| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Original Article
Volume 12, Number 12, December 2020, pages 787-793
The Impact of Different Induction Immunosuppressive Therapy on Long-Term Kidney Transplant Function When Measured by Iothalamate Clearance
Tambi Jarmia, c, Samir Khouzama, Nitika Shekhara, Meray Hosnia, Launia Whiteb, David O. Hodgeb, Martin L. Maia, Hani M. Wadeia
aDepartment of Transplant, Mayo Clinic Florida, Jacksonville, FL 32224, USA
bDepartment of Health Sciences Research, Mayo Clinic Florida, Jacksonville, FL 32224, USA
cCorresponding Author: Tambi Jarmi, Department of Transplant, Mayo Clinic Florida, 4500 San Pablo Road, Jacksonville, FL 32224, USA
Manuscript submitted October 21, 2020, accepted November 11, 2020, published online December 18, 2020
Short title: Induction Therapy and Kidney Allograft Function
doi: https://doi.org/10.14740/jocmr4369
| Abstract | ▴Top |
Background: Improvement in short-term outcomes after kidney transplant has been achieved by using different induction and maintenance therapeutic approaches. Long-term outcomes have not matched the expectations of the transplant stakeholders. Our study aimed to address the early impact of induction agents on long-term outcome of kidney transplant when measured by iothalamate clearance.
Methods: All adult kidney transplant recipients between January of 2012 and December of 2016 were reviewed. Six hundred forty-nine patients were divided into three groups based on the induction agent (basiliximab, alemtuzumab, and thymoglobulin). Protocoled 4 months and 48 months kidney allograft function evaluations with iothalamate clearance test were compared among the three groups.
Results: Patients who received basiliximab were significantly older with no difference among African American and Caucasians. The 48 months assessment showed significant decline in median iothalamate clearance in basiliximab group at 49 mL/min vs. alemtuzumab group 64.8 mL/min and thymoglobulin 60 mL/min with P = 0.007. The basiliximab group developed a significant higher proteinuria measured by spot urine to creatinine ratio at 48 months.
Conclusions: The use of basiliximab as an induction agent for kidney transplant is associated with significant decline in kidney function 4 years post transplantation when measured by iothalamate clearance.
Keywords: Induction; Iothalamate; Allografts
| Introduction | ▴Top |
With development of immunosuppressive medications and major advances in clinical care, short-term outcomes after kidney transplant are noticeably better [1, 2]. However, the main challenge is how to improve long-term outcomes [3, 4]. The introduction of potent and selective agents for the initiation of immunosuppression, as induction therapy, has reduced the incidence of acute rejection early post transplantation and improved 1-year graft survival [5-11]. However, long-term graft survival was less linked to induction therapy and more to maintenance immunosuppressive regimen and existence of medical comorbidities. The Kidney Disease Improving Global Outcomes (KDIGO) guidelines recommend basiliximab as a first-line induction therapy across all types of donor-recipient profiles to reduce acute rejection risk and allograft loss [12, 13]. However, there has been a lack of randomized clinical trials comparing induction agents when recipients are receiving a maintenance immunosuppressive therapy with tacrolimus (TAC) and mycophenolic acid (MPA) with or without steroids; and the recommendations are mainly made on the basis of meta-analysis data [11, 14]. Since the incidence of acute rejection is less than 10% in most reported for cause biopsies, the sample size required for randomized clinical trials that could determine small differences in observed outcomes between basiliximab, alemtuzumab and thymoglobulin has been estimated to be between 1,600 and 7,000 [15]. Such studies would be difficult to conduct from logistics standpoints. Given such an obstacle, we reviewed 649 kidney transplant recipients at Mayo Clinic Florida from 2012 to 2016 and compared the long-term kidney transplant function, based on the induction therapy used at the time of transplantation. Kidney allograft function was measured by iothalamate as a standard clearance test [16].
| Materials and Methods | ▴Top |
Patient population
After obtaining required Institutional Review Board (IRB) approval, all adult patients who received kidney transplant at Mayo Clinic Florida between January 2012 and December 2016 were reviewed in this study. Seventy hundred ninety-seven adult patients were identified. We excluded patients who received combined kidney and other solid organ transplant, kidney after previous solid organ transplant, and second or third kidney transplant. Totally, 649 patients were included in this study.
