The mean age was 56.76 years vs. 55.67 years (P = 0.68) in the normal albumin vs. the low albumin group. We noticed an inverse relationship between serum albumin at presentation and serious outcomes. The low albumin group had a higher composite outcome (93.88% vs. 6.12%, P < 0.05) and higher mortality (13.87% vs. 2.38%, P < 0.05) in comparison to the normal albumin group. The multivariate logistic regression analysis revealed higher odds of having composite outcomes with lower albumin group (odds ratio (OR) 10.88, 95% confidence interval (CI) 4.74 - 24.97, P < 0.05). In the subgroup analysis, the multivariate logistic regression analysis revealed higher odds of having composite outcomes with the very low albumin group (OR 7.94, 95% CI 1.70 - 37.14, P < 0.05).
Conclusions: Low serum albumin on presentation in COVID-19 infection is associated with serious outcomes not limited to mortality. The therapeutic option of albumin infusion should be investigated.
Keywords: COVID-19; SARS-CoV-2; Hypoalbuminemia; Serious outcomes; Mortality; Albumin; ARDS; Hypercoagulopathy
| Introduction | ▴Top |
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is a novel viral disease first discovered in December 2019 in Wuhan, China. Within 1 month, it was confirmed in 19 countries [1] and the World Health Organization declared it a pandemic shortly thereafter [2]. As of now, it has been established as a highly contagious viral disease, primarily affecting the respiratory system but with complications including kidney injury, liver injury, cardiac injury, stroke, encephalopathy, post-viral debility, coagulopathy, long hospital stay, and significant risk of mortality [3-12]. The majority of the patients survive the infection without complications, but a notable proportion of patients develop serious complications. Since the beginning, high levels of acute inflammatory markers were observed in the patients who had poor outcomes but the factors that lead to serious complications remain inadequately understood [3-5].
Albumin serves as a major anti-inflammatory agent in our body [13, 14], and one of the lesser discussed properties in the literature are its anti-oxidative and anti-thrombotic property [15, 16]. Albumin is a major defense that protects the host cells from the oxidative burst that occurs against the infection or inflammation [17-19]. Albumin has a long half-life (3 weeks) and 90% of it is in plasma [20]. The level of plasma is dropped rapidly during acute inflammation due to transcapillary leakage, consumption, and other mechanisms [13, 19, 21, 22].
As of now, we lack proven and effective therapeutic options to treat serious complications of COVID-19 infection. We also lack the ability to predict the serious outcomes of COVID-19 infection. In many studies, low levels of albumin, in addition to high inflammatory markers had been observed with those patients that had poor outcomes. Some studies have suggested that low serum albumin can predict mortality in COVID-19 infection [23-27].
We conducted this study to explore this relationship further with the hypothesis that low serum albumin at presentation can predict serious outcomes in COVID-19 infection.
| Materials and Methods | ▴Top |
The study was approved by Cape Fear Valley Medical Center’s Institutional Review Board (IRB ID number 319-20). This study was conducted in compliance with the ethical standards of the IRB on human subjects.
Study design and population
The study was a single-center retrospective case-control study. We reviewed the charts of patients with the discharge diagnosis of “COVID-19, virus identified” (ICD code U07.1) from March 1 to July 31, 2020 in a tertiary care center in North Carolina, United States of America (USA). We identified 796 charts that had confirmed SARS-CoV-2 reverse transcriptase-polymerase chain reaction (RT-PCR) results of nasopharyngeal swab. After exclusions, a total of 352 charts of unique patients were included in the study (Fig. 1). Inclusion criteria included: 1) patients with SARS-CoV-2 detected using the RT-PCR method, 2) age 18 and above, and 3) admission date between March 1, 2020 and July 31, 2020. Patients with 1) emergency department (ED) visits only, 2) less than 18 years of age, 3) pregnancy, and 4) no serum albumin available at presentation in the ED were excluded (Fig. 1).
