| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Original Article
Volume 10, Number 10, October 2018, pages 765-771
Number of Nodules but not Size of Hepatocellular Carcinoma Can Predict Refractoriness to Transarterial Chemoembolization and Poor Prognosis
Kazuhiro Katayamaa, f, Toshihiro Imaia, Yutaro Abea, Tadatoshi Nawaa, Noboru Maedab, Katsuyuki Nakanishib, Hiroshi Wadac, Keisuke Fukuid, Yuri Itod, Isao Yokotae, Kazuyoshi Ohkawaa
aDepartment of Hepatobiliary and Pancreatic Oncology, Osaka International Cancer Institute, Osaka, Japan
bDepartment of Diagnostic and Interventional Radiology, Osaka International Cancer Institute, Osaka, Japan
cDepartment of Surgery, Osaka International Cancer Institute, Osaka, Japan
dDepartment of Medical Statistics, Research and Development Center, Osaka Medical College, Osaka, Japan
eDepartment of Biostatistics, Graduate School of Medical Science, Kyoto Prefectural University of Medicine, Kyoto, Japan
fCorresponding Author: Kazuhiro Katayama, Department of Hepatobiliary and Pancreatic Oncology, Osaka International Cancer Institute, 3-1-69, Otemae, Chuo-ku, Osaka 541-8567, Japan
Manuscript submitted August 2, 2018, accepted September 1, 2018
Short title: HCC Tumor Number and TACE Refractoriness
doi: https://doi.org/10.14740/jocmr3559w
| Abstract | ▴Top |
Background: To determine whether response to transarterial chemoembolization (TACE) predicts survival and to identify pretreatment factors associated with TACE response and prognosis.
Methods: Between April and September 2010, 50 patients underwent TACE for hepatocellular carcinoma. Response to TACE was assessed using post-treatment computed tomography (CT) and magnetic resonance imaging (MRI) scans and tumor marker levels and classified as Response Poor (P) and Non-poor (NP). Time zero was set to September 30, 2010, and survival rates were analyzed by landmarking. Cumulative survival rates were calculated using the Kaplan-Meier method and compared according to grades using the log-rank test; contributing factors to survival were analyzed using a Cox proportional hazards model. Pretreatment factors were analyzed for 109 TACE sessions performed until October 2017, using a multiple logistic regression model. Receiver operating characteristic (ROC) curves were generated to determine the best tumor number for predicting response P.
Results: Response P patients showed significantly lower cumulative survival rates than Response NP patients (P < 0.001). On multivariate analysis, tumor number (hazard ratio (HR), 1.475), protein-induced vitamin-K absence-II (HR, 4.539), and the number of previous TACE sessions (HR, 1.472) were identified as pretreatment factors contributing to Response P. Further, pre-treatment platelet count (HR, 0.876) and tumor number (HR, 1.330) were factors contributing to survival in multivariate analysis. ROC curve analysis revealed that the optimal cut-off value to discriminate Response P was 7.5.
Conclusions: Response to TACE can predict survival. Pretreatment tumor number is a useful factor for predicting both TACE response and prognosis.
Keywords: HCC; TACE; BCLC; Response; Prediction
| Introduction | ▴Top |
Hepatocellular carcinoma (HCC) is the third leading cause of cancer death worldwide and poses a significant challenge for public health [1-3]. The precise prediction of patient prognosis is an essential step in the management of HCC. The Barcelona Clinic Liver Cancer (BCLC) system is the most commonly used staging system for HCC prognosis prediction and treatment selection in Western countries [4]. Several studies have demonstrated the efficacy of transarterial chemoembolization (TACE) for BCLC stage B HCC, and TACE is the recommended treatment for these types of cancer [5]. However, failure or refractoriness to TACE has not clearly been defined, and there are no clear rules to determine when to terminate TACE. In the clinical setting, the efficacy of TACE and adverse reactions should be carefully considered and TACE should be discontinued when adverse reactions outweigh survival benefits. The efficacy of sorafenib in advanced HCC has recently been demonstrated, so discontinuation of TACE does not always mean the termination of HCC treatment [6, 7]. Numerous studies have reported the efficacy of sorafenib in cases of TACE failure [8-12]. Therefore, for patients not responding to TACE, even at stage B, switching to sorafenib is likely to be more effective than the continuation of TACE [5].