Study design
The study is a retrospective analysis of kidney transplant recipients at a single transplant center, Mayo Clinic Florida Transplant Center. The study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration, and received IRB approval before initiation. Patients were divided into three groups based on the induction agent used at the time of transplantation and according to immunosuppressive therapy protocols approved and adopted by Mayo Clinic Transplant Enterprise. Group 1 represent low immunological risk and received basiliximab (patients 65 years of age and older and/or two haplotype-matched donors or zero mismatch); group 2 received alemtuzumab (patients 64 years of age and younger and/or no detected donor specific antibodies (DSA) or detected DSA but with mean florescence intensity (MFI) < 2,000); group 3 represent high immunological risk and received thymoglobulin (patients 64 years of age and younger with DSA at MFI ≥ 2,000 or positive acceptable flow cytometry cross match with DSA at any value). Groups 1 and 3 will receive maintenance steroid with 5 mg prednisone on daily basis. Group 2 will not receive maintenance steroid. All transplant recipients were maintained on TAC and MPA for long-term immunosuppression.
Data points
Patients’ demographic data were collected. Allografts functional data points on 4 and 48 months were collected and included: Iothalamate clearance which is a standard of care at our center and performed on all kidney transplant recipients within the first 4 months and at 48 months post-transplant. Urine protein to creatinine ratio, time to first rejection, TAC level with first rejection and pathology score of first rejection.
Statistical analysis
Categorical variables were presented as the number of subjects and the percentages. Medians were used to summarize the continuous variables. Overall comparisons among the groups for categorical variables were completed using the χ2 test. The Kruskal-Wallis test was used to compare continuous variables. The cumulative probability of a rejection was estimated using the Kaplan-Meier method. These curves were compared between groups using the log-rank test. All analyses were completed using SAS version 9.4 (Cary, NC).
| Results | ▴Top |
Demographics and comorbidities
Out of the 649 reviewed patients, group 1 has 149 patients received basiliximab, group 2 has 264 received alemtuzumab and group 3 has 236 received thymoglobulin. Patients induced with basiliximab were significantly older as expected. African Americans patients were represented in each group with no significant differences when compared to White patients (Table 1). We analyzed the pre-transplant diabetes history (type I and II) and found no significant differences among the three induction groups (Table 1). All reviewed patients were also analyzed based on their smoking history documented on the initial evaluation visit pre-transplant and no differences in the incidence of smoking among the three groups (Table 1). Body mass index (BMI) was reviewed, all accepted patients for transplantation is recommended to have BMI of 32 and less according to Mayo Clinic Transplant evaluation protocol and all reviewed patients showed no differences in their BMI calculated at the time of transplantation (Table 1).
 Click to view | Table 1. Patients Demographics |
Kidney transplant long-term function based on induction agents
The protocoled 4 months iothalamate clearance showed no difference among the three groups (alemtuzumab 65.7 ± 26 mL/min, basiliximab 62.8 ± 29.6 mL/min and thymoglobulin 68.6 ± 29.6 mL/min, P = 0.13). However, it was significantly lower, at 48 months, in patients induced with basiliximab 52.6 ± 30 in comparison to alemtuzumab 64.8 ± 34 and thymoglobulin 61 ± 30.1 (P < 0.007) (Fig. 1a). When we compared the three groups at 48 months after excluding all patients who received deceased kidney allografts from donors with kidney donor profile index (KDPI) greater than 85%, we found similar significant decline in allograft function at 48 months in patients induced with basiliximab (52 mL/min) when compared with alemtuzumab (64.0 mL/min) and thymoglobulin (61 mL/min) (Fig. 1b). Overall, 48 months proteinuria, measured by spot urine protein to creatinine ratio, among the three groups was not high. However, the median protein creatinine ratio was significantly higher in patients induced with basiliximab (0.2) when compared to patients induced with alemtuzumab (0.1) and thymoglobulin (0.1) (P < 0.03) (Fig. 2).