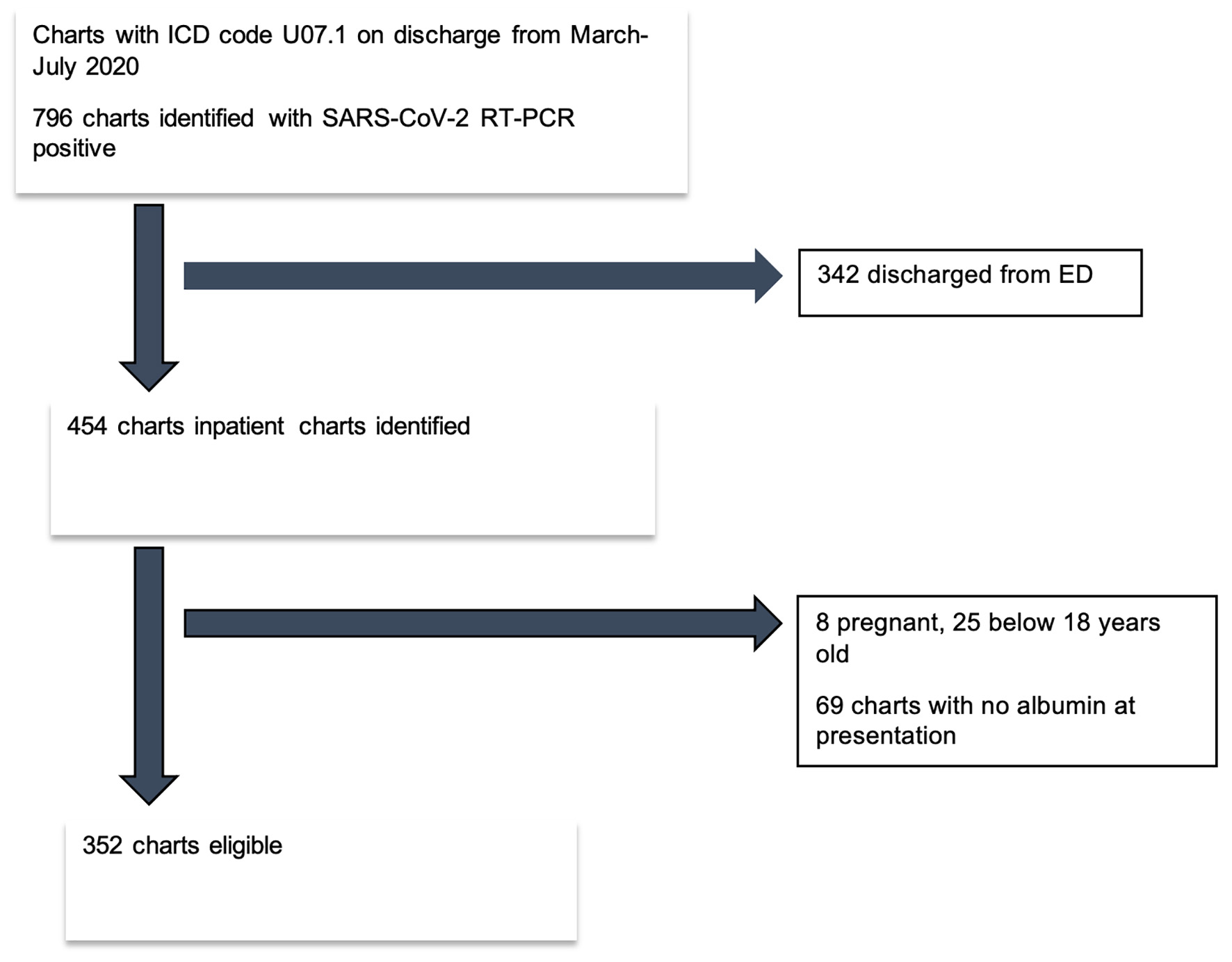 Click for large image | Figure 1. Flow diagram of eligible patient selection. RT-PCR: reverse transcriptase polymerase chain reaction; SARS-CoV-2: severe respiratory syndrome coronavirus 2. |
Included patients were classified into two groups based on serum albumin at presentation: normal serum albumin (NSA) group with a value of ≥ 3.5 g/dL, and low serum albumin (LSA) group < 3.5 g/dL. The low albumin group was further sub-grouped as mild low albumin (MLA) group with a value of 2.5 - 3.4 g/dL and very low albumin (VLA) group with a value of < 2.5 g/dL for subgroup analysis. The patients were followed up for 21 days or until death if it happened sooner.