Patients with BCLC stage B HCC represent a highly heterogeneous population with considerable differences in their response to TACE. Therefore, Bolondi et al proposed subgrouping these patients based on pretreatment conditions and determining treatment strategies according to respective subgroups [13]. Several researchers have attempted to determine whether TACE should be continued or discontinued based on patient response to TACE (an anti-tumor effect and/or changes in liver function) [14-16]. The Japan Society of Hepatology (JSH) defined “refractoriness or failure to TACE” as follows [14]: The first criterion applies to intrahepatic lesions, where refractoriness to TACE is defined as ≥ 2 consecutive ineffective responses of treated tumor responses (viable lesions > 50%) or ≥ 2 consecutive progressive increases in total tumor count, despite a change of chemotherapeutic agent or selection of the feeding artery. For an ineffective response, it is recommended to reevaluate the patient using computed tomography (CT) or magnetic resonance imaging (MRI) at 1 - 3 months after a selective TACE procedure. Additional criteria for refractoriness to TACE include the continuous elevation of tumor marker levels immediately after TACE and the new emergence of vascular invasion and extrahepatic spread after the procedure.
This study aimed to determine whether response to TACE, assessed using images and tumor marker levels after TACE, predicts survival prognosis, with reference to the JSH definition of TACE failure/refractoriness, and to identify pretreatment factors that predict patient response to TACE.
| Materials and Methods | ▴Top |
Subjects
TACE was used for the treatment of HCC in 62 patients at our hospital over 6 months, between April 2010 and September 2010 (inclusive). All patients were diagnosed as BCLC stage B, without extrahepatic spread or major vascular invasion, before the initiation of TACE. Of these patients, 12 were excluded because embolization was not performed in part of the tumors, due to technical issues and decreased liver function. The remaining 50 patients underwent TACE for all HCC lesions diagnosed during the TACE session. HCC treatment was continued beyond October 2010 and the patients underwent 109 TACE sessions, in total, by October 31, 2017.
Procedures of abdominal angiography and TACE
Digital subtraction angiography, computed tomography during hepatic arteriogram (CTHA), and computed tomography during arterial portography (CTAP) were performed to assess the number and size of tumors and identify tumor-feeding vessels.
A 4-Fr angiographic catheter (Selecon PA; Clinical Supply Co., Ltd., Gifu, Japan) was inserted through the femoral artery. Contrast material was injected into the common hepatic artery or the proper hepatic artery for hepatic arteriography and into the superior mesenteric artery for portography. CTAP was performed using 90 mL of iopamidol (Iopamiron 150; Bayer Pharmaceuticals Co. Ltd., Osaka, Japan), injected at a rate of 3 mL per second into the super mesenteric artery. CT scanning of the liver was initiated 30 s after starting the contrast medium injection. For digital subtraction angiography and CTHA, the catheter was placed in the common hepatic artery or the proper hepatic artery, and a contrast medium was injected. For a routine CTHA, 30 mL of iopamidol was injected at a rate of 1.5 mL per second and 5 s after initiation of the injection; the first-phase images were obtained. The second-phase images were obtained beginning 10 s after the end of the first-phase scan. A four-channel multi-detector CT scanner (Aquilion 4; Toshiba Medical Systems Co. Ltd., Otawara, Japan) was used. For more selective catheter placement, a microcatheter (Progreat; Terumo Co., Tokyo, Japan) was inserted through the 4-Fr catheter and placed in the peripheral arteries.