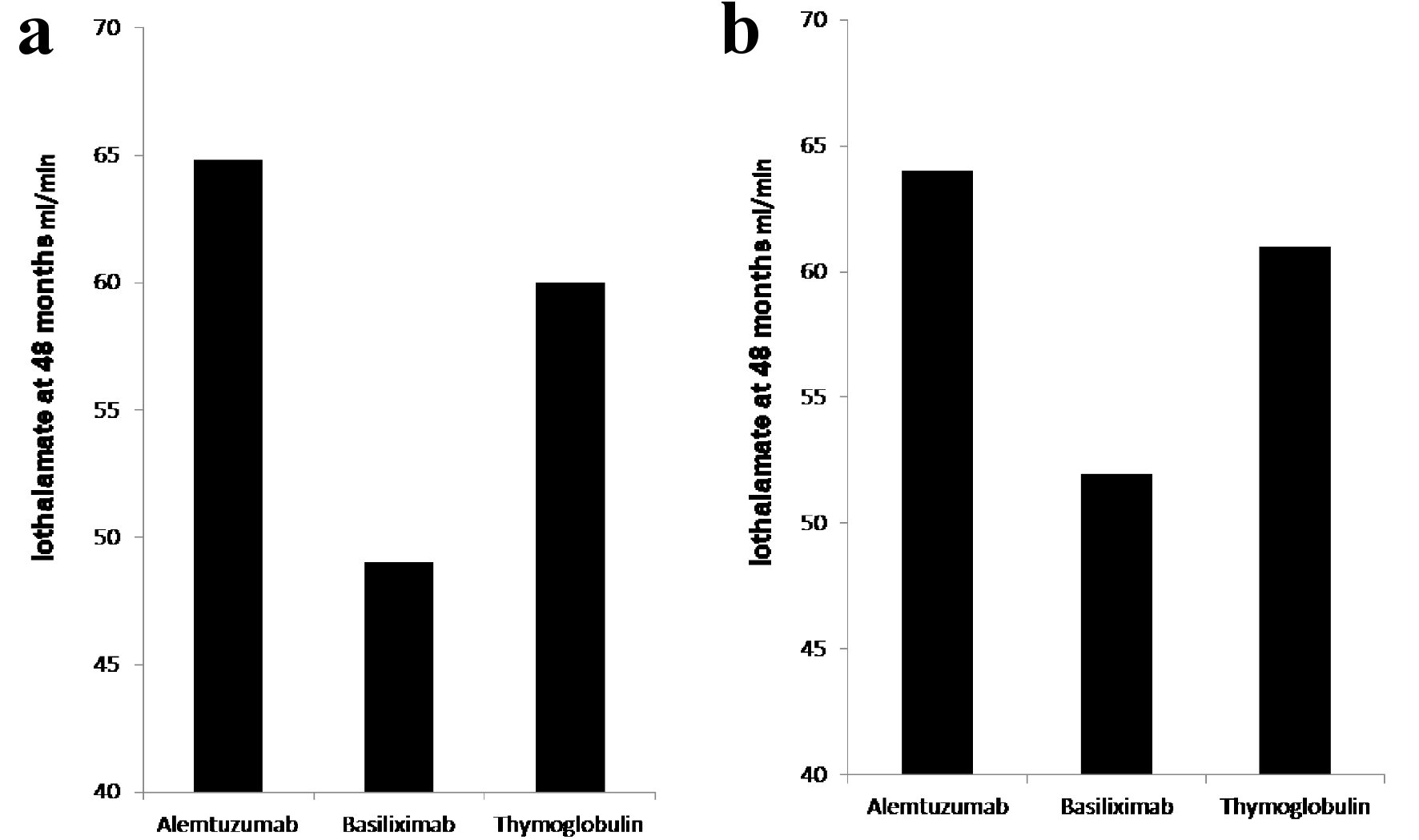 Click for large image | Figure 1. (a) Iothalamate clearance at 48 months. Recipients induced with basiliximab (149 patients) showed significant decline in iothalamate clearance at 49 when compared to alemtuzumab (264 patients) at 64.8 and thymoglobulin (236 patients) at 60 with P = 0.007. (b) Iothalamate clearance at 48 months, after excluding of recipients from donors with KDPI > 85%. Recipients induced with basiliximab (129 patients) showed significant decline in iothalamate clearance at 52 when compared to alemtuzumab (256 patients) at 64 and thymoglobulin (229 patients) at 61 with P = 0.02. |
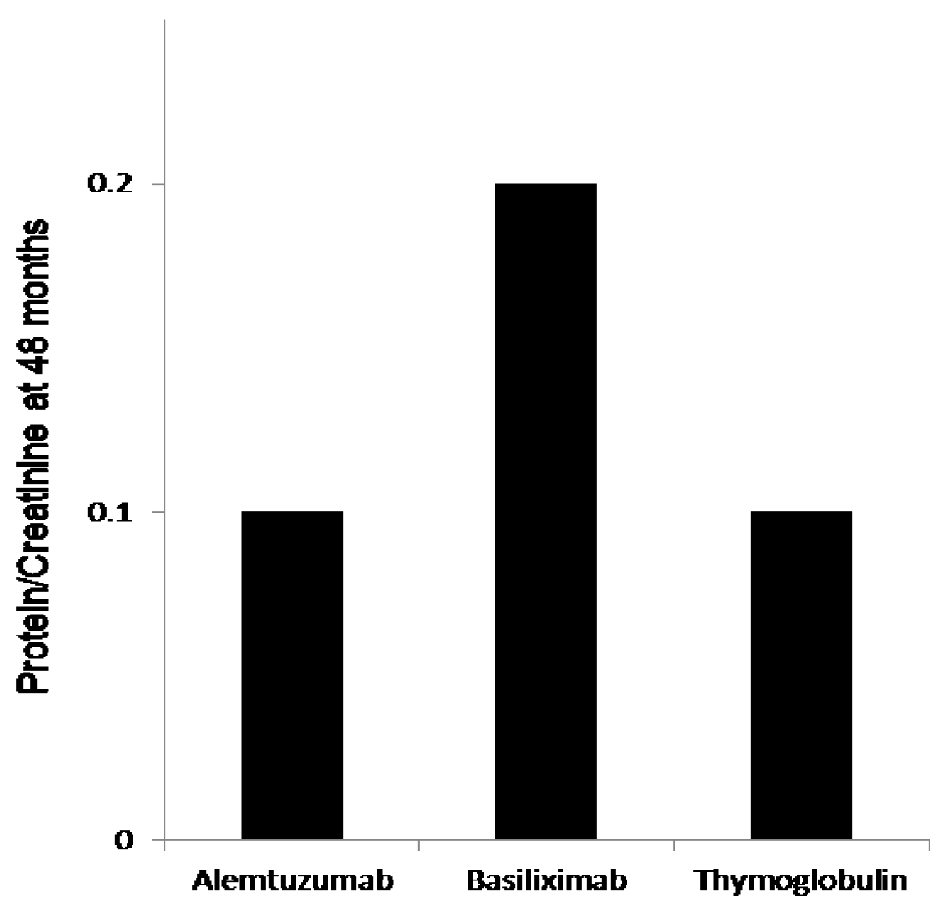 Click for large image | Figure 2. The 48 months protein creatinine ratio measurement showed significantly elevated mean protein/creatinine when measured at 48 months at 0.2 when compared to protein/creatinine of 0.1 with both alemtuzumab and thymoglobulin when used for induction, P < 0.03. |
Rejection, cytomegalovirus (CMV) and polyomavirus (BK) incidence
