| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Original Article
Volume 11, Number 2, February 2019, pages 89-97
Investigation of a Dipeptidyl Peptidase-4 Inhibitor/Thiazolidinedione Combination Drug for Patients With Type 2 Diabetes and Poor Glycemic Control: Difficulty With Patient Enrollment
Keisuke Okamuraa, Kazuyuki Shiraia, Motoyasu Miyazakib, Tetsu Okudaa, Yosuke Takamiyaa, Miwa Gotob, Hidenori Urataa, c
aDepartment of Cardiovascular Diseases, Fukuoka University Chikushi Hospital, Chikushino, Japan
bDepartment of Pharmacy, Fukuoka University Chikushi Hospital, Chikushino, Japan
cCorresponding Author: Hidenori Urata, Department of Cardiovascular Diseases, Fukuoka University Chikushi Hospital, 1-1-1 Zokumyoin, Chikushino-shi, Fukuoka 818-8502, Japan
Manuscript submitted August 3, 2018, accepted December 4, 2018
Short title: DPP4I/TZD for Type 2 Diabetes
doi: https://doi.org/10.14740/jocmr3558
| Abstract | ▴Top |
Background: One of the treatment options for type 2 diabetes mellitus (DM) is a combination drug (CD) that contains the dipeptidyl peptidase-4 inhibitor (DPP4I) alogliptin (AG) together with pioglitazone (PG). This CD can improve impaired insulin secretion and insulin resistance, which are the two major pathologic factors for type 2 DM, and is also expected to increase adherence to treatment. We conducted a multicenter open-label prospective study to examine the usefulness of this CD for routine management of type 2 DM.
Methods: In type 2 DM patients with poor glycemic control who had been taking a DPP4I for ≥ 1 month, PG (15 mg/day) was added (first point). When the safety of PG was confirmed after 1 - 3 months, the DPP4I and PG were switched to the CD containing AG (25 mg) and PG (15 mg) (second point). Three months after switching to the CD was defined as the final point. Evaluation of objective findings, laboratory test results, and medication adherence was performed at these three points.
Results: Nineteen subjects completed the study, but this was far short of the target (160 subjects). Compared to the first point, white blood cell count (WBC), aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyl transpeptidase (γ-GTP), and fasting blood glucose (FBG) all showed a significant decrease at both the second and final points. No change in medication adherence was observed throughout the study period. The most notable point about this study was the extremely small number of subjects enrolled. As a possible explanation, we considered whether the preferences of the study doctors for antidiabetic drugs differed between specialties. The study doctors were mainly gastroenterologists, followed by endocrinologists/diabetologists and cardiologists in equal numbers. As an additional investigation, we determined the percentages of specialist doctors prescribing DPP4Is, sodium-glucose cotransporter-2 inhibitors (SGLT2Is), PG, or biguanides (BGs) as the main treatment for DM in 1 month at our hospital. We found that a low percentage of endocrinologists/diabetologists prescribing PG compared to other drugs, while cardiologists prescribed PG frequently.
Conclusions: It was confirmed that the combination of DPP4I with PG was effective for the treatment of type 2 DM and improving metabolic function. Our data also showed that prescription of antidiabetic drugs differed between specialties, suggesting differences in their response to the results of various clinical studies and adverse reaction reports.
Keywords: Dipeptidyl peptidase-4 inhibitor; Combination drug; Alogliptin; Pioglitazone; Thiazolidinedione; Type 2 diabetes mellitus; Glycemic control
| Introduction | ▴Top |
According to many epidemiological studies, tight glycemic control is associated with a lower risk of the onset and progression of microangiopathy or macroangiopathy. In Japan, it is recommended to aim for a hemoglobin A1c (HbA1c) < 7.0% (National Glycohemoglobin Standardization Program (NGSP)) for prevention of microangiopathy. While strict glycemic control is required, adherence to treatment also needs to be considered because patients remain on medication indefinitely. It has been reported that treatment with a combination drug (CD) is effective for improving adherence to medication and many studies have already demonstrated better adherence in patients with hypertension (HT) receiving CD therapy. Good adherence to antihypertensive medication significantly improves blood pressure (BP) control and reduces the risk of cardiovascular disease [1-3], while simplifying medication and reducing the dosing frequency by switching to a CD was also reported to significantly improve BP [4, 5]. However, few CDs are available for treatment of diabetes mellitus (DM), and their effects have not been examined as often as those of CD for HT.
The first CD containing alogliptin (AG), a selective dipeptidyl peptidase-4 inhibitor (DDP4I), and pioglitazone (PG), a thiazolidinedione (TZD), was released in Japan in 2011. Since DPP4Is are rarely associated with hypoglycemia because insulin secretion is promoted in a blood glucose-dependent manner and also does not cause weight gain, these drugs have the advantage of being easily prescribed by non-specialist doctors, even though long-term evidence is insufficient. PG improves insulin resistance by targeting peripheral tissues such as fat and muscle, and evidence that it prevents macroangiopathy has been provided by studies such as the PROspective pioglitAzone Clinical Trial In macroVascular Events (PROactive Study) [6]. PG is considered to increase adiponectin secretion from fat tissue, and it acts directly on the peroxisome proliferator-activated receptor γ (PPARγ) expressed by macrophages that is involved in activation of cholesterol transport [7]. The combination of AG with PG can improve impaired insulin secretion and insulin resistance, which are the two major contributors to type 2 DM, and is also expected to improve adherence to treatment. Several CDs containing a DPP4I and a sodium-glucose cotransporter-2 inhibitor (SGLT2I) have become available recently, suggesting that the role of such drugs in managing DM is likely to be reassessed.
