| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Original Article
Volume 11, Number 10, October 2019, pages 676-681
Ezetimibe Monotherapy Reduces Serum Levels of Platelet-Activating Factor Acetylhydrolase in Patients With Dyslipidemia
Kanako Tanoa, d, Yasunori Suematsua, d, Kohei Tashiroa, Naoko Kumagai-Koyanagia, b, Yoshino Matsuoa, Takashi Kuwanoa, Shin-ichiro Miuraa, e, Zenith Trial Investigatorsc
aDepartment of Cardiology, Fukuoka University School of Medicine, Fukuoka, Japan
bDivision of Cardiology, Sata Hospital, Fukuoka, Japan
cKeijiro Saku, Keita Noda, Shin-ichiro Miura, Naoko Koyanagi, Rie Koyoshi, Atsushi Iwata (Fukuoka University Hospital, Fukuoka), Hidenori Urata (Fukuoka University Chikushi Hospital, Fukuoka), Masahiko Seki (Seki Internal Clinic), Toshiki Hiratsuka (Hiratsuka Clinic), Yoichi Tanabe (Tanabe Clinic), Kazuaki Fujisawa (Fujisawa Internal Clinic), Jin Miyawaki (Miyawaki Clinic), Fumihiro Hoshino (Murakamikarindo Hospital), Masatsugu Oga (Oga Internal Circulatory Clinic), Masaki Kohara (Kohara Clinic), Fumitada Hattori (Nagao Hospital), Yutaka Tachikawa (Tanaka Hospital)
dThese authors contributed equally to this manuscript.
eCorresponding Author: Shin-ichiro Miura, Department of Cardiology, Fukuoka University School of Medicine, 7-45-1 Nanakuma, Jonan-Ku, Fukuoka 814-0180, Japan
Manuscript submitted June 13, 2019, accepted August 20, 2019
Short title: Ezetimibe Reduces Serum Levels of PAF-AH
doi: https://doi.org/10.14740/jocmr3901
| Abstract | ▴Top |
Background: The combination of ezetimibe with statin therapy reduced cardiovascular events compared to statin monotherapy in IMPROVEIT study, and ezetimibe monotherapy attenuated atherosclerosis in basic study. We previously showed ezetimibe monotherapy was especially effective for metabolic syndrome (MetS) patients. We investigated the effects of ezetimibe monotherapy for high-density lipoprotein cholesterol (HDL-chol) function and platelet-activating factor acetylhydrolase (PAF-AH) activity.
Methods: Forty-two patients who initially received ezetimibe (10 mg/day) without statin treatment for 16 weeks from January 2009 to August 2011 were enrolled. Patients were divided into MetS and non-MetS groups, and serum levels of lipids, PAF-AH, and HDL-chol efflux capacity (HDL-CEC) at baseline and after 16 weeks of treatment were investigated. Serum PAF-AH, HDL-associated PAF-AH (HDL-PAF-AH), and LDL-associated PAF-AH (LDL-PAF-AH) were measured.
Results: In all patients, age, the percentages of males, and body mass index were 61.0 ± 8.8 years, 59.5% and 26.3 ± 3.4 kg/m2, respectively. Total cholesterol and low-density lipoprotein cholesterol (LDL-chol) were significantly decreased by ezetimibe monotherapy. Serum PAF-AH and LDL-PAF-AH were significantly decreased by ezetimibe monotherapy, whereas HDL-PAF-AH and HDL-CEC were not. There was no difference in the results of PAF-AH and HDL-CEC between MetS and non-MetS groups.
Conclusions: Ezetimibe monotherapy might prevent coronary heart disease (CHD) regardless of the presence of MetS, because PAF-AH was independent risk factor for CHD.
Keywords: Metabolic syndrome; Platelet-activating factor acetylhydrolase; Cholesterol efflux capacity
| Introduction | ▴Top |
Dyslipidemia causes cardiovascular disease (CVD) due to atherosclerosis of the arterial vessel wall, and is the foremost cause of premature mortality and disability-adjusted life years [1]. Ezetimibe selectively inhibits the intestinal absorption of cholesterol and related phytosterols. In the IMPROVE-IT study, the combination of ezetimibe plus simvastatin therapy in patients with post-acute coronary syndrome significantly reduced cardiovascular events compared to simvastatin monotherapy [2].