Although, Banff classification of first diagnosed rejection based on protocoled and indicated biopsies was not different among the three groups, patients induced with basiliximab showed 7.4% increase incidence of IIB rejection when compared to alemtuzumab 2.9% and thymoglobulin 0.0% with P < 0.3 (Table 2). Time for first rejection (Fig. 3a) was not different among the three induction groups (P > 0.05) and tacrolimus level (mean ± standard deviation (SD)) at the time of first rejection was at 7.7 ± 3.8 ng/mL with thymoglobulin, 6.8 ± 2.5 ng/mL with alemtuzumab and 7.3 ± 3.1 ng/mL with basiliximab group (Fig. 3b) (P > 0.05). Among the three groups of patients, incidence of viremia was higher with thymoglobulin and viremia incidence was higher with alemtuzumab. The overall incidence of CMV and BK was not significant among the three groups (Fig. 4).
 Click to view | Table 2. Rejection Severity |
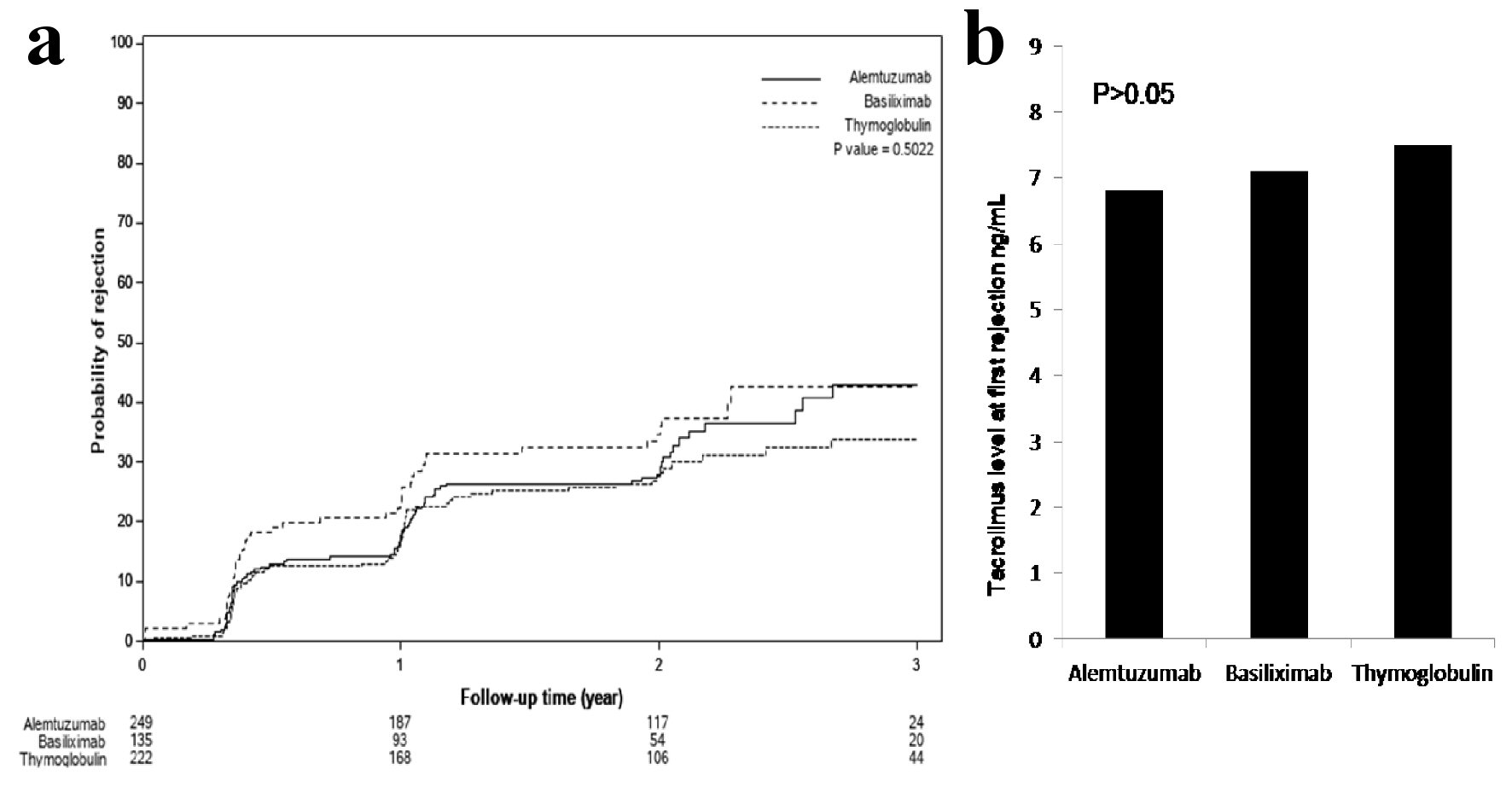 Click for large image | Figure 3. (a) Time to first rejection, based on protocoled or indicated biopsies, showed no significant differences among the