Accordingly, we examined improvement of glycemic control by this CD containing AG + PG, as well as its usefulness for promoting medication adherence and facilitating the routine management of type 2 DM.
| Methods | ▴Top |
This was a multicenter open-label prospective study. The subjects were outpatients with type 2 DM who were under management by doctors of the Chikushi Cardiovascular Disease Clinical Research Network (Chikushi-JRN), for which Fukuoka University Chikushi Hospital and our hospital are the base hospitals, and whose HbA1c (NGSP) was not controlled to < 7.0% despite treatment with a DPP4I. The exclusion criteria were as follows: age < 20 years, severe DM, type 1 DM, recent serious illness, a history of drug hypersensitivity, females who were possibly pregnant, and patients who were considered ineligible by the treating doctor for other reasons. In type 2 DM patients who had been on DPP4I therapy for 1 month or more, PG was added at 15 mg/day (first point). When the safety of adding PG was confirmed after 1 - 3 months, the DPP4I and PG (15 mg/day) were switched to the CD containing AG 25 mg + PG 15 mg (Fig. 1). We defined the time when PG was added to the DPP4I as the first point, and the time when the DPP4I and PG were switched to the CD 1 - 3 months later as the second point. In addition, the time 3 months after switching to the CD was defined as the final point. We examined the BP, pulse rate (PR), body weight (BW), HbA1c, lipid profile, adverse reactions, and adherence at these three time points. To evaluate adherence, patients completed a questionnaire about their use of medications. PG has been reported to improve nonalcoholic steatohepatitis (NASH), which is a non-alcoholic fatty liver disease (NAFLD) associated with advanced fibrosis. A decrease in the platelet count and an increase in fibrotic markers occurred in NASH. Accordingly, we examined the effect of switching to the CD on the fibrosis-4 (FIB-4) index, which is a screening marker for predicting the progression of hepatic fibrosis [8]. Concomitant drugs other than the DPP4I and PG were continued without changing the dosage. The study was discontinued if glycemic control was inadequate after addition of PG, if severe hypoglycemia occurred, if it was difficult to continue the investigation due to an adverse event, if adherence to treatment was poor, or if the study doctor considered discontinuation to be appropriate for other reasons.
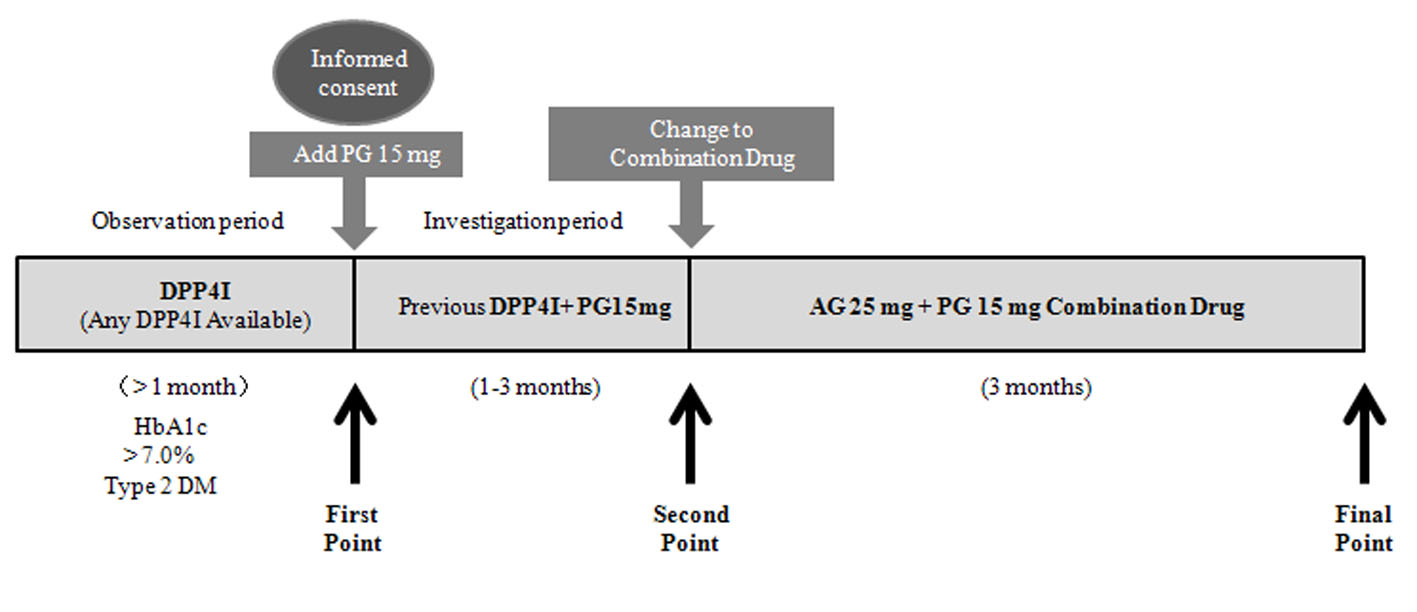 Click for large image | Figure 1. Study design (CHAT-LIO). CHAT: CHikushi Anti-Diabetes Mellitus Trial; LIO: LIOvel; DPP4I: dipeptidyl peptidase-4 inhibitor; HbA1c: hemoglobin A1c; DM: diabetes mellitus; PG: pioglitazone; AG: alogliptin. |
Target number of subjects
It was assumed that adding PG to a DPP4I would increase the rate of achieving HbA1c < 7.0% to 30%, with approximately half of the improvement being due to the placebo effect. When the power of detection was set at 90% and the level of significance at P < 0.05, the target number of subjects was calculated to be 160. The study period was from the date of approval by the Institutional Review Board in March 2013 to March 31, 2014.