Outcomes
The primary outcome was defined as a composite outcome of death or any serious complications. The variables of serious complications were death, acute encephalopathy, acute kidney injury, the requirement of new renal replacement therapy, acute hypercoagulability, acute circulatory failure, new-onset heart failure, acute cardiac injury, acute arrhythmia, acute respiratory distress syndrome (ARDS), high flow oxygen support, intensive care unit (ICU) stay, and mechanical ventilation. The study definitions and definitions of serious complications are outlined in Table 1. The secondary outcome was death due to any cause during the 21 days follow-up period. The abbreviations, normal ranges, and units of variables are outlined in Table 2.
 Click to view | Table 1. Study Definition |
 Click to view | Table 2. Abbreviations, Normal Ranges and Units |
Statistical analysis
A formal sample size calculation was not carried out as all patients meeting criteria in the pre-specified timeframe were included. The differences in categorical variables were analyzed using a Chi-square test, or Fisher’s exact test as appropriate. A Student’s t-test was utilized to evaluate differences in continuous variables that were normally distributed, and a Wilcoxon rank sum test was utilized if the data were not normally distributed. Continuous variables were expressed as mean ± SD and categorical variables were expressed as the frequency with percentages. The association of albumin level category and outcome was estimated in univariate and multivariate logistic regression analyses in terms of odds ratio (OR). Multivariate analyses were adjusted for age, sex, race, diabetes mellitus (DM), hypertension (HTN), chronic kidney disease (CKD), end-stage renal disease on hemodialysis (ESRD on HD), chronic obstructive pulmonary disease (COPD), other lung diseases, congestive heart failure (CHF), coronary artery disease (CAD), human immunodeficiency virus (HIV) infection, malignancy, smoking, alcohol dependency, obesity, and cirrhosis. A P-value of < 0.05 was considered significant. Data analysis was performed using STATA 13.1 (Stata Corp., College Station, TX).
| Results | ▴Top |
Demographic features
Baseline demographic and lab characteristics by albumin level categories are depicted in Table 3. The overall population was predominantly males (n = 184, 52.27%), and African-American (n = 176, 50%). Eighty-one percentage (n = 288) had at least one co-morbidity. Cough (62.78% vs. 73.81%, P = 0.16), fever (58.90% vs. 64.29%, P = 0.5), and shortness of breath (64.08% vs. 59.52%, P = 0.56) were the most common presenting symptoms in the LSA and NSA groups, respectively. The frequency of CKD was higher among the NSA group (35.71% vs. 17.10%, P < 0.05). Among the subgroups of low albumin, the presenting symptoms and co-morbidities were similar between the two groups (Table 3).
 Click to view | Table 3. Baseline Characteristics of Normal and Low Serum Albumin Groups |
Primary outcome
The LSA group had a significantly higher proportion of composite outcome compared to the NSA group (93.88% vs. 6.12%, P < 0.05). This difference was true in between the subgroups of only low albumin as well, where 96.72% of the VLA group had composite outcome as compared to 81.12% in the MLA group (P < 0.05) (Table 4).
 Click to view | Table 4. In-Hospital Complications and Duration of Stays in Normal and Low Serum Albumin Groups |
Logistic regression analysis revealed higher odds of having composite outcome with the lower albumin group during univariate (OR 7.83, 95% confidence interval (CI) 3.94 - 15.58, P < 0.05) and multivariate (OR 10.88, 95% CI 4.74 - 24.97, P < 0.05) analyses. Subgroup analysis also revealed a similar relationship (Table 5).
 Click to view | Table 5. Results From Logistic Regression Analyses - Odds of Composite Outcome Based on Albumin Level Category |
In comparison to the NSA group, the LSA group had higher length of stay (9.33 days vs. 4.48 days, P < 0.05), higher incidence of acute kidney injury (AKI) (42.90% vs. 14.29%, P < 0.05), acute encephalopathy (22.90% vs. 7.14%, P < 0.05), mechanical ventilation use (13.23% vs. 2.38%, P < 0.05), development of ARDS (19.35% vs. 2.39%, P < 0.05) and post-viral physical debility (24.19% vs. 3%, P < 0.05). Similar differences were observed between the VLA and MLA groups with a higher incidence among the VLA group (Table 4). Multivariate analysis revealed that malignancy had the strongest influence on the primary outcome (OR 11.34, 95% CI 2.05 - 62.64, P < 0.05) (Table 6).