three groups (P > 0.05). (b) Tacrolimus level checked within the 24 - 48 h prior to first diagnosed rejection showed acceptable therapeutic levels and no differences among the three groups: 7.7 ± 3.8 ng/mL with thymoglobulin, 6.8 ± 2.5 ng/mL with alemtuzumab and 7.3 ± 3.1 ng/mL with basiliximab group (P > 0.05). |
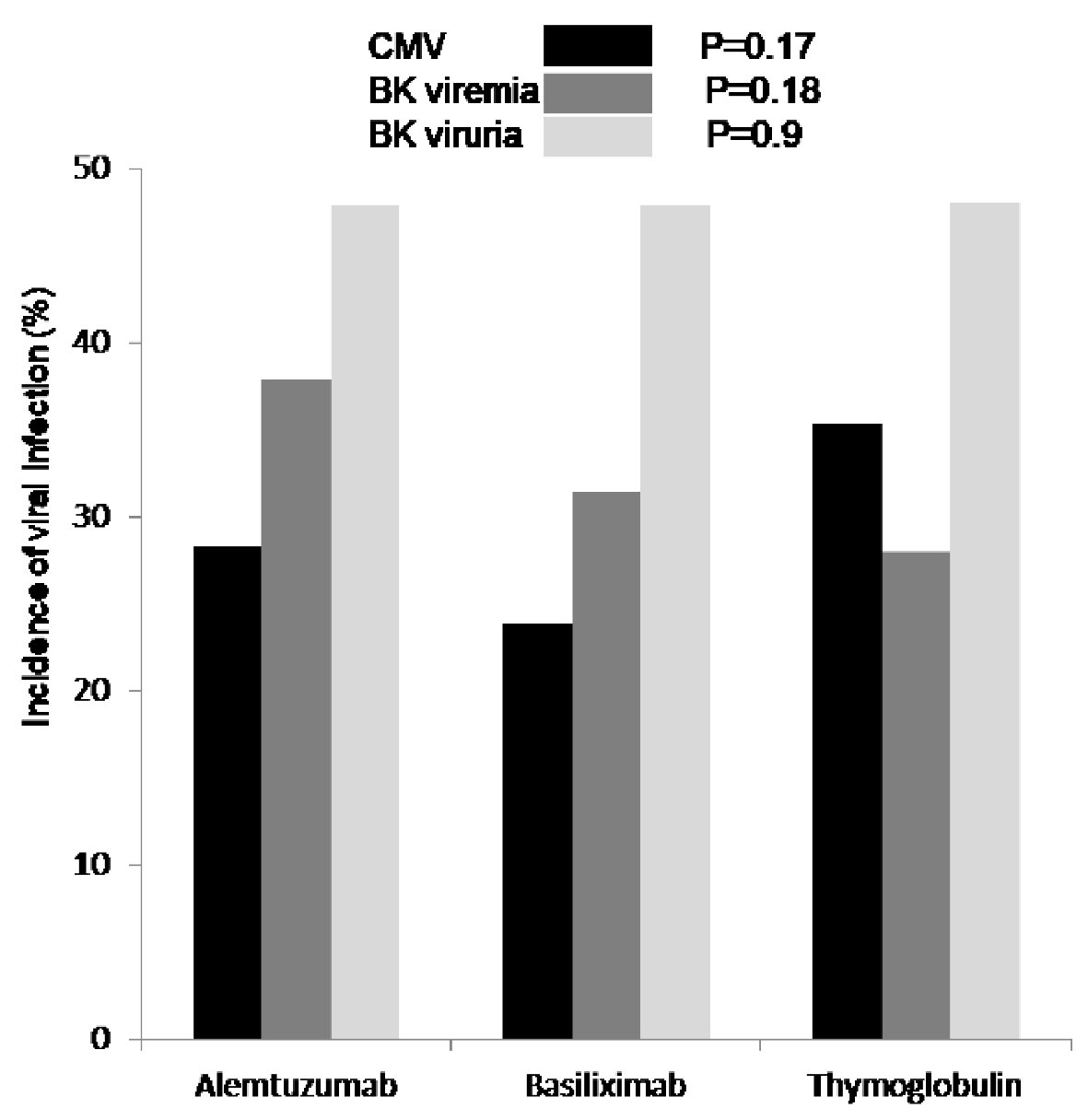 Click for large image | Figure 4. First diagnosed CMV viremia (black), based on screening test and/or symptoms, was reported as percentage of patients who tested positive and showed no difference among the three groups (P = 0.17). Incidence of BK viremia (grey) was higher in patients who received alemtuzumab for induction but not significant (P = 0.18). Incidence of BK viruria (white) was similar in all patients group (P = 0.9). CMV: cytomegalovirus; BK: polyomavirus. |
| Discussion | ▴Top |
Conventionally, the monoclonal antibodies directed against the activated interleukin-2 (IL-2) receptor such as basiliximab, have been usually reserved for low to moderate immunologic risk patients [17-19], whereas the depleting agents, such as thymoglobulin and alemtuzumab, have been used in high-risk recipients receiving kidney transplantation [7, 20, 21]. Our study is a retrospective analysis of kidney allograft’s long-term function based on immunosuppressive induction agents used at time of transplant. We found a significant drop in iothalamate clearance when measured at 48 months post-transplant. However, the 4 months iothalamate clearance was similar among all patients. This change from equivalent iothalamate clearance at 4 months between the groups to a drop in iothalamate clearance at 48 months in basiliximab group may be less related to donor factors and more to immunosuppressive approach. In order to correct for the donor factors, we repeated the 48 months analysis after excluding all recipients from all three groups who received kidney allografts from deceased donors with KDPI > 85%. The secondary analysis still showed significant drop in iothalamate clearance in patients received basiliximab for induction when compared to alemtuzumab and thymoglobulin.