Statistical analysis
Statistical analysis was performed at Fukuoka University using the IBM SPSS Statistics 23 package. To assess the significance of differences, we used the t-test for variables with a normal distribution with Levene’s test when variance was equal and Welch’s test when variance was not equal. For items where variables did not show a normal distribution, we examined changes over time by using the Wilcoxon signed-rank test. Numerical data are presented as the mean (standard deviation: SD), median (interquartile range: IQR), or frequency (proportion). In all analyses, P < 0.05 was considered significant.
Informed consent
This study was approved by the Ethical Committee of Fukuoka University Chikushi Hospital (approval number: R13-006). Written informed consent was obtained from all of the patients who participated. All of the researchers conducted the study in accordance with the Declaration of Helsinki.
| Results | ▴Top |
A total of 20 patients were registered, and 19 patients completed the study. One subject experienced an adverse event (leg pain) after starting PG treatment, after which PG was discontinued and the subject withdrew from the study. The adverse reaction observed in this subject was mild and transient, and did not require additional treatment. Averages of observation period, investigation period and CD period were 342 ± 313, 91 ± 42 and 92 ± 41 days, respectively.
Table 1 lists the characteristics of the patients at the first point. There were more men (69%) than women, and the body mass index (BMI) was slightly high at 26.3 ± 4.5 kg/m2. Many of the subjects (47%) were taking sulfonylureas at enrollment.
 Click to view | Table 1. Patient Characteristics at the First Point (n = 19) |
Table 2 shows the DPP4Is used by the subjects at the first point. Approximately half of them were taking vildagliptin.
 Click to view | Table 2. DPP4Is Used by Patients at the First Point (n = 19) |
Figure 2 displays the changes in BP and PR over time. When BP and PR were compared among the first point, second point, and final point, no significant changes were observed.
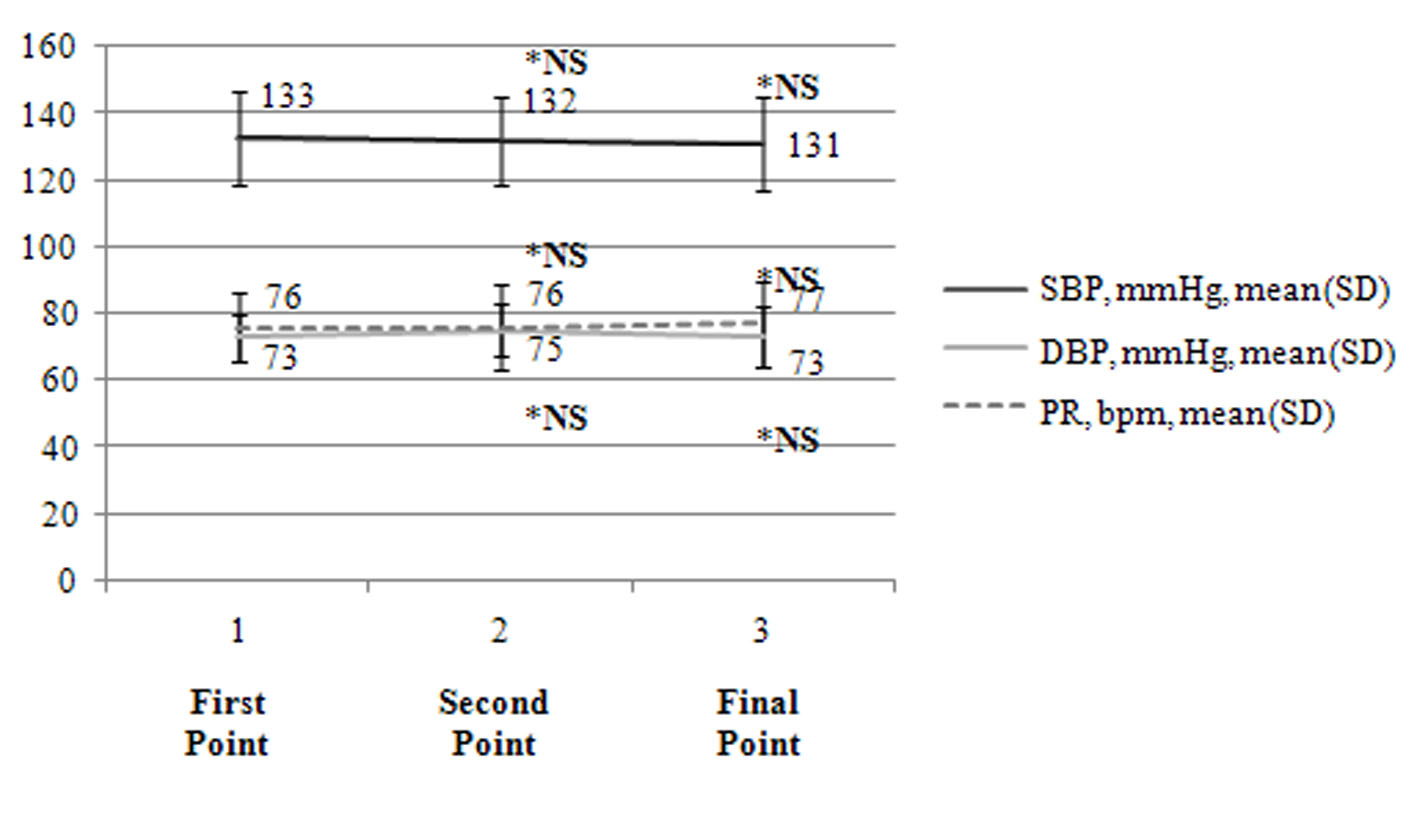 Click for large image | Figure 2. Changes in BP and PR (n = 19). The changes in BP and PR during the study are presented. BP: blood pressure; SBP: systolic BP; DBP: diastolic BP; PR: pulse rate; bpm: beats per minute; NS: not significant; *: compared to the first point. |
Table 3 details the laboratory test results obtained during the study. Compared to the first point, the white blood cell count (WBC), aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyl transpeptidase (γ-GTP) and fasting blood glucose (FBG) were significantly lower at the second and final points, while alkaline phosphatase (ALP) and triglycerides (TGs) were also decreased, but not significantly. At either the second or final point, Hb, lactate dehydrogenase (LDH), and HbA1c showed a significant decrease, while high-density lipoprotein cholesterol (HDL-C) was significantly increased. A significant change in the FIB-4 index was not observed.
