| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Original Article
Volume 13, Number 3, March 2021, pages 177-183
Palliative Thoracic Radiotherapy for Non-Small Cell Lung Cancer in Outpatients: Reasons for Unplanned Hospitalization and Its Impact on Survival
Carsten Niedera, b, d, Kristian S. Imingena, b, Ellinor Hauklanda, b, c
aDepartment of Oncology and Palliative Medicine, Nordland Hospital, 8092 Bodo, Norway
bDepartment of Clinical Medicine, Faculty of Health Sciences, University of Tromso, 9037 Tromso, Norway
cSHARE - Center for Resilience in Healthcare, Faculty of Health Sciences, Department of Quality and Health Technology, University of Stavanger, 4036 Stavanger, Norway
dCorresponding Author: Carsten Nieder, Department of Oncology and Palliative Medicine, Nordland Hospital, 8092 Bodo, Norway
Manuscript submitted February 9, 2021, accepted February 16, 2021, published online March 19, 2021
Short title: Hospitalization After NSCLC Radiotherapy
doi: https://doi.org/10.14740/jocmr4445
| Abstract | ▴Top |
Background: The aims of the study were to examine the rates of and reasons for unplanned hospitalization after start of palliative radiotherapy or chemoradiation (CRT), and to study whether unplanned hospitalization deteriorates patients’ prognosis. In addition, risk factors were identified.
Methods: A retrospective review of 136 patients treated with palliative radiotherapy or CRT was performed. Inclusion criteria were prescribed total dose at least 30 Gy and outpatient at the start of treatment. Uni- and multivariate analyses were employed.
Results: Fifty-eight patients (43%) were hospitalized within 3 months from start of radiotherapy or CRT. Their median overall survival was 6.7 months as compared to 11.1 months in non-hospitalized patients (P = 0.09). The median length of hospitalization was 8 days (range 1 - 61). In patients with possibly treatment-related hospitalization (n = 32), median survival was 5.0 months, significantly shorter than the 11.1 months observed in the remaining patients (P = 0.006). In multivariate analysis, only one variable was significantly associated with higher risk of unplanned hospitalization: previous hospitalization in the last 4 weeks before commencing radiotherapy or CRT.
Conclusions: Unplanned hospitalization occurred frequently in a standard care setting without early involvement of a dedicated palliative team. Patients with preceding hospitalization might represent a group that is particularly vulnerable, thus qualifying for a targeted intervention aiming at continued outpatient care.
Keywords: Lung cancer; Radiotherapy; Chemoradiation; Palliative radiation therapy; Hospitalization; Toxicity
| Introduction | ▴Top |
Palliative radiotherapy, increasingly in combination with platinum-based chemotherapy, continues to play an important role in the management of non-small cell lung cancer (NSCLC) [1-3]. Such treatment often provides symptom relief, objective imaging responses and improved survival [4]. However, side effects such as esophagitis, pneumonitis and chemotherapy-related infections may negatively impact on the patients’ overall well-being, and even cause hospitalization [5]. In a Norwegian randomized trial of chemotherapy versus chemoradiation (42 Gy in 15 fractions), which showed superiority of the combined approach, 40% of the patients in the combined arm were hospitalized once and 11% twice in relation to side effects [6]. In order to shed more light on the issue of unplanned hospitalization in the context of palliative radiotherapy and chemoradiation for NSCLC, we defined several research questions: 1) What are the rates of and reasons for hospitalization outside of a prospective clinical trial? 2) Does unplanned hospitalization deteriorate patients’ prognosis? 3) Who is at increased risk of unplanned hospitalization? These questions were addressed in a single center retrospective study that utilized an updated, previously established database [7, 8].