The Cochrane Collaboration published a meta-analysis of randomized controlled trials that compared IL-2 receptor antagonist, such as basiliximab, induction with placebo and thymoglobulin [22]. Acute rejection rates were lower with IL-2 receptor antagonist versus placebo and graft loss was reduced. However, thymoglobulin was not more effective in preventing rejection, and the safety profile favored IL-2 receptor antagonist induction. Based largely on these findings, KDIGO guidelines recommended that induction therapy with a biological agent be a routine part of the initial immunosuppressive regimen and that an IL-2 receptor antagonist agent to be the first-line therapy [23]. However, little information is available about the long-term effects of the IL-2 receptor antagonist basiliximab; most of the published studies addressed the short-term benefits and risks of basiliximab in terms of reducing incidence of rejection and monitoring for side effects [17]. In our study, we reviewed the long-term, 5 years, kidney allografts outcome when using basiliximab vs. alemtuzumab and thymoglobulin for induction. We showed no difference in time to first diagnosed rejection, no difference in the severity of first diagnosed rejection based on Banff classification [24] among patients who were induced with basiliximab when compared to alemtuzumab and thymoglobulin. We accounted for possible donor factors represented by high KDPI by repeating the analysis after excluding all patients who received kidney allografts from donors with KDPI > 85%. The iothalamate clearance [25] at 5 years post-transplant was significantly lower in patients who received basiliximab for induction when compared to alemtuzumab and thymoglobulin. This results remains the same even after we excluded old recipient of kidney allograft from donors with KDPI > 85%. Despite the similarities in serology and pathology findings among the three groups, the significant difference in iothalamate clearance at 5 years post-transplant in the basiliximab group is alarming. We could argue that the patients who received basiliximab had chronic smoldering and subclinical cellular injury that resulted in such decline in kidney allograft function and the increase in proteinuria [26, 27].
In this study, we analyzed the incidence of viral infection, mainly CMV and BK, among the three groups of patients based on the induction agent. Multiple studies have shown an increased risk of viral infection when T-cell depleting agent is used for induction therapy versus basiliximab [28-30]. However, we found no significant difference in the incidence of either CMV or BK disease among the different induction groups.
Conclusions
This study demonstrated potential effect of the induction agent used at the time of kidney transplantation on 5 years allograft function when measured by iothalamate clearance. Using basiliximab resulted in lower iothalamate clearance and increased proteinuria 5 years post-transplant.
Acknowledgments
Thanks to the Mayo Clinic Transplant Center.
Financial Disclosure
No associated finances that need to be disclosed. The study required no funding.
Conflict of Interest
None to declare.
Informed Consent
The study is a retrospective analysis and did not require informed consent.
Author Contributions
Tambi Jarmi contributed to the hypothesis, methods and design, data collection and analysis, data and results interpretation, and manuscript writing. Samir Khouzam: data collection. Nitika Shekhar: data collection. Meray Hosni: data collection. Launia White: data analysis. David O. Hodge: data analysis. Martin L. Mai: data and results interpretation, and manuscript writing. Hani M. Wadei: data and results interpretation, and manuscript writing.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
| References | ▴Top |
- Gaston RS. Improving long-term outcomes in kidney transplantation: towards a new paradigm of post-transplant care in the United States. Trans Am Clin Climatol Assoc. 2016;127:350-361.
- Wang JH, Skeans MA, Israni AK. Current status of kidney transplant outcomes: dying to survive. Adv Chronic Kidney Dis. 2016;23(5):281-286.
doi pubmed - Patel AK, Samaniego M. The struggle for optimization of long-term outcomes after kidney transplantation. Adv Chronic Kidney Dis. 2016;23(5):280.
doi pubmed - Brennan DC. Long-term trends in allograft survival. Adv Chronic Kidney Dis. 2006;13(1):11-17.
doi pubmed - Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med. 2004;351(26):2715-2729.
doi pubmed - Hellemans R, Bosmans JL, Abramowicz D. Induction therapy for kidney transplant recipients: do we still need Anti-IL2 receptor monoclonal antibodies? Am J Transplant. 2017;17(1):22-27.
doi pubmed - Hill P, Cross NB, Barnett AN, Palmer SC, Webster AC. Polyclonal and monoclonal antibodies for induction therapy in kidney transplant recipients. Cochrane Database Syst Rev. 2017;1:CD004759.
doi pubmed - Khalkhali HR, Ghafari A, Hajizadeh E, Kazemnejad A. Risk factors of long-term graft loss in renal transplant recipients with chronic allograft dysfunction. Exp Clin Transplant. 2010;8(4):277-282.
- Tanriover B, Jaikaransingh V, MacConmara MP, Parekh JR, Levea SL, Ariyamuthu VK, Zhang S, et al. Acute rejection rates and graft outcomes according to induction regimen among recipients of kidneys from deceased donors treated with tacrolimus and mycophenolate. Clin J Am Soc Nephrol. 2016;11(9):1650-1661.
doi pubmed - Van Loon E, Senev A, Lerut E, Coemans M, Callemeyn J, Van Keer JM, Daniels L, et al. Assessing the complex causes of kidney allograft loss. Transplantation. 2020;104(12):2557-2566.
doi pubmed - Wiseman AC. Induction therapy in renal transplantation: why? What agent? What dose? We may never know. Clin J Am Soc Nephrol. 2015;10(6):923-925.