After identification of the feeding arteries of the target tumor nodules by the above method, TACE was performed using anticancer drugs, poppy-seed oil (lipiodol; Guerbet Japan, Tokyo, Japan), and gelatin particles. Briefly, 10 to 50 mg of epirubicin (Farmorubicin; Kyowa Hakko Kogyo, Tokyo, Japan) or 10 to 50 mg of cisplatin (IA-call, Nippon Kayaku Co. Ltd., Tokyo, Japan) were mixed with 1 to 10 mL of iodized poppy seed oil and injected via a microcatheter. Next, gelatin sponge particles (Gelfoam; Pfizer Inc., New York, USA) or porous gelatin particles (Gelpart; Nippon Kayaku Co. Ltd., Tokyo, Japan) were infused until the feeding arteries were completely embolized. Doses of epirubicin or cisplatin and lipiodol were adjusted for the size, number, and site of tumors. When tumors were located in one segment, a microcatheter was placed in the appropriate segmental artery, and when tumors were located in multiple segments, a microcatheter was placed in each of the segments. When many tumors spread in either the left or the right lobe, a catheter was placed in the appropriate hepatic artery and TACE was performed.
Assessment of response to TACE and post-TACE follow-up procedures
TACE response of the 50 subjects were classified into two grades, using CT or MRI images and tumor marker levels, 1 to 3 months after TACE. The tumor markers used were alfa-fetoprotein (AFP) and protein induced by vitamin-K absence-II (PIVKA-II). The two groups were defined as follows: Response Poor (P), in which most of the tumors remained viable or a new lesion emerged on imaging, and tumor volume was ≥ 50% of the pretreatment tumor volume, or tumor marker levels did not decrease below 50% of the pretreatment levels; Response Non-Poor (NP) in which no viable (enhancing) residual tumors were identified on imaging, and tumor marker levels decreased below normal levels, or in which some tumors remained viable or a new lesion emerged on imaging, the volume of which was < 50% of the pretreatment tumor volume, or tumor marker levels decreased to < 50% of the pretreatment levels, but not to normal levels. After TACE, patients underwent follow-up blood tests and CT or MRI scans every 3 months, on average, at our outpatient clinic. If recurrence was detected, the size and number of tumors and liver function were evaluated, and appropriate additional treatment was planned. TACE was performed 109 times, in total, by the time of last follow-up visit on October 30, 2017.
Statistical analysis
Cumulative survival rates were compared between the groups using a landmark method [17], where the time zero was set to September 30, 2010. All 50 patients were alive on September 30, 2010, and the first TACE session before this date was used for response evaluation. Cumulative survival rates were analyzed using the Kaplan-Meier method and compared using the log-rank test. Table 1 shows patient characteristics before TACE. If the tumor number was ≥ 11, the tumor number was treated as 11 in the analyses. Univariate and multivariate Cox proportional hazards models were used to assess which patient characteristics contributed to survival.
 Click to view | Table 1. Patient Characteristics |
For the 109 TACE sessions, univariate and multivariate analyses were performed using a multiple logistic regression model to evaluate the impact of pre-TACE factors on response to TACE. Additionally, receiver operating characteristic (ROC) curves were generated to determine the best tumor number for predicting patient response to TACE.
Ethical review
The present retrospective study was approved by the Ethics Review Committee of our hospital (No. 1409115121). Written informed consent was waived by the Institutional Review Board.
| Results | ▴Top |
TACE and cumulative survival
Figure 1 showed the cumulative survival rates according to the response, analyzed using the Kaplan-Meier method. The cumulative survival rate of Response NP was significantly higher than that of Response P in the log-rank test (P < 0.001). The median survival time was 51.7 months in the Response NP group and 12.6 months in the Response P group. Analysis by Cox proportional hazard model showed that survival of Response NP group was significantly better than that of Response P group (hazard ratio, 0.228; 95% confidence interval (CI), 0.111 - 0.470).