Ezetimibe monotherapy has been shown to reduce atherothrombotic complications after superficial plaque erosion [3] and to attenuate atherosclerosis associated with lipid reduction and the inhibition of inflammation [4]. We previously reported that ezetimibe monotherapy for dyslipidemia patients reduced the serum levels of total cholesterol (T-chol), low-density lipoprotein cholesterol (LDL-chol), and non-high-density lipoprotein cholesterol (non-HDL-chol), especially in metabolic syndrome (MetS) (Zenith Trial) [5]. But ezetimibe is not recommended as a fist choice for dyslipidemia.
Platelet-activating factor acetylhydrolase (PAF-AH) activity is a circulating enzymatic biomarker of inflammation that is primarily bound to LDL-chol [6]. We previously reported that PAF-AH activity was associated with paroxysmal atrial fibrillation [6] and HDL-associated PAF-AH activity was associated with diabetes mellitus (DM) and the coronary artery calcification score [7]. PAF-AH activity was also associated with both unstable and stable coronary heart disease [8] and was an independent predictor of coronary artery disease (CAD) [9].
HDL-chol efflux capacity (HDL-CEC), one of the functions of HDL-chol, has a strong inverse association with atherosclerosis [10]. We previously reported the efficacy of HDL-CEC against atherosclerosis and CAD [11-15].
In this study, we investigated PAF-AH and HDL-CEC in dyslipidemia patients receiving ezetimibe monotherapy.
| Materials and Methods | ▴Top |
Study design
Kumagai et al previously reported the effects of ezetimibe on dyslipidemia patients with MetS (Zenith Trial) [5]. Each subject signed an informed consent form after the protocol was explained. Patients who were treated with ezetimibe for dyslipidemia from January 2009 to August 2011 at Fukuoka University Hospital and its related hospitals in the Kyushu area of Japan were enrolled. Patients who were pretreated with statin after a 4-week washout period were enrolled. Serum samples were collected at baseline and after 16 weeks of treatment with ezetimibe. The patients were divided into MetS and non-MetS groups. Male, the presence of MetS, and a lower ratio of LDL-chol to HDL-chol were independent factors that predicted a good response to ezetimibe monotherapy. For this investigation, the Zenith Trial including the current investigation was approved by the Independent Review Board (IRB) of Fukuoka University (2017M160) and conducted according to the Declaration of Helsinki regarding investigations in humans. We excluded 16 patients from the Zenith Trial due to insufficient blood sample volumes, and finally analyzed 42 patients.
PAF-AH activity
Serum lipoprotein-associated phospholipase A2 was measured by a spectrophotometric assay using an automatic analyzer (JCA-BM6010, JEOL, Ltd, Tokyo, Japan) [16]. HDL-associated PAF-AH (HDL-PAF-AH) activity was measured in apolipoprotein B-depleted serum, which was separated by precipitation of apo-B using phosphotungstate acid/MgCl2. The value of LDL-associated PAF-AH (LDL-PAF-AH) activity was taken to be the value of PAF-AH activity in whole serum minus the value of HDL-PAF-AH activity, as previously described [6, 7].
HDL-CEC
HDL-CEC was measured by an ex vivo technique, as previously described [11-15]. 3H-cholesterol-labeled J774 macrophage cells were incubated with apoB-depleted serum samples for 4 h. Radiolabeled cholesterol counts in macrophage and medium were measured by a liquid scintillation analyzer (Tri-Carb 2900TR, Perkin Elmer Co., Ltd, MA, USA). HDL-CEC was calculated as HDL-CEC (%) = (radioactivity in the medium/radioactivity in the medium + macrophages) × 100/cholesterol efflux activity in serum-free medium.
Measurements of various parameters
Age, gender, body mass index (BMI), history of hypertension (HT), DM, and CAD were evaluated. BMI was calculated as weight (kg)/height2 (m2).