 Click to view | Table 3. Changes in BW and Laboratory Data (n = 19) |
Table 4 shows adherence to medication during the study. There were no significant changes in the percentage of subjects who remembered to take their medication.
 Click to view | Table 4. Changes in Adherence to Medication (n = 19) |
| Discussion | ▴Top |
Major findings include: 1) No serious adverse events were reported; 2) There were no significant changes in BP or PR during the study; 3) Compared with before switching to the CD (first point), there was a significant decrease in WBC, AST, ALT, γ-GTP, and FBG at the second and final points, as well as a significant decrease in Hb, LDH, and HbA1c at either point and a significant increase in HDL-C; 4) A significant change in the FIB-4 index was not observed; 5) There was no significant improvement of adherence to medication; 6) No serious complications were reported and the safety of this CD was confirmed, and 7) Only 20 subjects were enrolled in this study (one subject discontinued), which was far short of the target number of 160.
Many subjects switched from vildagliptin in this study, possibly because vildagliptin is administered twice daily and patients wished to switch to a once-daily drug.
An antihypertensive effect of TZDs has been shown by various clinical studies, including the PROactive study [6, 9], and the mechanism is thought to involve rennin-angiotensin-aldosterone system (RAAS) blockade via inhibition of ATIR expression on vascular smooth muscle cells by PPARγ [10]. In this study, no antihypertensive effect was observed after adding PG to basal DPP4I therapy, possibly because the BP was not high at the start of treatment and the number of subjects was small.
There have been various reports about the effects of DPP4Is on BP. GLP1 reduces BP by increasing the production of ANP, causing vasodilation through relaxation of vascular smooth muscle via cGMP, and increasing urinary sodium excretion [11, 12]. It was reported that the BP decreased in rats administered sitagliptin [13], but meta-analysis has not found a BP-lowering effect of DPP4I therapy in humans [14]. No antihypertensive effect was observed after adding PG to basal DPP4I therapy in this study, possibly because the Na excretory effect of the DPP4I and Na resorptive effect of PG offset each other, but further examination of this issue is needed.
PG was previously reported to decrease TG and increase HDL-C [15]. Similar changes in HDL-C and TG were confirmed in this study, and the lipid profile was improved along with a decrease in HbA1c. PG has been reported to break down fats through PPARγ activation, thus improving insulin resistance [16]. It has also been reported that the TG-lowering mechanism of TZDs involves changes in lipoprotein lipase (LPL) expression by fat cells [17].
Furthermore, it was reported that the combination of AG with PG increases gastric inhibitory polypeptide (GIP) receptor expression and activity in pancreatic B-cells, as well as improving blood glucose and lipid parameters [18, 19]. In a prospective three-arm study of AG monotherapy (25 mg), PG monotherapy (30 mg), and AG + PG combination therapy, HbA1c showed a durable decrease in the combination therapy group and the combination of a DPP4I and PG was concluded to be useful [20]. Thus, glucose metabolism is improved by adding PG to basal DPP4I therapy, as was observed in our study.
In a study conducted in Japan, HbA1c decreased by approximately 1% after treatment with AG (25 mg) was added in patients showing poor glycemic control on PG, and the decrease in HbA1c was greater in the patients with higher baseline HbA1c levels [21]. In the present study, we compared the changes in HbA1c after dividing the subjects into a high HbA1c group (HbA1c ≥ 8.4, n = 7) and a low HbA1c group (HbA1c < 8.4, n = 12) or into a high BW group (BW ≥ 68.4 kg, n = 9) and a low BW group (BW < 68.4 kg, n = 10), but no significant differences were observed (data not shown). Based on data from this study, the severity of DM or presence of obesity does not seem to influence the decrease in HbA1c after adding PG to AG.
In patients with NAFLD and elevation of γ-GTP or ALT, the incidence of cardiovascular events is reported to be high [22, 23]. A decrease in γ-GTP or ALT was observed in the present study after addition of PG, supporting the decrease in cardiovascular events reported in the PROactive Study [6]. It is considered that obesity underlies NASH, the severe form of NAFLD, which is associated with a high incidence of metabolic syndrome. A meta-analysis of four randomized comparative studies showed that treatment with PG could improve hepatocyte ballooning degeneration, parenchymal inflammation, fatty degeneration, and fibrosis [24]. In our study, no change in the FIB-4 index, a marker of NASH, was observed after addition of PG. It has occasionally been reported that PG therapy is effective for inhibiting hepatic fibrosis. However, a multicenter randomized study conducted in the USA to investigate the histological improvement of NASH with PG treatment found no significant improvement in the PG group compared to the placebo group [25], and although short-term improvement of insulin resistance was observed, the effect did not persist [26].