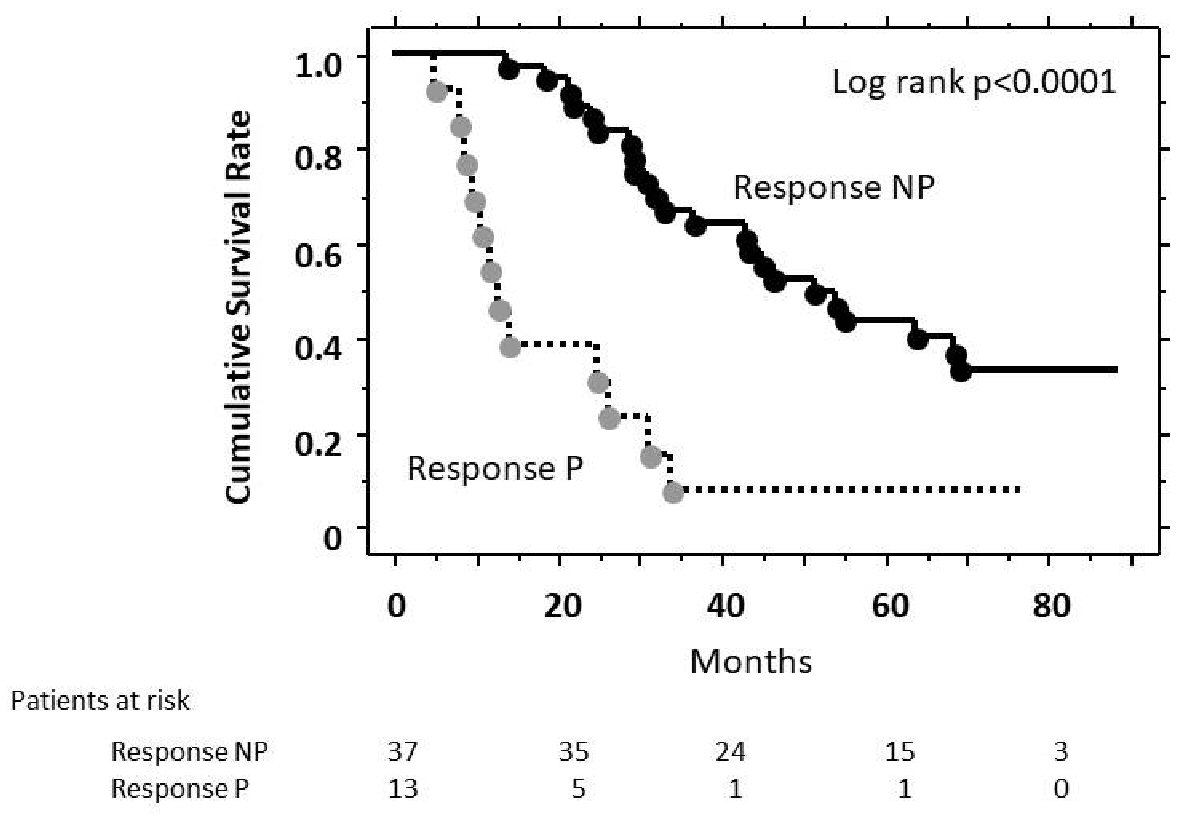 Click for large image | Figure 1. Estimates of cumulative survival rates according to response to TACE (Kaplan-Meier method). There was a significant difference in cumulative survival (P < 0.001, log-rank test) between Response P patients and Response NP patients. |
Pre-TACE factors contributing to TACE response
Response to TACE was found to be a useful factor for predicting patient prognosis. Thus, to determine whether pre-TACE factors predict response to TACE, a multiple logistic regression analysis was performed. Factors contributing to Response P versus Response NP were analyzed (Table 2). On univariate analysis, significant factors were total bilirubin, platelet count, PIVKA-II, tumor number, the presence of up-to-7 criteria, and the number of previous TACE sessions. Multivariate analysis revealed that PIVKA-II, tumor number, and the number of previous TACE sessions were significant factors contributing to TACE response.
 Click to view | Table 2. Univariate and Multivariate Analyses to Investigate Factors Predicting Response P Versus NP |
Pre-TACE factors and the post-TACE survival
Multiple logistic regression analysis of pre-TACE factors contributing to survival revealed that the following were significant: total bilirubin level, prothrombin time, platelet count, tumor number, and the presence of up-to-7 criteria on univariate analysis. There were two significant factors on the multivariate analysis: platelet count and tumor number (Table 3).