Serum levels of white blood cell (WBC), high-sensitivity C-reactive protein (hs-CRP), blood urea nitrogen (BUN), creatinine (Cr), aspartate aminotransferase (AST), alkaline phosphatase (ALT), T-chol, TG, LDL-chol, HDL-chol, remnant-like particle-cholesterol (RLP-chol), and adiponectin were evaluated at the Fukuoka University Hospital Laboratory Unit or by SRL Corporation, as reported previously [5].
Statistical analyses
All data analyses were performed using the SAS (Statistical Analysis System) Software Package (Ver. 9.4, SAS Institute Inc., Cary, NC, USA) at Fukuoka University (Fukuoka, Japan). Continuous variables with a normal distribution were expressed as mean ± standard deviation and compared between groups by Student’s t-test. Continuous variables with a non-normal distribution were expressed as median (interquartile range) and compared between groups by the Wilcoxon rank sum test. Categorical variables were compared between groups by a Chi-square analysis. The baseline laboratory data and the data after 16 weeks of treatment were compared by the paired t-test for continuous variables with a normal distribution and by the Wilcoxon signed rank test for continuous variables with a non-normal distribution. A value of P < 0.05 was considered significant.
| Results | ▴Top |
Baseline patient characteristics
Table 1 shows the patient characteristics. In all patients, average age, BMI, and the percentages of male, HT, DM, and CAD were 61.0 ± 8.8 years, 26.3 ± 3.4 kg/m2, 59.5% (n = 25), 87.8% (n = 36), 57.1% (n = 24), and 11.9% (n = 5), respectively. The percentage of DM in the MetS group was significantly greater than that in non-MetS group (MetS group: 77.3% vs. non-MetS group: 35.0%, P = 0.006).
 Click to view | Table 1. Patient Characteristics |
Laboratory data
Laboratory data at baseline and after 16 weeks of treatment with ezetimibe are shown in Table 2. At baseline, TG and RLP-chol in the MetS group were significantly higher than those in the non-MetS group (TG: 178 ± 61 mg/dL vs. 120 ± 64 mg/dL, P = 0.004 and RLP-chol: 7.4 (6.8 - 9.1) mg/dL vs. 4.6 (3.7 - 5.5) mg/dL, P = 0.0002). T-chol, HDL-chol, LDL-chol, and adiponectin in the MetS group were not significantly different than those in the non-MetS group. T-chol and LDL-chol were significantly decreased during 16 weeks of treatment with ezetimibe in all patients (T-chol: baseline 242 ± 33 mg/dL, 16 weeks of treatment: 208 (191 - 223) mg/dL, P < 0.0001; and LDL-chol: 165 ± 29 mg/dL, 127 (113 - 144) mg/dL, P < 0.0001) and in both the MetS (T-chol: 249 ± 35 mg/dL, 213 ± 39 mg/dL, P < 0.0001 and LDL-chol: 171 ± 30 mg/dL, 134 ± 31 mg/dL, P < 0.0001) and non-MetS (T-chol: 234 ± 29 mg/dL, 210 ± 21 mg/dL, P < 0.0001 and LDL-chol: 158 ± 26 mg/dL, 132 ± 24 mg/dL, P < 0.0001) groups. RLP-chol was significantly decreased after 16 weeks of treatment with ezetimibe in all patients (6.4 (4.4 - 7.8) mg/dL, 4.8 (4.0 - 6.9) mg/dL, P = 0.003) and the MetS group (7.4 (6.8 - 9.1) mg/dL, 6.5 ± 2.5 mg/dL, P < 0.0001), but not the non-MetS group (4.6 (3.7 - 5.5) mg/dL, 4.5 ± 1.4 mg/dL, P = 0.1). After 16 weeks of treatment with ezetimibe, TG and RLP-chol in the MetS group were still significantly higher than those in the non-MetS group (TG: 166 ± 51 mg/dL vs. 118 (96 - 129) mg/dL, P = 0.01 and RLP-chol: 6.5 ± 2.5 mg/dL vs. 4.5 ± 1.4 mg/dL, P = 0.01).
