| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Original Article
Volume 12, Number 5, May 2020, pages 300-306
Treating Japanese Patients With Pembrolizumab for Platinum-Refractory Advanced Urothelial Carcinoma in Real-World Clinical Practice
Nobuki Furubayashia, e, Kentaro Kuroiwab, Noriaki Tokudac, Toshihisa Tomodad, Futoshi Morokumac, Yoshifumi Horib, Takahito Negishia, Tomohiro Inouea, Masatoshi Kumagaia, Motonobu Nakamuraa
aDepartment of Urology, National Hospital Organization Kyushu Cancer Center, Fukuoka, Japan
bDepartment of Urology, Miyazaki Prefectural Miyazaki Hospital, Miyazaki, Japan
cDepartment of Urology, Saga-ken Medical Centre Koseikan, Saga, Japan
dDepartment of Urology, Oita Prefectural Hospital, Oita, Japan
eCorresponding Author: Nobuki Furubayashi, Department of Urology, National Hospital Organization Kyushu Cancer Center, Notame 3-1-1, Minami-ku, Fukuoka 811-1395, Japan
Manuscript submitted April 10, 2020, accepted April 21, 2020
Short title: Pembrolizumab for Advanced Urothelial Carcinoma
doi: https://doi.org/10.14740/jocmr4162
| Abstract | ▴Top |
Background: Since December 2017, pembrolizumab has been approved in Japan as a second-line treatment for radical unresectable urothelial carcinoma (UC) that has become exacerbated after chemotherapy by the international randomized phase 3 trial, KEYNOTE-045. The aim of this study was to evaluate the oncological efficacy and safety of pembrolizumab after failure of platinum-based chemotherapy in Japanese patients with advanced UC in real-world clinical practice.
Methods: A total of 34 patients who received pembrolizumab after the failure of platinum-based chemotherapy for advanced urothelial carcinoma at four institutions between January 2018 and August 2019 were retrospectively evaluated. In all patients, UC was histopathologically diagnosed, and disease progression after platinum-based chemotherapy was radiologically confirmed.
Results: The median follow-up period was 7.7 months. The objective response rate, median progression-free survival, and median overall survival were 20.6%, 3.3 months, and 11.7 months, respectively. Regarding the toxicities associated with pembrolizumab, adverse events (AEs) of any grade occurred in 61.8%, and grade 3 AEs occurred in 23.5%; grade ≥ 4 AEs did not occur in any patients. Univariate analyses revealed that the Eastern Cooperative Oncology Group Performance Status, neutrophil/lymphocyte ratio, liver metastases, and time from previous chemotherapy were prognostic variables. Multivariate analyses revealed that liver metastases (positive: hazard ratio, 4.23; 95% confidence interval, 1.48 - 12.08; P < 0.01) and time from previous chemotherapy (≥ 3 months: hazard ratio, 5.06; 95% confidence interval, 1.43 - 17.91; P = 0.01) were independent prognostic factors.
Conclusions: In this real-world clinical study, these findings concerning the efficacy and safety of pembrolizumab for advanced UC in Japanese patients were comparable to those of the open-label, international, phase 3 trial KEYNOTE-045. Liver metastases and time from previous chemotherapy were independent prognostic factors in the present study.
Keywords: Pembrolizumab; Advanced urothelial carcinoma; Platinum-refractory; Japanese; Real-world clinical practice
| Introduction | ▴Top |
Urothelial carcinoma (UC), the most common histologic subtype of cancer arising from the transitional epithelium of the renal pelvis, ureter, bladder, or urethra, represents the fourth most common type of malignancy worldwide [1]. Approximately 30% of UC patients already present with muscle invasion and metastatic disease at the initial diagnosis [2]. Furthermore, despite curative surgery as local therapy for patients with muscle invasion, more than one-third of these patients eventually develop metastases [3]. Systemic chemotherapy with cisplatin-based regimen is the gold-standard treatment for patients with advanced or metastatic UC as the first-line treatment. Combined chemotherapy with gemcitabine and cisplatin (GC) is currently widely used for advanced UC, since GC therapy showed a similar overall survival (OS) and time to progression with less toxicity than combined chemotherapy with methotrexate, vinblastine, doxorubicin and cisplatin in a randomized phase 3 trial [4]. However, no standard second-line treatment had been established, and following the failure of first-line chemotherapy, metastatic UC is a fatal disease with an OS of 6 - 7 months [5].