It is generally expected that switching to a CD will reduce the cost of medications and improve adherence to treatment. It has been reported that adherence was improved in patients with DM by using a CD [27], and good adherence medication improves glycemic control [28]. However, no improvement of adherence to medication was observed after switching to the CD in our study. As an exploratory analysis, we divided the subjects into a good adherence group and a poor adherence group at the first point for comparison of the change in HbA1c, but we found no significant difference between these two groups (data not shown). This outcome may have been related to concomitant lifestyle-related diseases in the majority of subjects, resulting in polypharmacy.
The one of the most impressive points of this study was the very small number of subjects enrolled, since only 20 patients were recruited versus a target of 160. Compared to the CHAT Study using a similar medication for DM that was also conducted at our hospital [29, 30], the number of subjects was extremely small. The reason may have been caution regarding the adverse reactions caused by PG, which are reported to include an increase in BW, edema, congestive heart failure (CHF) [31], and an increased risk of fracture [32].
Edema is an especially problematic adverse reaction to PG, which has a relatively high frequency of 8% [31], and occurs because TZDs promote sodium reabsorption by the kidneys. The decrease in Hb and WBC after PG was added in this study is considered to be due to hemodilution resulting from an increase in intravascular fluid.
The specialties of the study doctors may influence selection of medications for DM, e.g. non-cardiologists may hesitate to prescribe PG because of the risk of CHF. Therefore, we investigated the specialties of the doctors participating in three studies on DPP4Is previously conducted at our hospital (CHAT-J, n = 1,153, approval number: R09-21; CHAT-N, n = 203, approval number: R11-013; and CHAT-T, n = 162, approval number: R13-017) and compared them with the doctors involved in this study. Contrary to our expectation, the percentages of doctors specializing in endocrinology/diabetology and cardiology were the same across the three DPP4I studies (Fig. 3a) and the present study employed PG (Fig. 3b). It was also surprising that a large number of registered doctors specialized in gastroenterology. They might have expected a beneficial effect of PG on NAFLD [33]. It is interesting that many doctors not specializing in internal medicine were involved in the previous three DPP4I studies. The probable reason for this finding is that doctors feel safer using DPP4Is because these drugs increase intrinsic incretin activity to regulate insulin and glucagon secretion in a blood glucose-dependent manner, so hypoglycemia is less likely to develop.
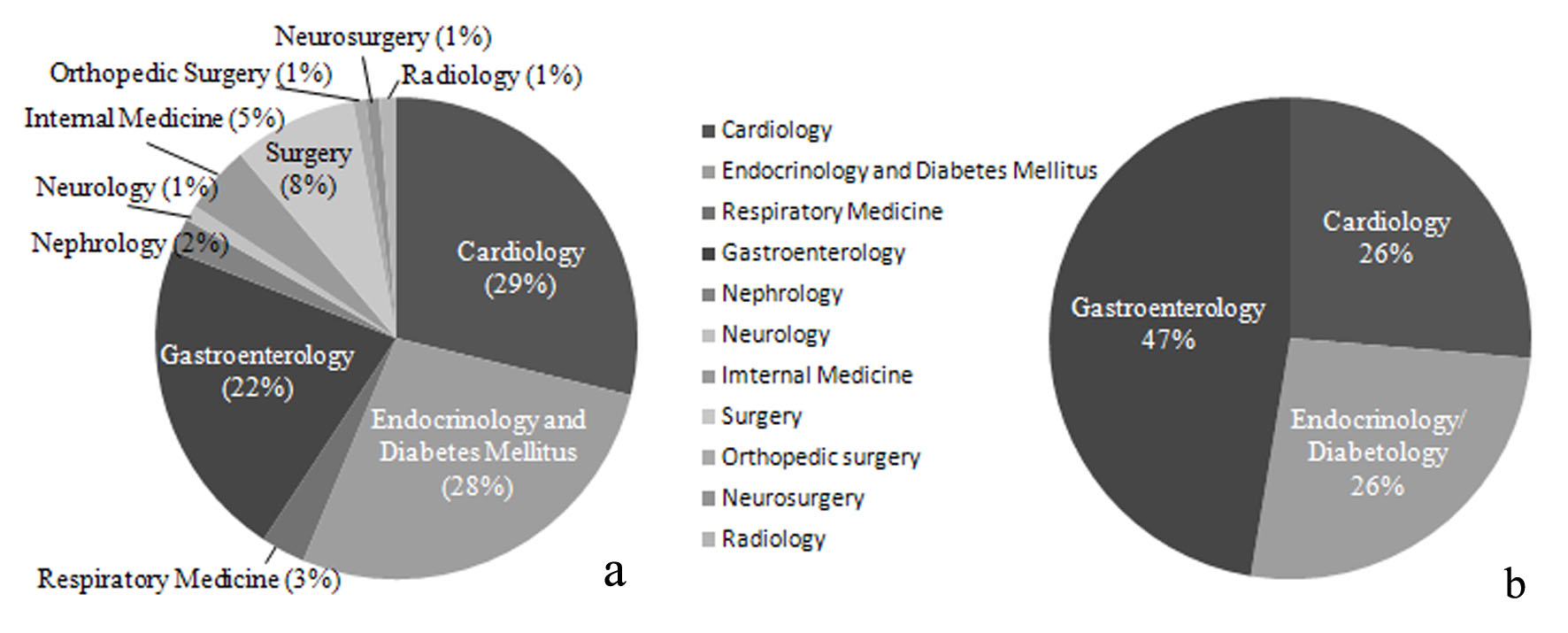 Click for large image | Figure 3. Specialties of doctors registered in previous studies of DPP4Is (CHAT-J, CHAT-N, and CHAT-T) and this study (CHAT-LIO). (a) Specialties of doctors registered in previous studies of DPP4Is (CHAT-J, CHAT-N, and CHAT-T) (n = 1,518). (b) Specialties of doctors registered in this study (CHAT-LIO) (n = 19). |
Accordingly, there may be different preferences for antidiabetic drugs among the specialties. To investigate this point, we calculated the percentages of different specialties prescribing DPP4I, SGLT2Is, TZDs, or biguanides (BGs) as the main antidiabetic drug in June 2017 (Fig. 4) (approval number: R18-030).