 Click to view | Table 2. Laboratory Data on Pre- and Post-Treatment With Ezetimibe for 16 Weeks |
PAF-AH and HDL-CEC
Data regarding PAF-AH and HDL-CEC are shown in Figure 1. There were no significant differences between the MetS and non-MetS groups at baseline. PAF-AH and LDL-PAF-AH were significantly decreased after 16 weeks of treatment with ezetimibe in all patients (PAF-AH: 521 ± 164 U/L, 450 ± 155 U/L, P < 0.0001 and LDL-PAF-AH: 499 ± 163 U/L, 428 ± 154 U/L, P < 0.0001) and in both the MetS (PAF-AH: 506 ± 108 U/L, 431 ± 119 U/L, P < 0.0001 and LDL-PAF-AH: 514 ± 109 U/L, 450 ± 119 U/L, P < 0.0001), and non-MetS (PAF-AH: 537 ± 203 U/L, 473 ± 180 U/L, P < 0.0001 and LDL-PAF-AH: 486 ± 202 U/L, 411 ± 178 U/L, P < 0.0001) groups. HDL-PAF-AH and HDL-CEC were not significantly changed by 16 weeks of treatment in either of all patients, the MetS group, or the non-MetS group.
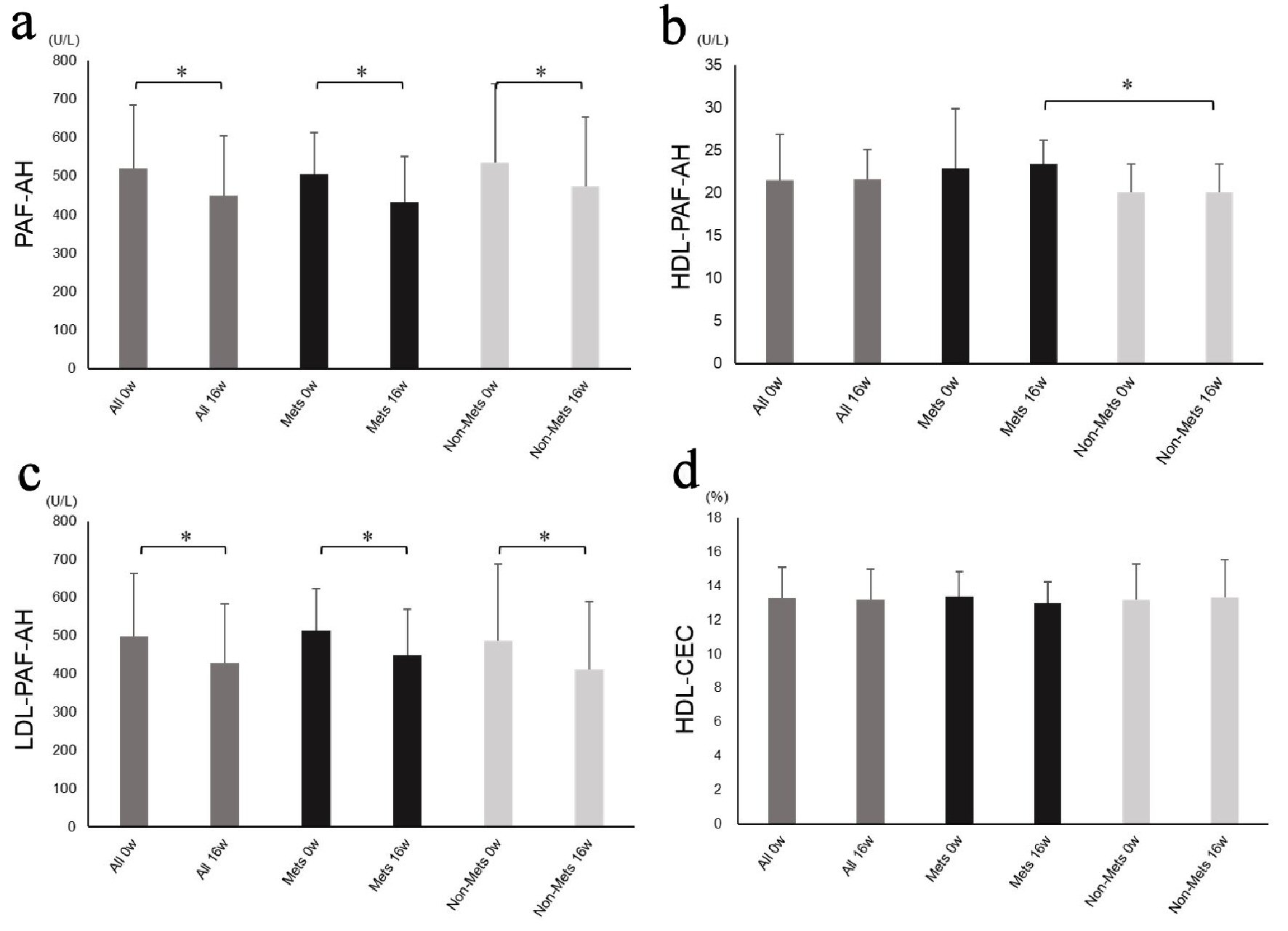 Click for large image | Figure 1. Platelet-activating factor acetylhydrolase activity and high-density lipoprotein cholesterol efflux capacity. PAF-AH (a), HDL-PAF-AH (b), LDL-PAF-AH activity (c) and HDL-CEC (d) at baseline and after 16 weeks of treatment in all patients and in the MetS and non-MetS groups. PAF-AH: platelet-activating factor acetylhydrolase; HDL-PAF-AH: high-density lipoprotein-associated PAF-AH; LDL-PAF-AH: low-density lipoprotein-associated PAF-AH; HDL-CEC: high-density lipoprotein cholesterol efflux capacity; MetS: metabolic syndrome. *P < 0.05. |
| Discussion | ▴Top |
We investigated the effects of ezetimibe monotherapy for PAF-AH and HDL-CEC in patients with dyslipidemia. Ezetimibe significantly reduced PAF-AH and LDL-PAF-AH in addition to reducing T-chol and LDL-chol regardless of the presence or absence of MetS, and did not change the levels of HDL-PAF-AH and HDL-CEC.