Pembrolizumab, a humanized monoclonal antibody that targets programmed death receptor-1, was associated with a significantly longer OS (by approximately 3 months) and a lower rate of treatment-related adverse events (AEs) than chemotherapy as second-line therapy for platinum-refractory advanced UC in the phase 3 trial KEYNOTE-045 [6]. Since December 2017, pembrolizumab has been approved in Japan as a second-line treatment for radical unresectable UC that has become exacerbated after chemotherapy [7]. However, information regarding the efficacy and safety of pembrolizumab is limited to the results of clinical trials [6, 8]. In addition, there are still few reports concerning the data of pembrolizumab in real-world Japanese clinical practice [9, 10].
In this study, we retrospectively assessed the tolerability, efficacy and prognostic factors for the OS of pembrolizumab therapy in patients who received pembrolizumab treatment for platinum-refractory advanced UC in Japanese.
| Materials and Methods | ▴Top |
The data of 34 patients who received pembrolizumab after the failure of platinum-based chemotherapy for advanced UC at four institutions between January 2018 and August 2019 were retrospectively evaluated. In all patients, UC was histopathologically diagnosed, and disease progression after platinum-based chemotherapy was radiologically confirmed [11]. Pembrolizumab was administered to all patients after platinum-based chemotherapy was found to be unsuccessful unless they had an autoimmune disease, and it was administered intravenously on day 1 at a dose of 200 mg, and the cycle was basically repeated every 21 days. This treatment was continued until disease progression or unacceptable AEs occurred. Tumor measurements were generally performed by computed tomography before and after every four to six cycles of pembrolizumab. Decisions regarding AEs were made based on the Common Terminology Criteria for Adverse Events, version 5.0 [12].
The tumor response was evaluated as the best response according to the Response Evaluation Criteria in Solid Tumors, version 1.1 [13]. The clinicopathological data, including the age, sex, Eastern Cooperative Oncology Group Performance Status (ECOG-PS), primary tumor site, histologic testing, number of prior chemotherapy regimens, hemoglobin (Hb) concentration, neutrophil/lymphocyte ratio (NLR), levels of lactate dehydrogenase (LDH) and C-reactive protein (CRP), visceral metastases (especially liver metastases) and time from previous chemotherapy, were collected. According to a previous study, the cut-off values of the Hb concentration, ECOG-PS and time from previous chemotherapy were < 10 g/dL, > 1 and < 3 months [6]. The cut-off values of the albumin, LDH and CRP levels were set as the upper limit of the normal range, which were < 4.1 g/dL, ≤ 222 U/L and ≤ 0.14 mg/dL, respectively.
Statistical analyses
The statistical analyses were carried out using the JMP® Pro, version 14.2.0 software package (SAS Institute, Inc., Cary, NC, USA). The OS and progression-free survival (PFS) were estimated using the Kaplan-Meier method. In the analysis of the OS, patients who were alive or lost to follow-up had their data censored at the time of last contact. In the analysis of the PFS, patients who were alive and without disease progression or who were lost to follow-up had their data censored at the time of last tumor assessment. Between-group differences in the OS were calculated using a log-rank test. The significance of associations between the clinical parameters and the OS was assessed using the Cox proportional hazards regression model. P < 0.05 was considered to indicate a statistically significant difference.
Ethics approval
The present study was approved by the Institutional Review Board of National Hospital Organization Kyushu Cancer Center (2014-99), and written informed consent was obtained from the patient.
| Results | ▴Top |
Patient characteristics
The clinical characteristics of the 34 (male, n = 28; female, n = 6; median age, 71 years; range, 57 - 82 years old) patients are listed in Table 1. All patients received pembrolizumab after the failure of platinum-based chemotherapy (more than one regimen) for advanced UC. Twenty-two patients received pembrolizumab after the first-line regimen, six received it after the second-line regimen, and six received it after the third-line regimen. Thirteen patients had bladder UC, 12 had upper urinary tract UC, and nine had both types of UC. Fourteen patients (41.2%) had an ECOG-PS of ≥ 1, 11 (32.4%) had liver metastasis, 14 (41.2%) had anemia (Hb < 10 g/dL), 10 (29.4%) had a high LDH (> 222 U/L) and time from previous chemotherapy was < 3 months in 27 patients (79.4%).