 Click to view | Table 6. Results From MV Analysis in Normal and Low Albumin Groups |
Secondary outcome
The LSA group was found to have higher mortality within 21 days as compared to the NSA group (13.87% vs. 2.38%, P < 0.05). Subgroup analysis of the low albumin group revealed 31.15% mortality in the VLA group vs. 9.64% in the MLA group (P < 0.05) (Table 4). We were unable to perform the regression analysis on mortality outcomes due to the very low number of mortalities in the normal albumin group.
| Discussion | ▴Top |
In this single-center study, we found that low serum albumin at the presentation in hospitalized patients with COVID-19 infection predicted higher mortality. Lower serum albumin at presentation was found to be an independent predictor of serious outcomes even after controlling for the multitude of factors such as age, sex, and presence of co-morbid conditions.
Low albumin level has been reported in hospitalized patients with COVID-19 that had poor outcomes from the beginning of the pandemic [3-5, 28]. A similar trend was observed in other studies that were conducted outside of China as well [26, 27, 29]. A few studies that compared low albumin level with mortality concluded that low albumin can predict mortality in COVID-19 patients [23-25]. In addition to low albumin, high levels of inflammatory markers were also noted in those studies. The findings of our study are consistent with these prior findings.
Low albumin has been found to predict higher mortality, a longer length of stay in hospitalized patients, and the general elderly population [30-34]; normalization of albumin prior to discharge was found to lower the mortality [33]. Albumin therapy has been shown to improve the oxygenation in ARDS [35, 36], and improve mortality in spontaneous bacterial peritonitis patients [37]. In the letter to the editor by Wiedermann et al and its response, the authors reported pooled analysis of three large-scale randomized controlled trials on sepsis and found that albumin therapy had a significant reduction in mortality which was attributed partly to albumin’s anti-oxidative and anti-immunosuppressive property [38]. The mechanistic theory for this includes albumin’s ability to bind reactive oxygen and reactive nitrogen species (ROS and RNS) preventing cellular damage and tissue injury during overwhelming inflammatory response [17-19]. These findings have given rise to the concept of the potential therapeutic use of albumin for COVID-19 infection [27, 39].
One of the limitations of our study is being a single-center study. More than 50% of the charts needed to be excluded decreasing the sample size. However, our study sample size was comparable to many similar studies done recently. The population was of mixed races unlike many studies done in China or Europe. We could not do the multivariate analysis of mortality because there was only one death in the normal albumin group.
COVID-19 has been diagnosed in more than 200 countries [40]. In the USA alone more than 30 million people were infected, and 556 thousand had died [41]. So far, there is no effective treatment for COVID-19 infection [40, 42-47], and the success of developed vaccines being unanswered due to emerging new strains of SARS-CoV-2. Due to recurrent waves of infection caused by emerging strains of SARS-CoV-2 the South Asian region, South American, Europe and other parts of the world had been through series of lockdowns. In addition, the vaccines are still out of reach of many low-medium income countries. We believe that this study will help health providers recognize that lower albumin carries a higher risk of severe complications and mortality in COVID-19 infection. It will be interesting to see the studies that utilize albumin as a treatment for serious COVID-19 illness.
Conclusion
Low serum albumin on presentation in COVID-19 infection is associated with a higher incidence of serious outcomes like kidney injury, cardiac injury, hypercoagulability, post-viral physical debility, and encephalopathy; and higher mortality. Further investigation of the utility of albumin replacement as a treatment of COVID-19 infection should be done as soon as possible.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
The informed consent was waived by IRB as the study was a retrospective study.
Author Contributions
RA designed the study, portions of statistical analysis, manuscript writing; DP and RB did the statistical analysis and contributed to manuscript writing; RA, AP, ES, MB, RP, SS, MB and SK did the portions of statistical analysis, and contributed to manuscript writing; KL and IL contributed to manuscript writing and supervision of the study. All authors read and approved the final manuscript.
Data Availability
The database used and/or analyzed during the current study is available from the corresponding author on reasonable request.
| References | ▴Top |
- Adhikari SP, Meng S, Wu YJ, Mao YP, Ye RX, Wang QZ, Sun C, et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect Dis Poverty. 2020;9(1):29.
doi pubmed - WHO Director-General’s opening remarks at the media briefing on COVID-19. March 11, 2020 [Internet]. [cited Oct 9, 2020]. Available from: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020.