Ezetimibe improved T-chol and LDL-chol in all patients and in both the MetS and non-MetS groups. These are reasonable effects of ezetimibe due to the selective inhibition of intestinal absorption of cholesterol. Ezetimibe also improved RLP-chol in all patients and the MetS group, but not the non-MetS group, because the level of RLP-chol in the non-MetS group was within the normal range at baseline. The incidence of diabetes in the MetS group was significantly higher than that in the non-MetS group, because diabetes is one of the diagnostic criteria of MetS [17].
Effect for PAF-AH by ezetimibe monotherapy
Packard et al reported that inflammatory markers, especially PAF-AH, are predictors of coronary events [9]. Tsimikas et al reported that an increased PAF-AH level was associated with MetS, and the incidences of both fatal and non-fatal CVD [18]. In this study, ezetimibe significantly reduced PAF-AH in both the MetS and non-MetS groups. Ezetimibe selectively inhibits the intestinal absorption of cholesterol and decreases serum LDL-chol. Since PAF-AH is reportedly strongly influenced by LDL-chol [18], it is possible that ezetimibe significantly reduced PAF-AF due to its effect of lowering LDL-chol. These results indicate that ezetimibe monotherapy may have a potential role in suppressing CVD regardless of the presence or absence of MetS. We previously reported the efficacies of HDL-PAF-AH and LDL-PAF-AH [6, 7]. In this study, HDL-PAF-AH was not significantly improved after 16 weeks of treatment with ezetimibe monotherapy because almost no PAF-AH was detected in apoB-depleted serum (LDL-PAF-AH). In this study, isolation of PAF-AH into HDL- and LDL-PAF-AH might be unrelated to the efficacy of ezetimibe monotherapy.