 Click to view | Table 3. Univariate and Multivariate Analyses to Investigate Factors Associated With Post-TACE Survival |
Association between tumor number and TACE response
Pre-TACE tumor number was found to be a useful factor to predict response to TACE and post-TACE prognosis. Thus, ROC curves were generated to determine the best tumor number for discriminating poor response (Fig. 2). The ROC curve for discriminating Response P from Response NP yielded a high area under the curve (AUC) value of 0.877, and the optimal cut-off value for tumor number was 7.5. That is, a patient undergoing TACE with ≥ 8 pre-TACE tumors would be assessed as Response P. Overall, 27 TACE sessions were performed in patients with a pre-TACE tumor number ≥ 8. Of those sessions, 21 (77.8%) resulted in Response P.
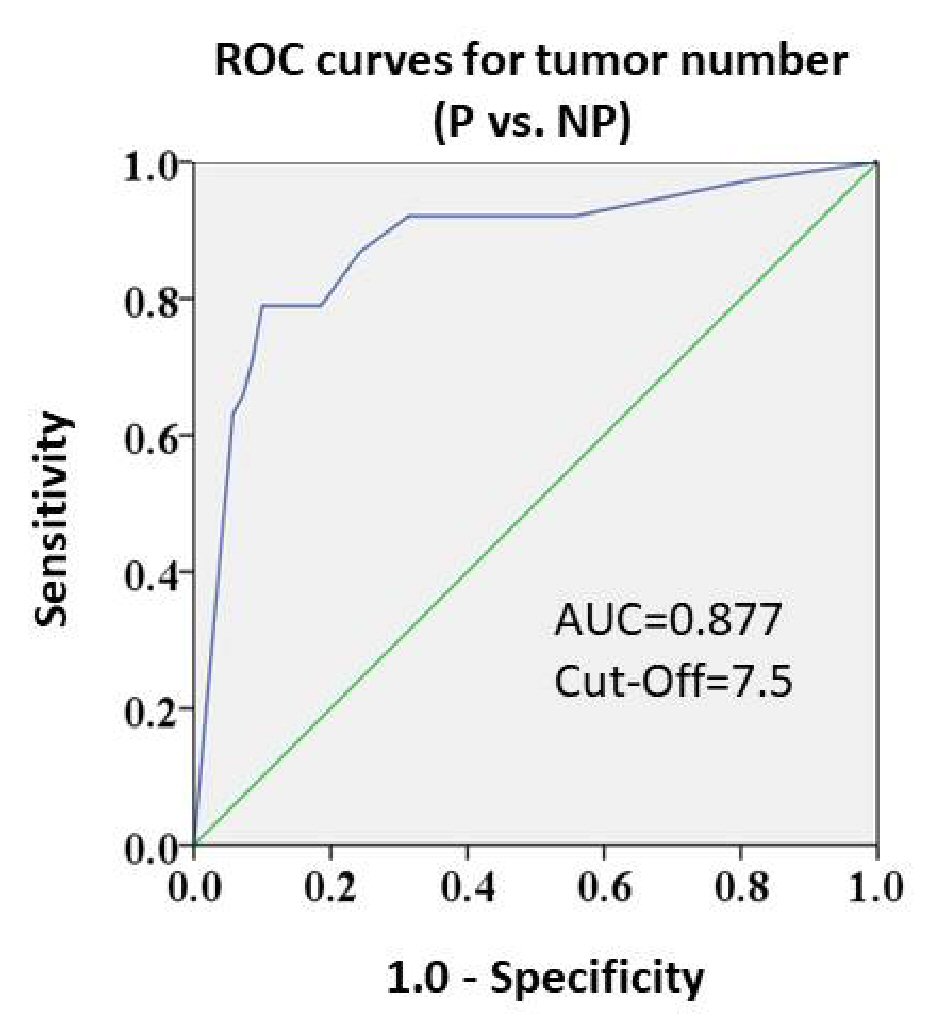 Click for large image | Figure 2. Receiver operating characteristic curves for response grades and pre-TACE tumor numbers (Response P vs. NP). The AUC value was high (0.877). The cut-off value was 7.5. |
Tumor number and post-TACE cumulative survival
Figure 3 showed the cumulative survival rates according to the pre-TACE tumor number, analyzed using the Kaplan-Meier method. The cumulative survival rate was higher among patients with a tumor number ≤ 7 than those of ≥ 8, and showed a significant overall difference in the log-rank test (P < 0.001).