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506.
doi - Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062.
doi - Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, Ma K, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091.
doi pubmed - Chen YT, Shao SC, Hsu CK, Wu IW, Hung MJ, Chen YC. Incidence of acute kidney injury in COVID-19 infection: a systematic review and meta-analysis. Crit Care. 2020;24(1):346.
doi pubmed - Long B, Brady WJ, Koyfman A, Gottlieb M. Cardiovascular complications in COVID-19. Am J Emerg Med. 2020;38(7):1504-1507.
doi pubmed - Levi M, Thachil J, Iba T, Levy JH. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7(6):e438-e440.
doi - Klok FA, Kruip M, van der Meer NJM, Arbous MS, Gommers D, Kant KM, Kaptein FHJ, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-147.
doi pubmed - Middeldorp S, Coppens M, van Haaps TF, Foppen M, Vlaar AP, Muller MCA, Bouman CCS, et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18(8):1995-2002.
doi pubmed - Mao R, Qiu Y, He JS, Tan JY, Li XH, Liang J, Shen J, et al. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5(7):667-678.
doi - Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683-690.
doi pubmed - Artigas A, Wernerman J, Arroyo V, Vincent JL, Levy M. Role of albumin in diseases associated with severe systemic inflammation: Pathophysiologic and clinical evidence in sepsis and in decompensated cirrhosis. J Crit Care. 2016;33:62-70.
doi pubmed - Rozga J, Piatek T, Malkowski P. Human albumin: old, new, and emerging applications. Ann Transplant. 2013;18:205-217.
doi pubmed - Grigoriadis G, Stewart AG. Albumin inhibits platelet-activating factor (PAF)-induced responses in platelets and macrophages: implications for the biologically active form of PAF. Br J Pharmacol. 1992;107(1):73-77.
doi pubmed - Kim SB, Chi HS, Park JS, Hong CD, Yang WS. Effect of increasing serum albumin on plasma D-dimer, von Willebrand factor, and platelet aggregation in CAPD patients. Am J Kidney Dis. 1999;33(2):312-317.
doi - Rabbani G, Ahn SN. Structure, enzymatic activities, glycation and therapeutic potential of human serum albumin: A natural cargo. Int J Biol Macromol. 2019;123:979-990.
doi pubmed - Galley HF. Oxidative stress and mitochondrial dysfunction in sepsis. Br J Anaesth. 2011;107(1):57-64.
doi pubmed - Caraceni P, Tufoni M, Bonavita ME. Clinical use of albumin. Blood Transfus. 2013;11(Suppl 4):s18-25.
- Garcovich M, Zocco MA, Gasbarrini A. Clinical use of albumin in hepatology. Blood Transfus. 2009;7(4):268-277.
- Moshage HJ, Janssen JA, Franssen JH, Hafkenscheid JC, Yap SH. Study of the molecular mechanism of decreased liver synthesis of albumin in inflammation. J Clin Invest. 1987;79(6):1635-1641.
doi pubmed - Brenner DA, Buck M, Feitelberg SP, Chojkier M. Tumor necrosis factor-alpha inhibits albumin gene expression in a murine model of cachexia. J Clin Invest. 1990;85(1):248-255.
doi pubmed - Huang J, Cheng A, Kumar R, Fang Y, Chen G, Zhu Y, Lin S. Hypoalbuminemia predicts the outcome of COVID-19 independent of age and co-morbidity. J Med Virol. 2020;92(10):2152-2158.
doi pubmed - Violi F, Cangemi R, Romiti GF, Ceccarelli G, Oliva A, Alessandri F, Pirro M, et al. Is Albumin Predictor of Mortality in COVID-19? Antioxid Redox Signal. 2020.
doi pubmed - Li J, Li M, Zheng S, Li M, Zhang M, Sun M, Li X, et al. Plasma albumin levels predict risk for nonsurvivors in critically ill patients with COVID-19. Biomark Med. 2020;14(10):827-837.
doi pubmed - de la Rica R, Borges M, Aranda M, Del Castillo A, Socias A, Payeras A, Rialp G, et al. Low albumin levels are associated with poorer outcomes in a case series of covid-19 patients in Spain: a retrospective cohort study. Microorganisms. 2020;8(8):1106.
doi pubmed - Herlekar R, Sur Roy A, Matson M. Hypoalbuminaemia in COVID-19 infection: A predictor of severity or a potential therapeutic target? J Med Virol. 2021;93(1):83-84.