Effect for HDL-CEC by ezetimibe monotherapy
HDL-CEC is one of the effects of HDL-chol against atherosclerosis [10]. In this study, ezetimibe monotherapy was expected to improve HDL-CEC, since previous reports demonstrated that ezetimibe affects reverse cholesterol transport [19, 20]. On the other hand, it was reported that treatment with a combination of atorvastatin plus ezetimibe did not increase HDL-CEC [21, 22]. Ezetimibe monotherapy did not improve HDL-CEC in this study. Although we previously reported that total CEC was positively correlated with HDL-chol levels [12], ezetimibe monotherapy did not change the levels of HDL-chol in this study. Taken together the results of PAF-AH, ezetimibe monotherapy had an effect for LDL-chol-associated factors rather than HDL-chol-associated factors.
Limitation
This study has several limitations. For example, a sufficient sample size was needed for several investigations. Although we discontinued statin treatment with a 4-week washout period before enrollment, statins may exert a legacy effect for CVD [23]. Ezetimibe monotherapy decreased levels of both LDL-chol and LDL-PAF-AH, and delta LDL-chol was significantly associated with changes in LDL-PAF-AH (data not shown). The reduction of LDL-PAF-AH might be due to the reduction of LDL-chol. We can expect that ezetimibe monotherapy would have an atheroprotective effect in addition to an LDL-chol-lowering effect, since PAF-AH has been shown to be an independent predictor of atherosclerosis regardless of the lipid profile [9].
In conclusion, ezetimibe significantly reduced PAF-AH in addition to reducing T-chol and LDL-chol regardless of the presence or absence of MetS.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
In the Zenith Trial, each subject signed an informed consent form after the protocol was explained. For this investigation, the Zenith Trial including the current investigation was approved by the Independent Review Board (IRB) of Fukuoka University (2017M160). In current investigation, there was no informed consent because the current investigation was a retrospective study.
Author Contributions
Acquisition: YS, K. Tano and NKK; conception and design: YS and SM; analysis: K. Tano, YM, YS and NKK; interpretation: K. Tashiro, TK and SM.
| References | ▴Top |
- Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, Hoes AW, et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J. 2016;37(39):2999-3058.
doi pubmed - Murphy SA, Cannon CP, Blazing MA, Giugliano RP, White JA, Lokhnygina Y, Reist C, et al. Reduction in Total Cardiovascular Events With Ezetimibe/Simvastatin Post-Acute Coronary Syndrome: The IMPROVE-IT Trial. J Am Coll Cardiol. 2016;67(4):353-361.
doi pubmed - Honda K, Matoba T, Antoku Y, Koga JI, Ichi I, Nakano K, Tsutsui H, et al. Lipid-Lowering therapy with ezetimibe decreases spontaneous atherothrombotic occlusions in a rabbit model of plaque erosion: a role of serum oxysterols. Arterioscler Thromb Vasc Biol. 2018;38(4):757-771.
doi pubmed - Tie C, Gao K, Zhang N, Zhang S, Shen J, Xie X, Wang JA. Ezetimibe attenuates atherosclerosis associated with lipid reduction and inflammation inhibition. PLoS One. 2015;10(11):e0142430.
doi pubmed - Naoko Kumagai S-iM, Bo Zhang, Keita Noda, Keijiro Saku, Zenith Trial Investigators. Effects of ezetimibe on hypercholesterolemia in the lipid profile in patients with metabolic syndrome: Zenith Trial. IJC Metabolic & Endocrine. 2013;1:7-12.
doi - Okamura K, Miura S, Zhang B, Uehara Y, Matsuo K, Kumagai K, Saku K. Ratio of LDL- to HDL-associated platelet-activating factor acetylhydrolase may be a marker of inflammation in patients with paroxysmal atrial fibrillation. Circ J. 2007;71(2):214-219.
doi pubmed - Mitsutake R, Miura S, Zhang B, Saku K. HDL-associated factors provide additional prognostic information for coronary artery disease as determined by multi-detector row computed tomography. Int J Cardiol. 2010;143(1):72-78.
doi pubmed - Samsamshariat S, Basati G, Movahedian A, Pourfarzam M, Sarrafzadegan N. Elevated plasma platelet-activating factor acetylhydrolase activity and its relationship to the presence of coronary artery disease. J Res Med Sci. 2011;16(5):674-679.