| Materials and Methods | ▴Top |
A retrospective review of 136 consecutive patients treated with palliative three-dimensional conformal radiotherapy or chemoradiation between 2009 and 2019 was performed. Inclusion criteria were prescribed total dose 30 - 54 Gy, and not hospitalized at the start of radiotherapy. Figure 1 shows the study flow chart. Patients treated with low-dose radiotherapy, primarily 2 fractions of 8.5 Gy, were excluded. All 136 study patients had Eastern Cooperative Oncology Group (ECOG) performance status 0 - 2. In case of chemoradiation, most patients received the Norwegian CONRAD regime (15 fractions of 2.8 Gy, carboplatin/vinorelbine before and during radiotherapy) [6]. Clinical information throughout follow-up after cancer diagnosis was abstracted from our electronic patient record system in order to capture hospitalization before, during or after radiotherapy. The system also captures hospitalization at all other hospitals in our healthcare region, thus providing complete data. This aspect is important, because many patients live remote from the region’s main hospital, which provides all radiotherapy. To capture hospitalization as result of treatment-related acute side effects, we limited registration of events to the first 3 months after start of radiotherapy [8]. After assessing the reason for hospitalization from the discharge letter, we dichotomized reasons into “possibly treatment-related side effect” and “unrelated”, as an expansion of a previous analysis of thoracic toxicity [8]. In this context, we opted for a liberal definition of “possibly related”, bearing in mind that for example radiation pneumonitis can be difficult to distinguish from other pulmonary conditions. All patients were followed during treatment by oncology nurses (after 50% of planned sessions) and physicians (day before final session). After the first follow-up visit at 6 - 8 weeks from the final session, intervals were increased to 3 months. Blood tests results were obtained approximately 1 week before radiotherapy, when treatment planning scans were taken. Treatment plans were calculated with Varian Eclipse TPS® and no intensity-modulated or arc-based techniques were employed. IBM SPSS v.25 was employed for statistical analyses. The latter included Chi-square test and binary logistic regression for associations between unplanned hospitalization (yes/no) and clinical and dosimetric variables. Significant variables, i.e., P < 0.05 in two-sided tests, were then included in multi-nominal logistic regression analysis. Actuarial overall survival was calculated according to the Kaplan-Meier method and the log-rank test was employed for comparison of survival curves. At the time of analysis, 35 patients were alive (censored observations after a median follow-up of 13.8 months). Date of death was known for all remaining patients. This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration. As a retrospective quality of care analysis, no approval from the Regional Committee for Medical and Health Research Ethics (REK Nord) was necessary.