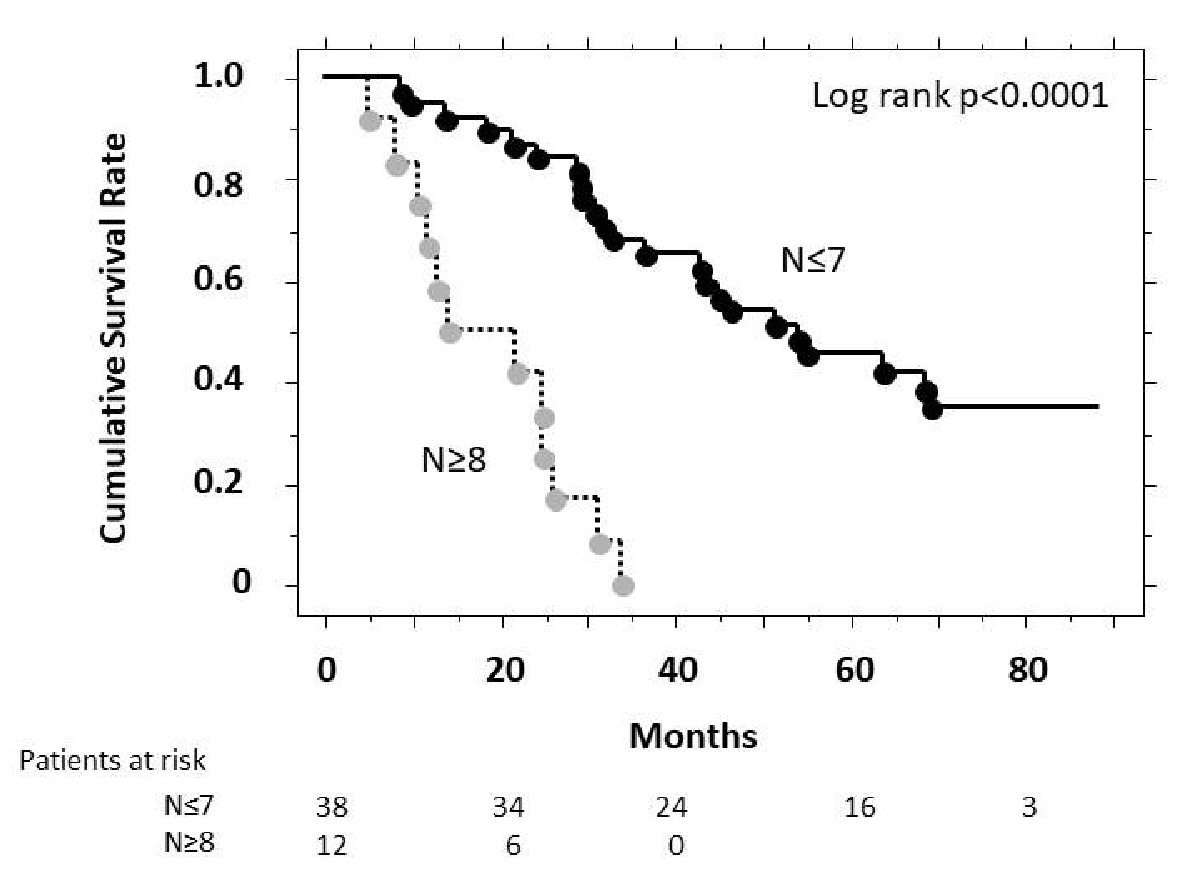 Click for large image | Figure 3. Estimates of cumulative survival rates according to pre-treatment tumor number (Kaplan-Meier method). There was a significant difference in cumulative survival (P < 0.001, log-rank test) between patients with a tumor number > 8 and those with a tumor number ≤ 7. N represents the number of tumor nodules. |
| Discussion | ▴Top |
This retrospective study investigated the response of patients with HCC to TACE treatment, which was assessed using images and tumor marker levels between 1 and 3 months after the procedure. We found that poor response to TACE was a useful predictor of poor prognosis. The accurate prediction of patient prognosis is an essential step in the management of HCC. The efficacy of sorafenib in advanced HCC has recently been demonstrated, so discontinuation of TACE does not always mean the termination of HCC treatment [6, 7]. Numerous studies have reported the efficacy of sorafenib in TACE failure cases [8-12]. Thus, for non-responders to TACE, even at stage B, switching to sorafenib is likely to be more effective than the continuation of TACE [5]. These findings indicate that accurate diagnosis of TACE refractoriness is increasingly relevant.