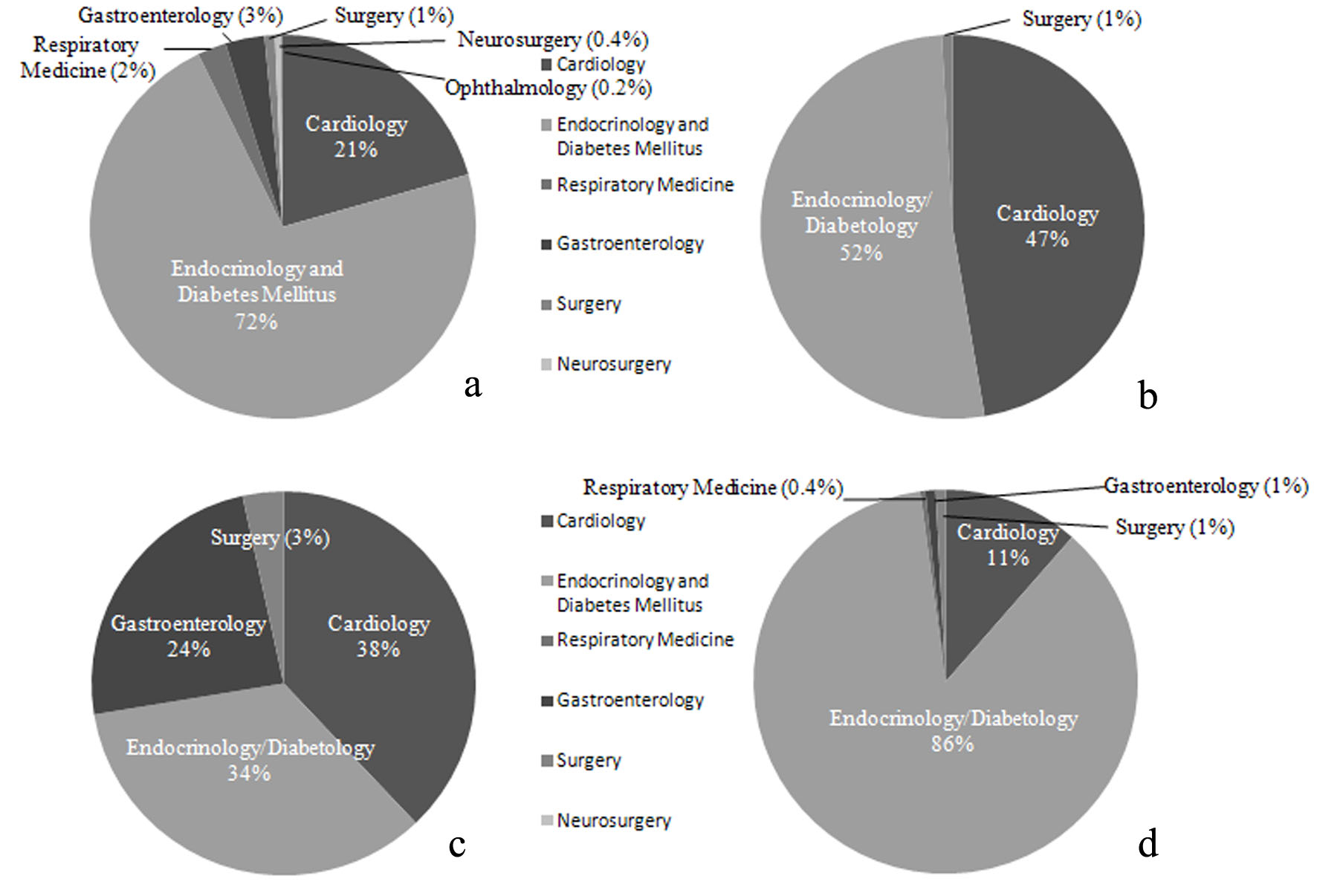 Click for large image | Figure 4. Proportions of specialties of doctors prescribing DM drugs during 1 month at our hospital. (a) Percentage of doctors in each specialty prescribing DPP4Is (n = 456). (b) Percentage of doctors in each specialty prescribing SGLT2Is (n = 112). (c) Percentage of doctors in each specialty prescribing PG (n = 29). (d) Percentage of doctors in each specialty prescribing BGs (n = 236). |
While DPP4Is were largely prescribed by doctors specializing in endocrinology/diabetology, these drugs were unexpectedly also prescribed by many doctors who did not specialize in internal medicine (Fig. 4a). It was also surprising that SGLT2Is were mainly prescribed by doctors specializing in cardiology and endocrinology/diabetology (Fig. 4b). The high proportion of cardiologists may have been due to reports that SGLT2I therapy reduced cardiovascular events in the EMPA-REG OUTCOME Study and the CANVAS Study [34, 35]. As initially expected, only a small percentage of doctors specializing in endocrinology/diabetology prescribed TZDs compared to other drugs, while the rate of TZD use was relatively high among cardiologists (Fig. 4c). The higher rate of TZD prescription by cardiologists may have occurred because of evidence that TZD inhibits macroangiopathy [6] and because these doctors are not concerned about the development of heart failure. BGs were chiefly prescribed by doctors specializing in endocrinology/diabetology and were less frequently prescribed by cardiologists, probably because tests using contrast medium such as coronary angiography are frequently conducted in cardiology departments (Fig. 4d).