doi pubmed - Packard CJ, O'Reilly DS, Caslake MJ, McMahon AD, Ford I, Cooney J, Macphee CH, et al. Lipoprotein-associated phospholipase A2 as an independent predictor of coronary heart disease. West of Scotland Coronary Prevention Study Group. N Engl J Med. 2000;343(16):1148-1155.
doi pubmed - Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364(2):127-135.
doi pubmed - Imaizumi S, Miura S, Takata K, Takamiya Y, Kuwano T, Sugihara M, Ike A, et al. Association between cholesterol efflux capacity and coronary restenosis after successful stent implantation. Heart Vessels. 2016;31(8):1257-1265.
doi pubmed - Norimatsu K, Kuwano T, Miura SI, Shimizu T, Shiga Y, Suematsu Y, Miyase Y, et al. Significance of the percentage of cholesterol efflux capacity and total cholesterol efflux capacity in patients with or without coronary artery disease. Heart Vessels. 2017;32(1):30-38.
doi pubmed - Shimizu T, Miura S, Tanigawa H, Kuwano T, Zhang B, Uehara Y, Saku K. Rosuvastatin activates ATP-binding cassette transporter A1-dependent efflux ex vivo and promotes reverse cholesterol transport in macrophage cells in mice fed a high-fat diet. Arterioscler Thromb Vasc Biol. 2014;34(10):2246-2253.
doi pubmed - Takata K, Imaizumi S, Kawachi E, Suematsu Y, Shimizu T, Abe S, Matsuo Y, et al. Impact of cigarette smoking cessation on high-density lipoprotein functionality. Circ J. 2014;78(12):2955-2962.
doi pubmed - Uehara Y, Ando S, Yahiro E, Oniki K, Ayaori M, Abe S, Kawachi E, et al. FAMP, a novel apoA-I mimetic peptide, suppresses aortic plaque formation through promotion of biological HDL function in ApoE-deficient mice. J Am Heart Assoc. 2013;2(3):e000048.
doi pubmed - Kosaka T, Yamaguchi M, Soda Y, Kishimoto T, Tago A, Toyosato M, Mizuno K. Spectrophotometric assay for serum platelet-activating factor acetylhydrolase activity. Clin Chim Acta. 2000;296(1-2):151-161.
doi - Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444(7121):881-887.
doi pubmed - Tsimikas S, Willeit J, Knoflach M, Mayr M, Egger G, Notdurfter M, Witztum JL, et al. Lipoprotein-associated phospholipase A2 activity, ferritin levels, metabolic syndrome, and 10-year cardiovascular and non-cardiovascular mortality: results from the Bruneck study. Eur Heart J. 2009;30(1):107-115.
doi pubmed - Briand F, Naik SU, Fuki I, Millar JS, Macphee C, Walker M, Billheimer J, et al. Both the peroxisome proliferator-activated receptor delta agonist, GW0742, and ezetimibe promote reverse cholesterol transport in mice by reducing intestinal reabsorption of HDL-derived cholesterol. Clin Transl Sci. 2009;2(2):127-133.
doi pubmed - Davidson MH, Voogt J, Luchoomun J, Decaris J, Killion S, Boban D, Glass A, et al. Inhibition of intestinal cholesterol absorption with ezetimibe increases components of reverse cholesterol transport in humans. Atherosclerosis. 2013;230(2):322-329.
doi pubmed - Nicholls SJ, Ray KK, Ballantyne CM, Beacham LA, Miller DL, Ruotolo G, Nissen SE, et al. Comparative effects of cholesteryl ester transfer protein inhibition, statin or ezetimibe on lipid factors: The ACCENTUATE trial. Atherosclerosis. 2017;261:12-18.
doi pubmed - Lee CJ, Choi S, Cheon DH, Kim KY, Cheon EJ, Ann SJ, Noh HM, et al. Effect of two lipid-lowering strategies on high-density lipoprotein function and some HDL-related proteins: a randomized clinical trial. Lipids Health Dis. 2017;16(1):49.
doi pubmed - Kashef MA, Giugliano G. Legacy effect of statins: 20-year follow up of the West of Scotland Coronary Prevention Study (WOSCOPS). Glob Cardiol Sci Pract. 2016;2016(4):e201635.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