 Click to view | Table 1. Patients’ Characteristics |
Efficacy of pembrolizumab
The median follow-up period after pembrolizumab administration was 7.7 months (range, 0.6 - 18.6 months). Among the 34 patients who received pembrolizumab, a complete response (CR) was confirmed in one patient, and six patients showed a partial response (PR), with an overall response rate of 20.6%. The disease control rate (defined by the achievement of CR, PR or stable disease (SD)), was 38.2%. The PFS and OS for pembrolizumab are shown in Figure 1. The median PFS was 3.3 months (95% confidence interval (CI), 2.8 - 7.4 months) and the median OS was 11.7 months (95% CI, 5.9 - 15.7 months). The median OS when pembrolizumab was used as second-line therapy was 11.9 months (95% CI, 4.6 - 16.6 months), and the median OS when pembrolizumab was used as third-line or later therapy was 11.6 months (95% CI, 3.1 - 15.7 months). The difference between the second-line and the third-line or later groups was not statistically significant according to the log-rank test (P = 0.94) (Fig. 1).
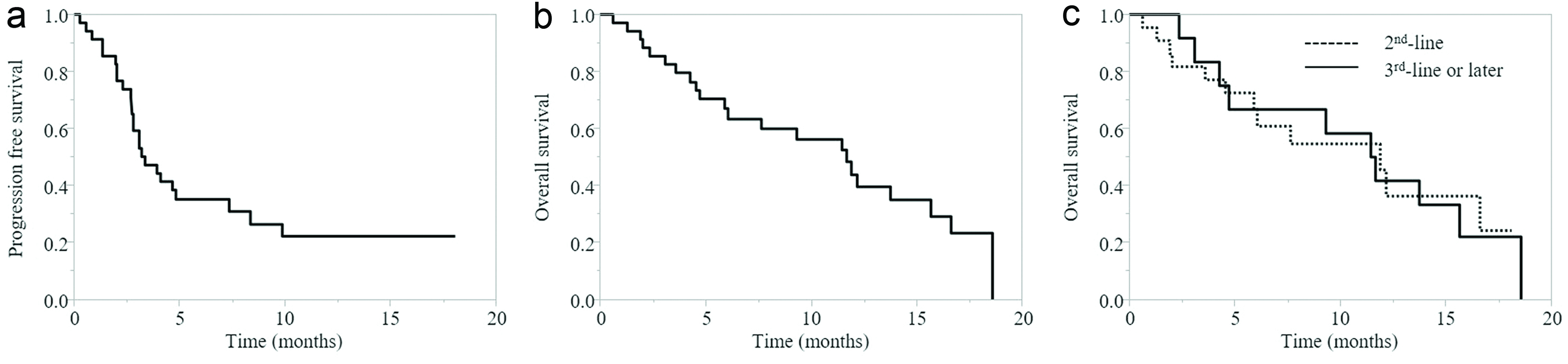 Click for large image | Figure 1. (a) PFS of all cases. (b) OS of all cases. (c) OS according to pembrolizumab treatment line. PFS: progression-free survival; OS: overall survival. |
Safety of pembrolizumab
Table 2 shows the toxicities associated with pembrolizumab. AEs occurred in 21 patients (61.8%), and grade 3 AEs occurred in eight patients (23.5%), while grade ≥ 4 AEs did not occur in any patients. Eight patients discontinued pembrolizumab due to AEs, regardless of grade. There were no treatment-related deaths among the 34 patients.