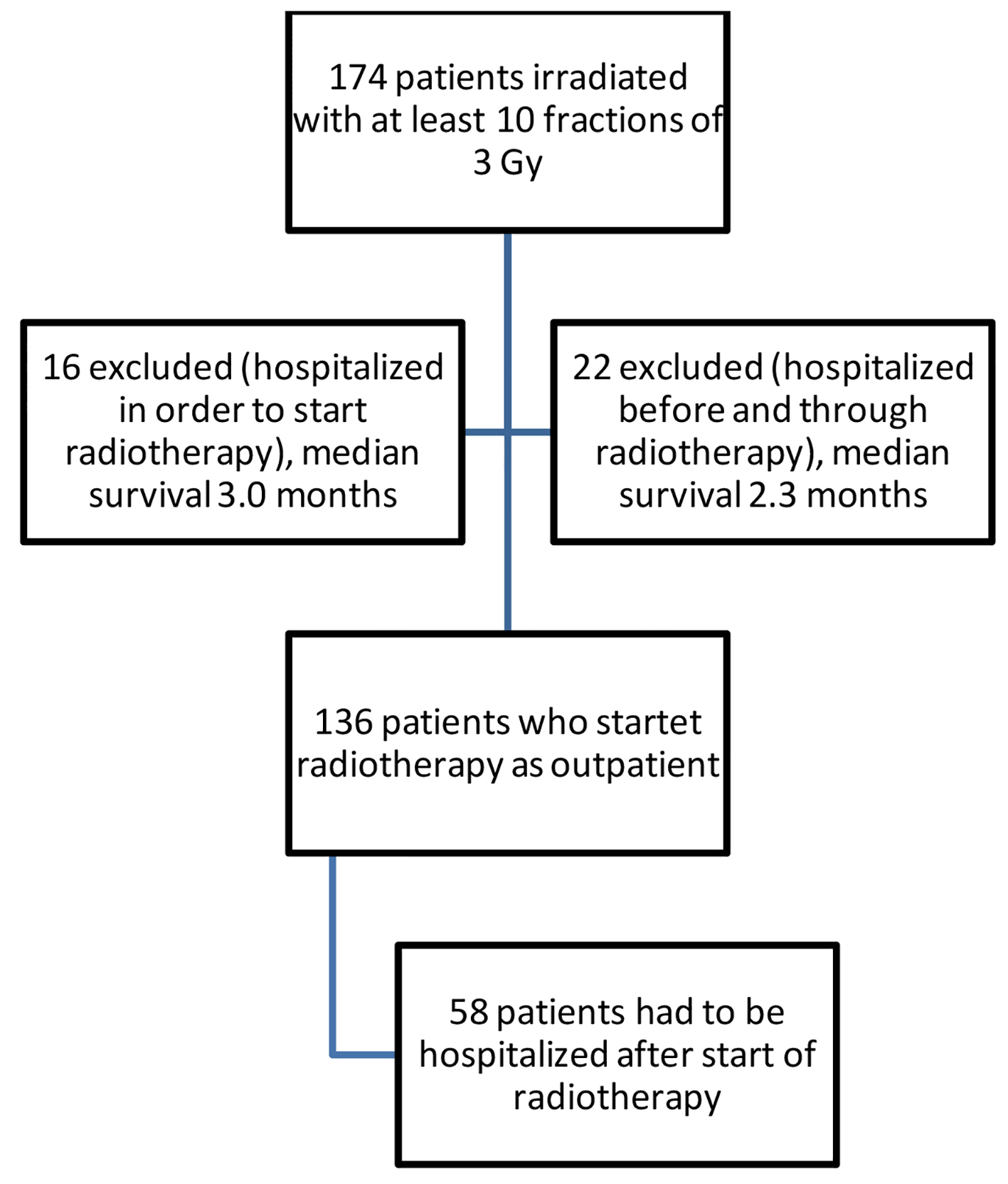 Click for large image | Figure 1. Study flow chart. |
| Results | ▴Top |
The median age was 69 years (range 41 - 90). Seventy-eight patients (57%) were men (Table 1). Stage and histology distribution were as follows: less than III in 8%, III in 47%, IV in 45%, adenocarcinoma in 42% and squamous cell carcinoma in 40% (other or unspecified in 18%). T3 and T4 tumors were treated in 37% and 26%, respectively (N2: 40%, N3: 32%). The median size of the clinical target volume (CTV) was 134 cm3 (range 19 - 1,185). Anemia was present in 44%, defined as serum hemoglobin below institutional limit of normal. Elevated C-reactive protein (CRP) was present in 72%, defined as > 4 mg/L (above institutional upper limit of normal). Low albumin was present in only 1% (< 36 g/L, below institutional limit of normal). Only 4% were never smokers and 20% were active smokers at the time of radiotherapy. Twenty-seven percent had a diagnosis of chronic obstructive pulmonary disease (COPD). Other comorbidity was not available in the database. Thirty percent were treated with 10 fractions of 3 Gy (“low” dose), 17% with “intermediate” dose (e.g., 13 fractions of 3 Gy) and 53% with “high” dose (e.g., 15 fractions of 2.8 or 3 Gy). Forty percent were chemotherapy-naive when they received radiotherapy. Concomitant chemoradiation was given in 35%. Fifty-two percent received any systemic therapy after they had finished radiotherapy. Any type of oral steroid medication was used concomitant to radiotherapy in 33% of patients. Reasons included comorbidity, reduced appetite, presence of brain metastases, etc. Dose and duration of steroid treatment varied. Twenty-seven of these 136 non-hospitalized patients (20%) had previously been hospitalized in the last 4 weeks preceding radiotherapy (for any reason), but were discharged before commencing.
 Click to view | Table 1. Baseline Characteristics Before Palliative Radiotherapy in 136 Patients |
Fifty-eight patients (43%) were hospitalized within 3 months from start of outpatient radiotherapy or chemoradiation. Their median overall survival was 6.7 months as compared to 11.1 months in non-hospitalized patients (P = 0.09). The median length of hospitalization was 8 days (range 1 - 61). Table 2 displays the reasons for hospitalization, which included infections, esophagitis and need for palliative measures, among others. In patients with possibly treatment-related hospitalization (n = 32), median survival was 5.0 months, significantly shorter than the 11.1 months observed in the remaining patients (P = 0.006, Fig. 2).