This study showed that poor response to TACE (tumor volume was ≥ 50% of the pretreatment tumor volume, or tumor marker levels ≥ 50% of the pretreatment levels) could predict poor prognosis. Thus, although the evaluation of TACE response is useful to predict prognosis, one of the important adverse effects of TACE is deterioration of liver function. Therefore, accurate prediction of TACE response using pre-treatment factors could avoid non-effective TACE and allow the selection of effective second line treatments. We investigated the association between pre-treatment factors and response to TACE. From the multivariate analysis, there were significant factors contributing to TACE response: PIVKA-II, tumor number, and the number of past TACE sessions (Table 2).
Next, we investigated the pre-TACE factors contributing to post-TACE survival. Multivariate analysis revealed two significant factors: platelet count and tumor number (Table 3).
Based on these findings, the present results indicated that HCC tumor number was a useful predictor for both response to TACE and patient prognosis after TACE. Thus, the ROC curve was generated to determine the best tumor number for discriminating response P from response NP yielding a high AUC value of 0.877 and an optimal cut-off value of 7.5 was selected. Hence, when TACE is performed in patients with pre-TACE tumor numbers ≥ 8, the response to TACE would likely be assessed as Response P (poor response) with poor prognosis after TACE. In this analytical process, it is noteworthy that the tumor size was not selected as a useful predictor.
The present study supports evidence that patients with BCLC stage B HCC represent a highly heterogeneous population with considerable differences in response to TACE [13]. Researchers have attempted to classify patients with intermediate-stage HCC into subgroups and establish optimal treatment strategies for each subgroup. Bolondi et al [13] proposed the use of the “Up-to-7” criteria for subgrouping patients, which represents pretreatment tumor status based on the findings of Mazzaferro et al [18] Mazzaferro et al evaluated the survival of patients who underwent liver transplantation for HCC and reported that patients who fell within the “Up-to-7” criteria (with 7 being the sum of the size of the largest tumor (in cm) and the number of tumors), even with tumors beyond the Milano criteria, achieved survival durations that were comparable to those of patients with tumors meeting the Milan criteria. Bolondi et al postulated that the “Up-to-7” criteria could be useful for the subclassification of patients who would benefit from TACE [13]. According to the authors, as patients with tumors that do not meet the “Up-to-7” criteria are expected to show poor response to TACE, treatment options in addition to TACE, including transarterial radioembolization and sorafenib, should be considered [13].
Pre-TACE tumor numbers ≥ 8 is a factor for predicting poor responses to TACE and is more useful than the “Up-to-7” criteria. In the present study, we included the “Up-to-7” criteria in the analyses and demonstrated that it was a useful predictor in univariate analysis, but found that these criteria were not identified in multivariate analysis. Since the “Up-to-7” criteria were derived from the sum of the size and number of tumors, the number of tumors may be a more influential predictor of response to TACE than tumor size.
Most of the TACE series that were evaluated in this study were conventional TACE, although several studies have compared conventional TACE with TACE using drug-eluting beads. A meta-analysis of seven studies reported that there were no significant differences in tumor response between conventional TACE and TACE using drug-eluting beads therapies [19, 20]. However, sufficient data comparing TACE using drug-eluting beads with conventional TACE have not yet been collected, and further prospective studies are warranted.
There are several limitations to this study. First, this study was retrospective, and a prospective study that further builds upon these results should be conducted. Second, 90% of the subjects in this study showed good hepatic reserves (Child-Pugh class A), and the mean tumor size was 3.1 cm. In contrast, approximately 65% of the subjects in the ART score study [15] showed Child-Pugh class A, and the mean tumor size was 5.8 cm in the training cohort and 4.3 cm in the validation cohort. Therefore, as most of the subjects in this study had good hepatic reserve and were treated relatively early, it is necessary to determine whether the results of this study are reproducible in Western countries, where advanced HCC is more prevalent. Third, the sample size was small (n = 50), and the results of this study should be confirmed across a larger number of patients.