 Click to view | Table 2. Toxicities in Patients Treated With Pembrolizumab Treatment |
Results of univariate and multivariate analyses concerning the associations between various factors and the OS with pembrolizumab treatment
To identify the prognostic factors associated with the OS in pembrolizumab treatment, we performed univariate and multivariate analyses using the Cox proportional hazards model (Table 3). Univariate analyses for various factors revealed that the ECOG-PS, NLR, liver metastases and time from previous chemotherapy were prognostic variables. Multivariate analyses revealed that liver metastases (positive: hazard ratio (HR), 4.23; 95% CI, 1.48 - 12.08; P < 0.01) and time from previous chemotherapy (≥ 3 months: HR, 5.06; 95% CI, 1.43 - 17.91; P = 0.01) were independent prognostic factors.
 Click to view | Table 3. The Univariate and Multivariate Analyses of the Factors Associated With Overall Survival in Patients Receiving Pembrolizumab Treatment |
| Discussion | ▴Top |
Pembrolizumab was associated with a better survival benefit and objective response and a lower rate of any-grade treatment-related AEs than chemotherapy as second-line treatment for platinum-refractory advanced UC in the phase 3 trial KEYNOTE-045 [6].
In this real-world clinical study, we reported our initial experience with pembrolizumab for platinum-refractory advanced UC in Japanese patients. The overall response rate was 20.6%, and the median PFS and OS were 3.3 months (95% CI, 2.8 - 7.4 months) and 11.7 months (95% CI, 5.9 - 15.7 months), respectively. Regarding the toxicities associated with pembrolizumab, AEs of any grade occurred in 61.8%, grade 3 AEs occurred in 23.5%, and grade ≥ 4 AEs did not occur in any patients. All of these results are comparable to those of the open-label, international, phase 3 trial KEYNOTE-045 (OS: 10.3 months, AEs of any grade: 60.9%). The long-term safety and efficacy outcomes of KEYNOTE-045 have already been reported (median follow-up was 27.7 months), so pembrolizumab can be expected to achieve comparable results in Japanese clinical practice, even in the long term [14].
Several prognostic factors have been reported to predict the prognosis of patients with UC. Bajorin et al found that a Karnofsky Performance Status < 80% and the presence of visceral (liver, lung, and bone) metastasis were independent prognostic factors associated with the OS [15]. Bellmunt et al reported that a poor PS (> 1), low Hb levels (< 10 g/dL) and liver metastasis were independent poor prognostic factors in patients with advanced UC showing treatment failure with platinum-based chemotherapy [16]. Furthermore, Sonpavde et al revealed that a significant decrease in the OS for patients with a shorter time from previous chemotherapy (stratification less than 3 months) was observed, independent of ECOG-PS > 0, Hb < 10 g/dL and the presence of liver metastases in the largest, individual patient-level dataset of second-line therapy assembled [17]. In the phase 3 trial KEYNOTE-045, randomization was stratified according to the ECOG-PS score (0 or 1 vs. 2), presence of liver metastases (yes vs. no), Hb concentration (< 10 g/dL vs. ≥ 10 g/dL) and time since the last dose of chemotherapy (< 3 months vs. ≥ 3 months). In the present study, we also investigated several prognostic factors, including previously reported markers, for patients with advanced UC receiving pembrolizumab. The multivariate analyses eventually revealed that liver metastases (positive: HR, 4.23; 95% CI, 1.48 - 12.08; P < 0.01) and time from previous chemotherapy (≥ 3 months: HR, 5.06; 95% CI, 1.43 - 17.91; P = 0.01) were independent prognostic factors.
The presence of liver metastases has been previously reported to be a poor prognostic factor. The present study also suggested that it is better not to allow an interval between chemotherapy and pembrolizumab. One reason for this may be T cell exhaustion, which is a state of T cell dysfunction that arises in many chronic infections and cancer. It is defined by a poor effector function, the sustained expression of inhibitory receptors and a transcriptional state distinct from that of functional effector or memory T cells [18, 19]. The absence of cancer progression for 3 months after chemotherapy may indicate that T cells have suppressed the disease during that time. However, since T cells are exhausted, there is a possibility that sufficient effects cannot be obtained even if T cells are reactivated by administering pembrolizumab. Part of the rationale for combining checkpoint inhibitors with conventional cytotoxic therapies is the hope that circulating T cells will encounter higher levels of tumor-derived neoantigens, resulting in an enhanced anticancer immune response [20]. Administering pembrolizumab without delay after the completion of the chemotherapy regimen may help to avoid T cell exhaustion while obtaining a similar response of combining checkpoint inhibitors with conventional cytotoxic therapies in the hope that circulating T cells will enhance the anticancer immune response by encountering higher levels of tumor-derived neoantigens.