 Click to view | Table 2. Reasons for Hospitalization in 58 Patients (32 Possibly Related to Treatment) |
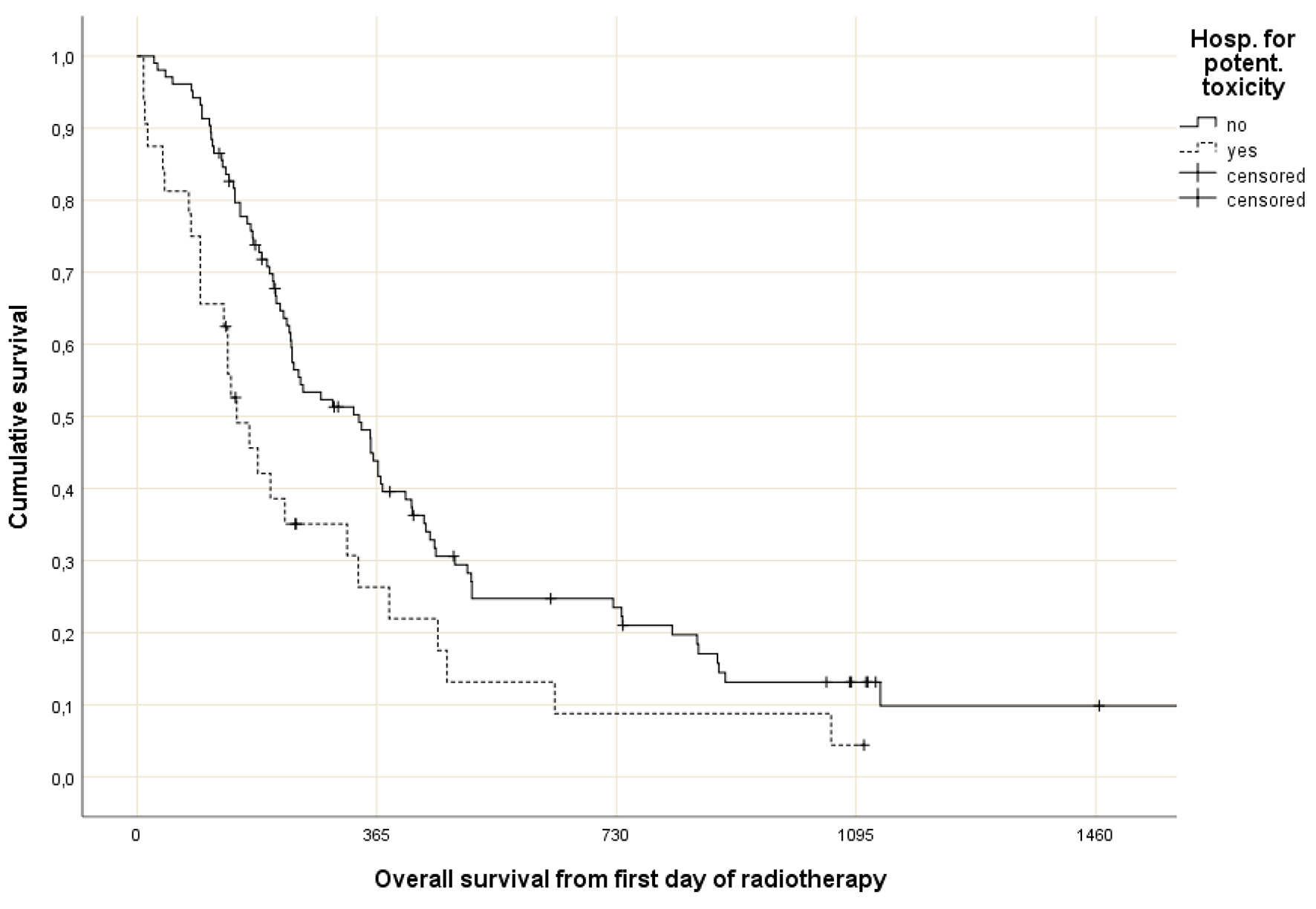 Click for large image | Figure 2. Actuarial overall survival (Kaplan-Meier curves) for 32 patients with possibly treatment-related hospitalization and 104 patients with unrelated hospitalization or no hospitalization at all (P = 0.006, log-rank test). |
The vast majority of baseline parameters and treatment-related factors were not significantly associated with unplanned hospitalization in univariate tests. Here, unplanned included any hospitalization, regardless of reason, and thus included all 58 patients. High P-values > 0.1 were found for T stage, N stage, M stage, age, active smoking, COPD, anemia, low albumin, radiation dose (three strata), preceding chemotherapy, concomitant chemotherapy, systemic treatment after radiotherapy, CTV size, mean lung dose, lung volume that received ≥ 20 Gy (V20), maximum heart dose, maximum esophageal dose and mean esophageal dose. Sex was not significantly associated either. However, the P-value was 0.07 (52% hospitalization in women, 36% in men). Table 3 shows the factors that had univariate P-values < 0.05 and were significantly associated with hospitalization, i.e., elevated serum CRP, steroid medication and previous hospitalization in the last 4 weeks before radiotherapy. Only previous hospitalization in the last 4 weeks before radiotherapy remained significant in the multi-nominal logistic regression analysis.