In conclusion, pre-TACE tumor number but not size is a useful factor for predicting response to TACE and survival after TACE in patients with BCLC stage B HCC. The use of pre-TACE tumor number to predict refractoriness to TACE and establish therapeutic strategies appears to be a promising approach for HCC treatment.
Conflict of Interest
We have no conflict of interest to declare.
Funding
This study was partly supported by a grant from Seijinnbyo-Yobou Kyokai.
| References | ▴Top |
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132(7):2557-2576.
doi pubmed - Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5-29.
doi pubmed - Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359-386.
doi pubmed - Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379(9822):1245-1255.
doi - Raoul JL, Gilabert M, Piana G. How to define transarterial chemoembolization failure or refractoriness: a European perspective. Liver Cancer. 2014;3(2):119-124.
doi pubmed - Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378-390.
doi pubmed - Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25-34.
doi - Bruix J, Raoul JL, Sherman M, Mazzaferro V, Bolondi L, Craxi A, Galle PR, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol. 2012;57(4):821-829.
doi pubmed - Raoul JL, Sangro B, Forner A, Mazzaferro V, Piscaglia F, Bolondi L, Lencioni R. Evolving strategies for the management of intermediate-stage hepatocellular carcinoma: available evidence and expert opinion on the use of transarterial chemoembolization. Cancer Treat Rev. 2011;37(3):212-220.
doi pubmed - European Association For The Study Of The L, European Organisation For R, Treatment Of C. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908-943.
doi pubmed - Ogasawara S, Chiba T, Ooka Y, Kanogawa N, Motoyama T, Suzuki E, Tawada A, et al. Efficacy of sorafenib in intermediate-stage hepatocellular carcinoma patients refractory to transarterial chemoembolization. Oncology. 2014;87(6):330-341.
doi pubmed - Arizumi T, Ueshima K, Minami T, Kono M, Chishina H, Takita M, Kitai S, et al. Effectiveness of sorafenib in patients with Transcatheter Arterial Chemoembolization (TACE) refractory and intermediate-stage hepatocellular carcinoma. Liver Cancer. 2015;4(4):253-262.
doi pubmed - Bolondi L, Burroughs A, Dufour JF, Galle PR, Mazzaferro V, Piscaglia F, Raoul JL, et al. Heterogeneity of patients with intermediate (BCLC B) Hepatocellular Carcinoma: proposal for a subclassification to facilitate treatment decisions. Semin Liver Dis. 2012;32(4):348-359.
pubmed - Kudo M, Izumi N, Kokudo N, Matsui O, Sakamoto M, Nakashima O, Kojiro M, et al. Management of hepatocellular carcinoma in Japan: Consensus-Based Clinical Practice Guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis. 2011;29(3):339-364.
doi pubmed - Sieghart W, Hucke F, Pinter M, Graziadei I, Vogel W, Muller C, Heinzl H, et al. The ART of decision making: retreatment with transarterial chemoembolization in patients with hepatocellular carcinoma. Hepatology. 2013;57(6):2261-2273.
doi pubmed - Bruix J, Reig M, Rimola J, Forner A, Burrel M, Vilana R, Ayuso C. Clinical decision making and research in hepatocellular carcinoma: pivotal role of imaging techniques. Hepatology. 2011;54(6):2238-2244.
doi pubmed - Crowley D. Handbook of statistics in clinical oncology. New York: Mancel Dekker, 2001.
- Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10(1):35-43.
doi - Varela M, Real MI, Burrel M, Forner A, Sala M, Brunet M, Ayuso C, et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007;46(3):474-481.
doi pubmed - Gao S, Yang Z, Zheng Z, Yao J, Deng M, Xie H, Zheng S, et al. Doxorubicin-eluting bead versus conventional TACE for unresectable hepatocellular carcinoma: a meta-analysis. Hepatogastroenterology. 2013;60(124):813-820.
pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