Recently, the idea has been proposed that chemotherapy causes cell death, which is termed immunogenic cell death (ICD). ICD is now used to refer to a functionally peculiar form of apoptosis that is sufficient for immunocompetent hosts to mount an adaptive immune response against dead cell-associated antigens. Several drugs have been ascribed the ability to provoke ICD when employed as standalone therapeutic interventions [21]. Suzuki et al showed that the chemotherapeutic drug gemcitabine, when given at a dose similar to the equivalent dose used in patients, was able to dramatically and specifically reduce the number of myeloid suppressor cells found in the spleens of animals bearing large tumors with no significant reductions in cluster of differentiation 4 (CD4)+ T cells, CD8+ T cells, natural killer (NK) cells, macrophages or B cells. The loss of myeloid suppressor cells was accompanied by an increase in the antitumor activity of CD8+ T cells and activated NK cells [22]. Therefore, there is a possibility that the administration of pembrolizumab without prolonging the period of chemotherapy may result in improved antitumor effects.
A previous clinical trial found that pembrolizumab improved the OS when administered as second-line treatment for advanced UC after platinum-based chemotherapy [6]. However, the prognosis period in which the effect of pembrolizumab treatment was better than that of other chemotherapy treatments was only approximately 3 months, and the overall response rate was only 21.1%, so the outcome of advanced UC remains unsatisfactory. Our real-world clinical practice retrospective study included six patients (18%) who had been treated with three prior chemotherapy regimens. The difference in the OS between the second-line and third-line or later groups was not statistically significant, according to the log-rank test (P = 0.94). Pembrolizumab may therefore achieve almost the same therapeutic response even in the later-regimen setting because of its different antitumor mechanisms in comparison to conventional cytotoxic therapies. Therefore, pembrolizumab as third-line treatment may also be an option in cases where certain effects can be obtained by chemotherapy.
The phase 3 KEYNOTE-045 trial included various races. The breakdown was as follows: white, 73.9%; Asian, 21.3%; black or African American, 1.5%; multiple, 0.4%; and missing, 2.9% [23]. The present study focused on Japanese, and similar results may be obtained in other countries or races.
Some limitations relevant to this study are worth mentioning. First, the data related to the efficacy and tolerability of pembrolizumab for platinum-refractory advanced UC in Japanese were evaluated retrospectively, not in a randomized trial but in real-world clinical practice. Second, the study population was relatively small. However, there are still few reports of pembrolizumab for advanced UC in Japanese, and it will be necessary to confirm our data in a larger study population in further studies.
Conclusions
In conclusion, we showed that pembrolizumab therapy for Japanese patients with advanced UC in real-world clinical practice, even in the later-regimen setting, had a comparable efficacy and safety profile to the KEYNOTE-045 trial. In addition, liver metastases and time from previous chemotherapy were poor prognostic variables in patients with advanced UC treated with pembrolizumab.
Acknowledgments
None to declare.
Financial Disclosure
No funding was received.
Conflict of Interest
The authors declare that they have no conflict of interest for this study.
Informed Consent
The patient provided written informed consent for the publication of any associated data.
Author Contributions
NF, KK and TN designed the study. FM, TT and YH extracted the data. NT, TI and MK assisted with the data processing and statistical analysis. NF and KK wrote the article. MN and KK supervised the study and critically reviewed the manuscript. All authors have read and approved the final version of the manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30.
doi pubmed - Vaidya A, Soloway MS, Hawke C, Tiguert R, Civantos F. De novo muscle invasive bladder cancer: is there a change in trend? J Urol. 2001;165(1):47-50; discussion 50.
doi pubmed - Stein JP, Lieskovsky G, Cote R, Groshen S, Feng AC, Boyd S, Skinner E, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001;19(3):666-675.