doi pubmed - Isakova T, Nickolas TL, Denburg M, Yarlagadda S, Weiner DE, Gutierrez OM, Bansal V, et al. KDOQI US Commentary on the 2017 KDIGO clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Am J Kidney Dis. 2017;70(6):737-751.
doi pubmed - Mandelbrot DA, Reese PP, Garg N, Thomas CP, Rodrigue JR, Schinstock C, Doshi M, et al. KDOQI US Commentary on the 2017 KDIGO clinical practice guideline on the evaluation and care of living kidney donors. Am J Kidney Dis. 2020;75(3):299-316.
doi pubmed - Williams AM, Barrett M, Smith AR, Kathawate RG, Woodside KJ, Sung RS. Variable benefits of antibody induction by kidney allograft type. J Surg Res. 2020;248:69-81.
doi pubmed - Willoughby LM, Schnitzler MA, Brennan DC, Pinsky BW, Dzebisashvili N, Buchanan PM, Neri L, et al. Early outcomes of thymoglobulin and basiliximab induction in kidney transplantation: application of statistical approaches to reduce bias in observational comparisons. Transplantation. 2009;87(10):1520-1529.
doi pubmed - Dowling TC, Frye RF, Fraley DS, Matzke GR. Comparison of iothalamate clearance methods for measuring GFR. Pharmacotherapy. 1999;19(8):943-950.
doi pubmed - Ponticelli C. Basiliximab: efficacy and safety evaluation in kidney transplantation. Expert Opin Drug Saf. 2014;13(3):373-381.
doi pubmed - Wang K, Xu X, Fan M. Induction therapy of basiliximab versus antithymocyte globulin in renal allograft: a systematic review and meta-analysis. Clin Exp Nephrol. 2018;22(3):684-693.
doi pubmed - Yakubu I, Ravichandran B, Sparkes T, Barth RN, Haririan A, Masters B. Comparison of alemtuzumab versus Basiliximab induction therapy in elderly kidney transplant recipients: a single-center experience. J Pharm Pract. 2019:897190019850934.
doi pubmed - Gundroo A, Zachariah M, Singh N, Sharma R. Alemtuzumab (Campath-1H) experience in kidney transplantation what we have learned; current practices; and scope for the future? Curr Opin Organ Transplant. 2015;20(6):638-642.
doi pubmed - Morgan RD, O'Callaghan JM, Knight SR, Morris PJ. Alemtuzumab induction therapy in kidney transplantation: a systematic review and meta-analysis. Transplantation. 2012;93(12):1179-1188.
doi pubmed - Webster AC, Ruster LP, McGee R, Matheson SL, Higgins GY, Willis NS, Chapman JR, et al. Interleukin 2 receptor antagonists for kidney transplant recipients. Cochrane Database Syst Rev. 2010;1:CD003897.
doi pubmed - Kidney Disease: Improving Global Outcomes Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9(Suppl 3):S1-155.
doi - Roufosse C, Simmonds N, Clahsen-van Groningen M, Haas M, Henriksen KJ, Horsfield C, Loupy A, et al. A 2018 Reference Guide to the Banff Classification of Renal Allograft Pathology. Transplantation. 2018;102(11):1795-1814.
doi pubmed - Soveri I, Berg UB, Bjork J, Elinder CG, Grubb A, Mejare I, Sterner G, et al. Measuring GFR: a systematic review. Am J Kidney Dis. 2014;64(3):411-424.
doi pubmed - Baldwin WM, 3rd, Valujskikh A, Fairchild RL. Mechanisms of antibody-mediated acute and chronic rejection of kidney allografts. Curr Opin Organ Transplant. 2016;21(1):7-14.
doi pubmed - Sellares J, de Freitas DG, Mengel M, Reeve J, Einecke G, Sis B, Hidalgo LG, et al. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant. 2012;12(2):388-399.
doi pubmed - Luan FL, Samaniego M, Kommareddi M, Park JM, Ojo AO. Choice of induction regimens on the risk of cytomegalovirus infection in donor-positive and recipient-negative kidney transplant recipients. Transpl Infect Dis. 2010;12(6):473-479.
doi pubmed - Pai D, Mann DM, Malik A, Hoover DR, Fyfe B, Mann RA. Risk factors for the development of BK virus nephropathy in renal transplant recipients. Transplant Proc. 2015;47(8):2465-2469.
doi pubmed - Bayraktar A, Catma Y, Akyildiz A, Demir E, Bakkaloglu H, Ucar AR, Dirim AB, et al. Infectious complications of induction therapies in kidney transplantation. Ann Transplant. 2019;24:412-417.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