 Click to view | Table 3. Risk Factors for Unplanned Hospitalization |
| Discussion | ▴Top |
Any hospitalization is associated with a certain risk of adverse events or iatrogenic harm, regardless if the patient has or has not a diagnosis of cancer [9]. A multinational, multicenter, non-interventional study was conducted in eight European countries in 2009 - 2010 [10]. Patients with confirmed NSCLC were enrolled and followed for 12 months or until death. Data were available for 3,508 patients (median age 65 years, stage IV in 49%). The overall mean number of hospitalization days was 16. Radiotherapy resulted in a mean of 12 hospitalization days. The majority of radiotherapy was palliative (56%), which resulted in fewer (mean 9.5) hospitalization days. The issue of unplanned hospitalization has been studied in different cancer-related settings [11, 12]. Hazell et al analyzed patients treated with definitive intent radiation (≥ 45 Gy) for lung cancer between 2008 and 2018 at a tertiary academic institution [13]. In addition to patient, tumor and treatment-related characteristics, specific baseline frailty markers were recorded. The 270 patients had a median age of 67 years. Cancer-related hospitalization incidence was 17%. On multivariable analysis, each 1 g/dL baseline drop in albumin was associated with a 2.4 times higher risk of any hospitalization (P = 0.01), and baseline hemoglobin ≤ 10 was associated with, on average, 2.7 more hospitalizations than having pre-treatment hemoglobin > 10 (P = 0.01). After controlling for baseline variables, cancer-related hospitalization was associated with 1.8 times increased risk of all-cause death (P = 0.04).
The present study was different from those discussed above, because it was limited to patients who received palliative radiotherapy or chemoradiation (according to national standards) and due to the availability of dosimetric factors and information about previous hospitalization before commencing radiotherapy. The median age was comparable to that in the studies discussed above. Our study population was heterogeneous with regard to tumor stage and fractionation regimen. Patients treated with low doses of radiotherapy, which are unlikely to cause side effects that may trigger hospitalization, were not included. Also, patients already hospitalized at the start of radiotherapy were excluded (Fig. 1). The latter groups had significantly shorter median survival (2.3 and 3.0 months, respectively; P = 0.0001) than our study patients, reflecting the fact that outpatients had an ECOG performance status of 0 - 2, whereas patients with worse performance status always were hospitalized for radiotherapy.
Expanding on a previous analysis [8], we stratified unplanned hospitalizations into two categories, i.e., unrelated to treatment and possibly related. The latter category included definitely related reasons such as esophagitis, but also others where the causality often is difficult to establish. In particular cardio-pulmonary side effects can have multifactorial pathogenesis [14]. Regardless of stratification, unplanned hospitalization was always associated with shorter survival (statistically significant if restricted to possibly related reasons). The fact that none of our hospitalized patients had a discharge diagnosis of radiation pneumonitis, an expected complication, supports our notion that this side effect likely has been classified in the COPD and pneumonia categories.