doi pubmed - von der Maase H, Hansen SW, Roberts JT, Dogliotti L, Oliver T, Moore MJ, Bodrogi I, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18(17):3068-3077.
doi pubmed - Bellmunt J, Theodore C, Demkov T, Komyakov B, Sengelov L, Daugaard G, Caty A, et al. Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J Clin Oncol. 2009;27(27):4454-4461.
doi pubmed - Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, Vogelzang NJ, et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med. 2017;376(11):1015-1026.
doi pubmed - Yuasa T, Urakami S, Yonese J. Recent advances in medical therapy for metastatic urothelial cancer. Int J Clin Oncol. 2018;23(4):599-607.
doi pubmed - Vaughn DJ, Bellmunt J, Fradet Y, Lee JL, Fong L, Vogelzang NJ, Climent MA, et al. Health-Related Quality-of-Life Analysis From KEYNOTE-045: A Phase III Study of Pembrolizumab Versus Chemotherapy for Previously Treated Advanced Urothelial Cancer. J Clin Oncol. 2018;36(16):1579-1587.
doi pubmed - Yasuoka S, Yuasa T, Nishimura N, Ogawa M, Komai Y, Numao N, Yamamoto S, et al. Initial Experience of Pembrolizumab Therapy in Japanese Patients With Metastatic Urothelial Cancer. Anticancer Res. 2019;39(7):3887-3892.
doi pubmed - Tamura D, Jinnouchi N, Abe M, Ikarashi D, Matsuura T, Kato R, Maekawa S, et al. Prognostic outcomes and safety in patients treated with pembrolizumab for advanced urothelial carcinoma: experience in real-world clinical practice. Int J Clin Oncol. 2020.
doi - Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur Urol. 2016;70(1):93-105.
doi pubmed - https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50.
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247.
doi pubmed - Fradet Y, Bellmunt J, Vaughn DJ, Lee JL, Fong L, Vogelzang NJ, Climent MA, et al. Randomized phase III KEYNOTE-045 trial of pembrolizumab versus paclitaxel, docetaxel, or vinflunine in recurrent advanced urothelial cancer: results of >2 years of follow-up. Ann Oncol. 2019;30(6):970-976.
doi pubmed - Bajorin DF, Dodd PM, Mazumdar M, Fazzari M, McCaffrey JA, Scher HI, Herr H, et al. Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy. J Clin Oncol. 1999;17(10):3173-3181.
doi pubmed - Bellmunt J, Choueiri TK, Fougeray R, Schutz FA, Salhi Y, Winquist E, Culine S, et al. Prognostic factors in patients with advanced transitional cell carcinoma of the urothelial tract experiencing treatment failure with platinum-containing regimens. J Clin Oncol. 2010;28(11):1850-1855.
doi pubmed - Sonpavde G, Pond GR, Fougeray R, Choueiri TK, Qu AQ, Vaughn DJ, Niegisch G, et al. Time from prior chemotherapy enhances prognostic risk grouping in the second-line setting of advanced urothelial carcinoma: a retrospective analysis of pooled, prospective phase 2 trials. Eur Urol. 2013;63(4):717-723.
doi pubmed - Virgin HW, Wherry EJ, Ahmed R. Redefining chronic viral infection. Cell. 2009;138(1):30-50.
doi pubmed - Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12(6):492-499.
doi pubmed - Crist M, Iyer G, Hsu M, Huang WC, Balar AV. Pembrolizumab in the treatment of locally advanced or metastatic urothelial carcinoma: clinical trial evidence and experience. Ther Adv Urol. 2019;11:1756287219839285.
doi pubmed - Pol J, Vacchelli E, Aranda F, Castoldi F, Eggermont A, Cremer I, Sautes-Fridman C, et al. Trial Watch: Immunogenic cell death inducers for anticancer chemotherapy. Oncoimmunology. 2015;4(4):e1008866.
doi pubmed - Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005;11(18):6713-6721.
doi pubmed - https://www.ema.europa.eu/en/documents/variation-report/keytruda-h-c-2830-ii-0023-g-epar-assessment-report-variation_en.pdf.
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