Prescription of PG may also have been reduced by the influence of reports about the risk of bladder cancer. Although an increased risk of bladder cancer due to PG was identified by the results of interim analysis at 5 years in the KPNC epidemiological study, it was not confirmed by the final analysis at 10 years [36]. The pan-European cohort study also found no increased risk of bladder cancer associated with PG therapy [37]. However, the current consensus is that the possibility of PG increasing the risk of bladder cancer has still not been excluded.
This study had some limitations. Because only a small number of subjects were enrolled in this study, effective analysis could not be conducted. The study period is too short for monitoring adverse events and efficacy. This was a single-arm prospective open label study, so future studies with a control group are needed.
Conclusions
This study confirmed the efficacy of PG for the treatment of type 2 DM in terms of improving glycemic control and systemic metabolism by practitioners. We also found that prescribing trends differed between specialties, possibly because of differences in the emphasis placed on the results of various studies and reports.
Acknowledgments
We thank Mrs. Nao Totake for her excellent technical assistance. We also thank the members of Chikushi-JRN (K Kobayashi, H Takaki, T Miake, R Mitsutake, and T Matsuzaki) for recruiting patients to participate in the study.
Financial Support
The authors received financial support from Takeda Pharmaceutical Company Limited.
| References | ▴Top |
- Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, White A, et al. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation. 2008;117(25):e510-526.
doi pubmed - Bramley TJ, Gerbino PP, Nightengale BS, Frech-Tamas F. Relationship of blood pressure control to adherence with antihypertensive monotherapy in 13 managed care organizations. J Manag Care Pharm. 2006;12(3):239-245.
doi - Corrao G, Parodi A, Nicotra F, Zambon A, Merlino L, Cesana G, Mancia G. Better compliance to antihypertensive medications reduces cardiovascular risk. J Hypertens. 2011;29(3):610-618.
doi pubmed - Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23(8):1296-1310.
doi - Thom S, Poulter N, Field J, Patel A, Prabhakaran D, Stanton A, Grobbee DE, et al. Effects of a fixed-dose combination strategy on adherence and risk factors in patients with or at high risk of CVD: the UMPIRE randomized clinical trial. JAMA. 2013;310(9):918-929.
doi pubmed - Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, Skene AM, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366(9493):1279-1289.
doi - Chawla A, Boisvert WA, Lee CH, Laffitte BA, Barak Y, Joseph SB, Liao D, et al. A PPAR gamma-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol Cell. 2001;7(1):161-171.
doi - Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ, Nash Clinical Research N. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7(10):1104-1112.
doi pubmed - Sarafidis PA, Lasaridis AN. Actions of peroxisome proliferator-activated receptors-gamma agonists explaining a possible blood pressure-lowering effect. Am J Hypertens. 2006;19(6):646-653.
doi pubmed - Benkirane K, Viel EC, Amiri F, Schiffrin EL. Peroxisome proliferator-activated receptor gamma regulates angiotensin II-stimulated phosphatidylinositol 3-kinase and mitogen-activated protein kinase in blood vessels in vivo. Hypertension. 2006;47(1):102-108.
doi pubmed - Liu Q, Adams L, Broyde A, Fernandez R, Baron AD, Parkes DG. The exenatide analogue AC3174 attenuates hypertension, insulin resistance, and renal dysfunction in Dahl salt-sensitive rats. Cardiovasc Diabetol. 2010;9:32.
doi pubmed - Kim M, Platt MJ, Shibasaki T, Quaggin SE, Backx PH, Seino S, Simpson JA, et al. GLP-1 receptor activation and Epac2 link atrial natriuretic peptide secretion to control of blood pressure. Nat Med. 2013;19(5):567-575.
doi pubmed - Liu L, Liu J, Wong WT, Tian XY, Lau CW, Wang YX, Xu G, et al. Dipeptidyl peptidase 4 inhibitor sitagliptin protects endothelial function in hypertension through a glucagon-like peptide 1-dependent mechanism. Hypertension. 2012;60(3):833-841.
doi pubmed - Monami M, Ahren B, Dicembrini I, Mannucci E. Dipeptidyl peptidase-4 inhibitors and cardiovascular risk: a meta-analysis of randomized clinical trials. Diabetes Obes Metab. 2013;15(2):112-120.
doi pubmed - Aronoff S, Rosenblatt S, Braithwaite S, Egan JW, Mathisen AL, Schneider RL. Pioglitazone hydrochloride monotherapy improves glycemic control in the treatment of patients with type 2 diabetes: a 6-month randomized placebo-controlled dose-response study. The Pioglitazone 001 Study Group. Diabetes Care. 2000;23(11):1605-1611.
doi pubmed - McLaughlin TM, Liu T, Yee G, Abbasi F, Lamendola C, Reaven GM, Tsao P, et al. Pioglitazone increases the proportion of small cells in human abdominal subcutaneous adipose tissue. Obesity (Silver Spring). 2010;18(5):926-931.