Unplanned hospitalization was common during the 3-month time period after commencing radiotherapy (43%). The 3-month cut-off was selected, because this time window is typically used to differentiate acute side effects from chronic side effects of radiotherapy. As shown in Table 1, many hospitalizations were necessary to palliate symptoms in this population where 45% of patients had metastatic disease, i.e., stage IV. However, side effects did also cause unplanned hospitalizations. Since we did not offer a standardized toxicity prevention program or early palliative care throughout the time period of this study, it is tempting to speculate that implementation of such interventions may have contributed to lower rates of hospitalization [15-18]. In this context, we would like to mention that our publicly-funded health care system allows for easy access to medically indicated hospital care and palliative care, without insurance-related barriers, copayments, etc. Hospitalization requires physician assessment rather than patient request alone.
Hazell et al suggested that anemia and low albumin may increase the risk of hospitalization in a different lung cancer scenario, i.e., definitive rather than palliative radiotherapy [13]. Only two patients in our study had low serum albumin. Interestingly, these two patients were hospitalized after the start of radiotherapy. However, larger databases are needed to draw firm conclusions. Overall, our results suggest a different main driver of unplanned hospitalization, namely a history of hospitalization in the immediate past, defined as 4 weeks before radiotherapy. This parameter has not been included in the other studies discussed here. It appears particularly interesting to consider early palliative care and intensified supportive measures, or continued hospital care, in this subgroup of patients who were likely to return to the hospital in our standard care setting (78% unplanned hospitalization). Surprisingly, concomitant chemoradiation and post-radiation systemic treatments did not increase the risk of unplanned hospitalization. Probably, their well-known toxicities were mainly limited to grade 1-2. In addition, patients selected for systemic treatment likely were younger and healthier. Dosimetric variables are known to correlate with toxicity, e.g., esophagitis [19, 20]. However, no significant impact on unplanned hospitalization has emerged in our study.
Despite inherent limitations of the retrospective study design, and the fact that our database did not include information about the anatomic pattern of metastatic disease and number of patients with hemoglobin ≤ 10 (as included in [13]), its main strength should also be considered, i.e., complete baseline and follow-up data due to the availability of a comprehensive regional electronic patient record, and the equal-access-to-care setting guaranteed by the Norwegian healthcare system.
Conclusion
Unplanned hospitalization occurred frequently in a standard care setting without early involvement of a dedicated palliative team. Patients with preceding hospitalization might represent a group that is particularly vulnerable, thus qualifying for a targeted intervention aiming at continued outpatient care.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
Not applicable.
Author Contributions
CN and KSI collected and analyzed the patient data. CN and EH drafted the manuscript. All authors read and approved the final manuscript.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
| References | ▴Top |
- Moeller B, Balagamwala EH, Chen A, Creach KM, Giaccone G, Koshy M, Zaky S, et al. Palliative thoracic radiation therapy for non-small cell lung cancer: 2018 Update of an American Society for Radiation Oncology (ASTRO) Evidence-Based Guideline. Pract Radiat Oncol. 2018;8(4):245-250.
doi pubmed - Brown S, Banfill K, Aznar MC, Whitehurst P, Faivre Finn C. The evolving role of radiotherapy in non-small cell lung cancer. Br J Radiol. 2019;92(1104):20190524.
doi pubmed - Nieder C, Tollali T, Yobuta R, Reigstad A, Flatoy LR, Pawinski A. Palliative thoracic radiotherapy for lung cancer: what is the impact of total radiation dose on survival? J Clin Med Res. 2017;9(6):482-487.
doi pubmed - Stevens R, Macbeth F, Toy E, Coles B, Lester JF. Palliative radiotherapy regimens for patients with thoracic symptoms from non-small cell lung cancer. Cochrane Database Syst Rev. 2015;1:CD002143.
doi - Reinfuss M, Mucha-Malecka A, Walasek T, Blecharz P, Jakubowicz J, Skotnicki P, Kowalska T. Palliative thoracic radiotherapy in non-small cell lung cancer. An analysis of 1250 patients. Palliation of symptoms, tolerance and toxicity. Lung Cancer. 2011;71(3):344-349.