doi pubmed - Zhang Y, Zheng L, Liu L, Zhao M, Xiao J, Zhao Q. Liver impairment in COVID-19 patients: A retrospective analysis of 115 cases from a single centre in Wuhan city, China. Liver Int. 2020;40(9):2095-2103.
doi pubmed - Li T, Guo Y, Zhuang X, Huang L, Zhang X, Wei F, Yang B. Abnormal liver-related biomarkers in COVID-19 patients and the role of prealbumin. Saudi J Gastroenterol. 2020;26(5):272-278.
doi pubmed - Sullivan DH, Roberson PK, Bopp MM. Hypoalbuminemia 3 months after hospital discharge: significance for long-term survival. J Am Geriatr Soc. 2005;53(7):1222-1226.
doi pubmed - Reinhardt GF, Myscofski JW, Wilkens DB, Dobrin PB, Mangan JE, Jr., Stannard RT. Incidence and mortality of hypoalbuminemic patients in hospitalized veterans. JPEN J Parenter Enteral Nutr. 1980;4(4):357-359.
doi pubmed - Sullivan DH, Roberson PK, Johnson LE, Mendiratta P, Bopp MM, Bishara O. Association between inflammation-associated cytokines, serum albumins, and mortality in the elderly. J Am Med Dir Assoc. 2007;8(7):458-463.
doi pubmed - Akirov A, Masri-Iraqi H, Atamna A, Shimon I. Low albumin levels are associated with mortality risk in hospitalized patients. Am J Med. 2017;130(12):1465 e1411-1465 e1419.
doi pubmed - Herrmann FR, Safran C, Levkoff SE, Minaker KL. Serum albumin level on admission as a predictor of death, length of stay, and readmission. Arch Intern Med. 1992;152(1):125-130.
doi pubmed - Martin GS, Moss M, Wheeler AP, Mealer M, Morris JA, Bernard GR. A randomized, controlled trial of furosemide with or without albumin in hypoproteinemic patients with acute lung injury. Crit Care Med. 2005;33(8):1681-1687.
doi pubmed - Uhlig C, Silva PL, Deckert S, Schmitt J, de Abreu MG. Albumin versus crystalloid solutions in patients with the acute respiratory distress syndrome: a systematic review and meta-analysis. Crit Care. 2014;18(1):R10.
doi pubmed - Salerno F, Navickis RJ, Wilkes MM. Albumin infusion improves outcomes of patients with spontaneous bacterial peritonitis: a meta-analysis of randomized trials. Clin Gastroenterol Hepatol. 2013;11(2):123-130 e121.
doi pubmed - Wiedermann CJ, Joannidis M. Albumin replacement in severe sepsis or septic shock. N Engl J Med. 2014;371(1):83.
doi - Mani Mishra P, Uversky VN, Nandi CK. Serum albumin-mediated strategy for the effective targeting of SARS-CoV-2. Med Hypotheses. 2020;140:109790.
doi pubmed - Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782-793.
doi pubmed - CDC. COVID Data Tracker [Internet]. Centers for Disease Control and Prevention. 2020 [cited 2021 Apr 8]. Available from: https://covid.cdc.gov/covid-data-tracker.
- Cavalcanti AB, Zampieri FG, Rosa RG, Azevedo LCP, Veiga VC, Avezum A, Damiani LP, et al. Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate Covid-19. N Engl J Med. 2020;383(21):2041-2052.
doi pubmed - Campochiaro C, Della-Torre E, Cavalli G, De Luca G, Ripa M, Boffini N, Tomelleri A, et al. Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study. Eur J Intern Med. 2020;76:43-49.
doi pubmed - Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, et al. Remdesivir for the treatment of COVID-19 - final report. N Engl J Med. 2020;383(19):1813-1826.
doi pubmed - RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. Dexamethasone in hospitalized patients with COVID-19 - preliminary report. N Engl J Med. 2020.
- Rosenberg ES, Dufort EM, Udo T, Wilberschied LA, Kumar J, Tesoriero J, Weinberg P, et al. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York State. JAMA. 2020;323(24):2493-2502.
doi pubmed - Li L, Zhang W, Hu Y, Tong X, Zheng S, Yang J, Kong Y, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA. 2020;324(5):460-470.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