doi pubmed - Schoonjans K, Peinado-Onsurbe J, Lefebvre AM, Heyman RA, Briggs M, Deeb S, Staels B, et al. PPARalpha and PPARgamma activators direct a distinct tissue-specific transcriptional response via a PPRE in the lipoprotein lipase gene. EMBO J. 1996;15(19):5336-5348.
doi pubmed - Kyle KA, Willett TL, Baggio LL, Drucker DJ, Grynpas MD. Differential effects of PPAR-{gamma} activation versus chemical or genetic reduction of DPP-4 activity on bone quality in mice. Endocrinology. 2011;152(2):457-467.
doi pubmed - Gupta D, Peshavaria M, Monga N, Jetton TL, Leahy JL. Physiologic and pharmacologic modulation of glucose-dependent insulinotropic polypeptide (GIP) receptor expression in beta-cells by peroxisome proliferator-activated receptor (PPAR)-gamma signaling: possible mechanism for the GIP resistance in type 2 diabetes. Diabetes. 2010;59(6):1445-1450.
doi pubmed - Rosenstock J, Inzucchi SE, Seufert J, Fleck PR, Wilson CA, Mekki Q. Initial combination therapy with alogliptin and pioglitazone in drug-naive patients with type 2 diabetes. Diabetes Care. 2010;33(11):2406-2408.
doi pubmed - Kaku K, Itayasu T, Hiroi S, Hirayama M, Seino Y. Efficacy and safety of alogliptin added to pioglitazone in Japanese patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial with an open-label long-term extension study. Diabetes Obes Metab. 2011;13(11):1028-1035.
doi pubmed - Ruttmann E, Brant LJ, Concin H, Diem G, Rapp K, Ulmer H, Vorarlberg Health M, et al. Gamma-glutamyltransferase as a risk factor for cardiovascular disease mortality: an epidemiological investigation in a cohort of 163,944 Austrian adults. Circulation. 2005;112(14):2130-2137.
doi pubmed - Schindhelm RK, Dekker JM, Nijpels G, Bouter LM, Stehouwer CD, Heine RJ, Diamant M. Alanine aminotransferase predicts coronary heart disease events: a 10-year follow-up of the Hoorn Study. Atherosclerosis. 2007;191(2):391-396.
doi pubmed - Boettcher E, Csako G, Pucino F, Wesley R, Loomba R. Meta-analysis: pioglitazone improves liver histology and fibrosis in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2012;35(1):66-75.
doi pubmed - Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362(18):1675-1685.
doi pubmed - Bell LN, Wang J, Muralidharan S, Chalasani S, Fullenkamp AM, Wilson LA, Sanyal AJ, et al. Relationship between adipose tissue insulin resistance and liver histology in nonalcoholic steatohepatitis: a pioglitazone versus vitamin E versus placebo for the treatment of nondiabetic patients with nonalcoholic steatohepatitis trial follow-up study. Hepatology. 2012;56(4):1311-1318.
doi pubmed - Vanderpoel DR, Hussein MA, Watson-Heidari T, Perry A. Adherence to a fixed-dose combination of rosiglitazone maleate/metformin hydrochloride in subjects with type 2 diabetes mellitus: a retrospective database analysis. Clin Ther. 2004;26(12):2066-2075.
doi pubmed - Krapek K, King K, Warren SS, George KG, Caputo DA, Mihelich K, Holst EM, et al. Medication adherence and associated hemoglobin A1c in type 2 diabetes. Ann Pharmacother. 2004;38(9):1357-1362.
doi pubmed - Nomiyama T, Akehi Y, Takenoshita H, Nagaishi R, Terawaki Y, Nagasako H, Kudo T, et al. Contributing factors related to efficacy of the dipeptidyl peptidase-4 inhibitor sitagliptin in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract. 2012;95(2):e27-28.
doi pubmed - Okamura K, Shirai K, Totake N, Okuda T, Urata H. Prospective direct comparison of antihypertensive effect and safety between high-dose amlodipine or indapamide in hypertensive patients uncontrolled by standard doses of angiotensin receptor blockers and amlodipine. Clin Exp Hypertens. 2018;40:2:99-106.
- Kawamori R, Kadowaki T, Onji M, Seino Y, Akanuma Y, PRACTICAL Study Group. Hepatic safety profile and glycemic control of pioglitazone in more than 20,000 patients with type 2 diabetes mellitus: postmarketing surveillance study in Japan. Diabetes Res Clin Pract. 2007;76(2):229-235.
doi pubmed - Habib ZA, Havstad SL, Wells K, Divine G, Pladevall M, Williams LK. Thiazolidinedione use and the longitudinal risk of fractures in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2010;95(2):592-600.
doi pubmed - Gastaldelli A, Harrison SA, Belfort-Aguilar R, Hardies LJ, Balas B, Schenker S, Cusi K. Importance of changes in adipose tissue insulin resistance to histological response during thiazolidinedione treatment of patients with nonalcoholic steatohepatitis. Hepatology. 2009;50(4):1087-1093.
doi pubmed - Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128.
doi pubmed - Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644-657.
doi pubmed - Lewis JD, Habel LA, Quesenberry CP, Strom BL, Peng T, Hedderson MM, Ehrlich SF, et al. Pioglitazone Use and Risk of Bladder Cancer and Other Common Cancers in Persons With Diabetes. JAMA. 2015;314(3):265-277.
doi pubmed - Korhonen P, Heintjes EM, Williams R, Hoti F, Christopher S, Majak M, Kool-Houweling L, et al. Pioglitazone use and risk of bladder cancer in patients with type 2 diabetes: retrospective cohort study using datasets from four European countries. BMJ. 2016;354:i3903.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