doi pubmed - Strom HH, Bremnes RM, Sundstrom SH, Helbekkmo N, Flotten O, Aasebo U. Concurrent palliative chemoradiation leads to survival and quality of life benefits in poor prognosis stage III non-small-cell lung cancer: a randomised trial by the Norwegian Lung Cancer Study Group. Br J Cancer. 2013;109(6):1467-1475.
doi pubmed - Nieder C, Imingen KS, Mannsaker B, Yobuta R, Haukland E. Risk factors for esophagitis after hypofractionated palliative (chemo) radiotherapy for non-small cell lung cancer. Radiat Oncol. 2020;15(1):91.
doi pubmed - Nieder C, Imingen K. Early high-grade thoracic toxicity after palliative radiotherapy for non-small cell lung cancer. Cureus. 2021;13(1):e12494.
doi - Haukland EC, von Plessen C, Nieder C, Vonen B. Adverse events in hospitalised cancer patients: a comparison to a general hospital population. Acta Oncol. 2017;56(9):1218-1223.
doi pubmed - Vergnenegre A, Carrato A, Thomas M, Jernigan C, Medina J, Cruciani G. Real-world healthcare resource utilization in a European non-small cell lung cancer population: the EPICLIN-Lung study. Curr Med Res Opin. 2014;30(3):463-470.
doi pubmed - Whitney RL, Bell JF, Tancredi DJ, Romano PS, Bold RJ, Wun T, Joseph JG. Unplanned hospitalization among individuals with cancer in the year after diagnosis. J Oncol Pract. 2019;15(1):e20-e29.
doi pubmed - Waddle MR, Chen RC, Arastu NH, Green RL, Jackson M, Qaqish BF, Camporeale J, et al. Unanticipated hospital admissions during or soon after radiation therapy: Incidence and predictive factors. Pract Radiat Oncol. 2015;5(3):e245-e253.
doi pubmed - Hazell SZ, Mai N, Fu W, Hu C, Friedes C, Negron A, Voong KR, et al. Hospitalization and definitive radiotherapy in lung cancer: incidence, risk factors and survival impact. BMC Cancer. 2020;20(1):334.
doi pubmed - Hanania AN, Mainwaring W, Ghebre YT, Hanania NA, Ludwig M. Radiation-Induced Lung Injury: Assessment and Management. Chest. 2019;156(1):150-162.
doi pubmed - Duggan KJ, Wiltshire J, Strutt R, Boxer MM, Berthelsen A, Descallar J, Vinod SK. Palliative care and psychosocial care in metastatic non-small cell lung cancer: factors affecting utilisation of services and impact on patient survival. Support Care Cancer. 2019;27(3):911-919.
doi pubmed - Razvi Y, Chan S, Zhang L, Tsao M, Barnes E, Danjoux C, Sousa P, et al. A review of the Rapid Response Radiotherapy Program in patients with advanced cancer referred for palliative radiotherapy over two decades. Support Care Cancer. 2019;27(6):2131-2134.
doi pubmed - Ambroggi M, Biasini C, Toscani I, Orlandi E, Berte R, Mazzari M, Cavanna L. Can early palliative care with anticancer treatment improve overall survival and patient-related outcomes in advanced lung cancer patients? A review of the literature. Support Care Cancer. 2018;26(9):2945-2953.
doi pubmed - De Ruysscher D, Faivre-Finn C, Nackaerts K, Jordan K, Arends J, Douillard JY, Ricardi U, et al. Recommendation for supportive care in patients receiving concurrent chemotherapy and radiotherapy for lung cancer. Ann Oncol. 2020;31(1):41-49.
doi pubmed - Fleming C, Cagney DN, O'Keeffe S, Brennan SM, Armstrong JG, McClean B. Normal tissue considerations and dose-volume constraints in the moderately hypofractionated treatment of non-small cell lung cancer. Radiother Oncol. 2016;119(3):423-431.
doi pubmed - McDermott RL, Armstrong JG, Thirion P, Dunne M, Finn M, Small C, Byrne M, et al. Cancer Trials Ireland (ICORG) 06-34: A multi-centre clinical trial using three-dimensional conformal radiation therapy to reduce the toxicity of palliative radiation for lung cancer. Radiother Oncol. 2018;127(2):253-258.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


