| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Letter to the Editor
Volume 10, Number 8, August 2018, pages 668-669
Bofutsushosan Significantly Ameliorated Betamethasone-Induced Cushing’s Syndrome
Hidekatsu Yanai
Department of Internal Medicine, National Center for Global Health and Medicine Kohnodai Hospital. 1-7-1 Kohnodai, Ichikawa, Chiba 272-8516, Japan
Manuscript submitted May 18, 2018, accepted June 1, 2018
Short title: Bofutsushosan Ameliorated Betamethasone-Induced CS
doi: https://doi.org/10.14740/jocmr3483w
| ▴Top |
Bofutsushosan (Fangfengtongshengsan in Chinese, BTS) is a formula in traditional Japanese Kampo medicine that is used for treating obesity and metabolic syndrome. Recent study reported that BTS significantly suppressed an increase in body weight and amounts of visceral fat and subcutaneous fat and serum concentrations of triglyceride, glucose, and hemoglobin A1c (HbA1c) in the mice fed with a high-fat diet as compared with the control group in 1 month [1]. However, the effects of BTS on metabolic parameters in humans have not been fully understood. Here, I show a significant effect of BTS on body weight, subcutaneous and visceral fat and HbA1c in a patient with betamethasone-induced Cushing’s syndrome.
A 53-year old man was referred to my department due to moon face and central obesity. He had been treated by betamethasone (0.5 mg/day) due to allergic dermatitis. His serum cortisol and adrenocorticotropic hormone could not be detected, which suggested the diagnosis of betamethasone-induced Cushing’s syndrome. I recommended him to reduce daily dose of betamethasone and to gradually stop betamethasone use. He reduced daily dose of betamethasone to 0.25 mg/day, however, could not stop the betamethasone use due to severity of his dermatitis. Reduction of daily dose of betamethasone did not ameliorate his symptoms of Cushing’s syndrome. Therefore, I started to use BTS while using betamethasone (0.25 mg/day). BTS constantly reduced his body weight and also reduced HbA1c levels (Fig. 1). Furthermore, BTS remarkably reduced both subcutaneous and visceral fat area which was measured by abdominal computed tomography (Fig. 2).
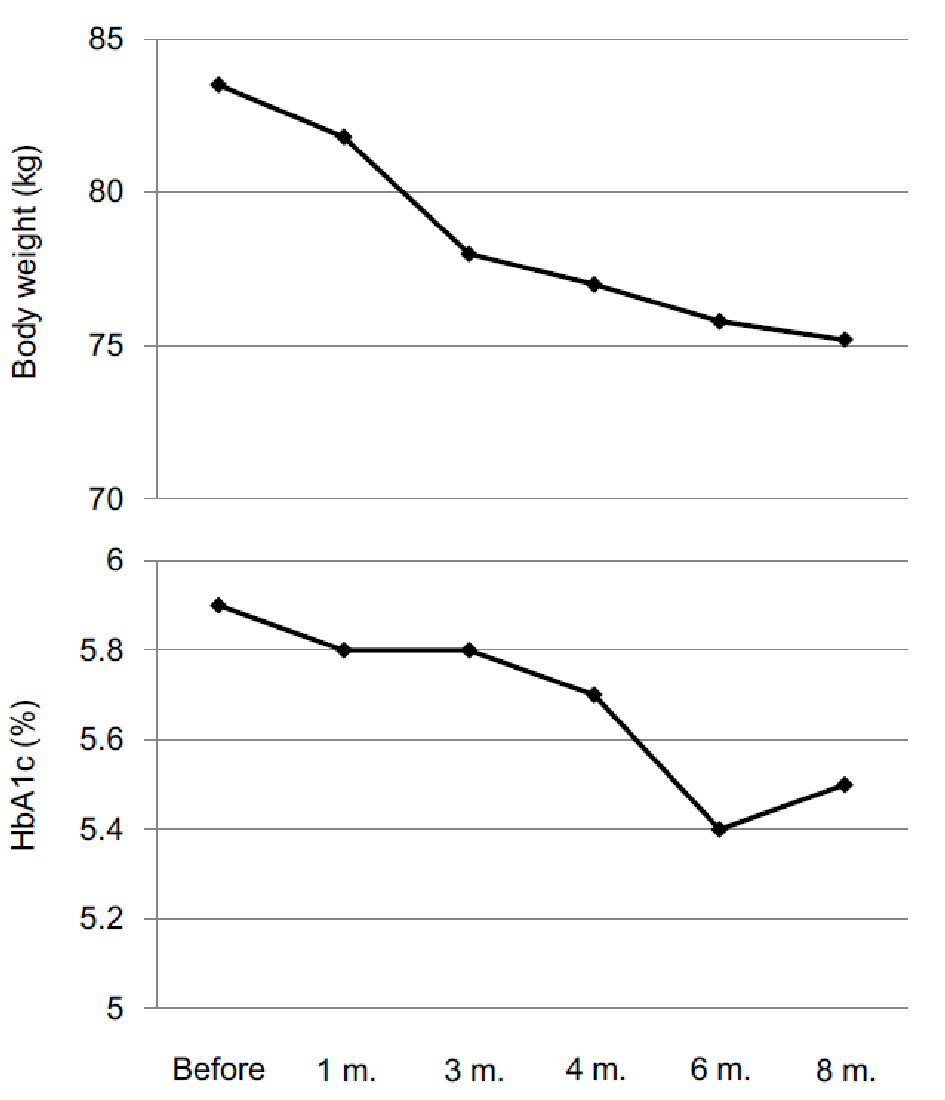 Click for large image | Figure 1. Changes in body weight and HbA1c after the start of Bofutsushosan use. |
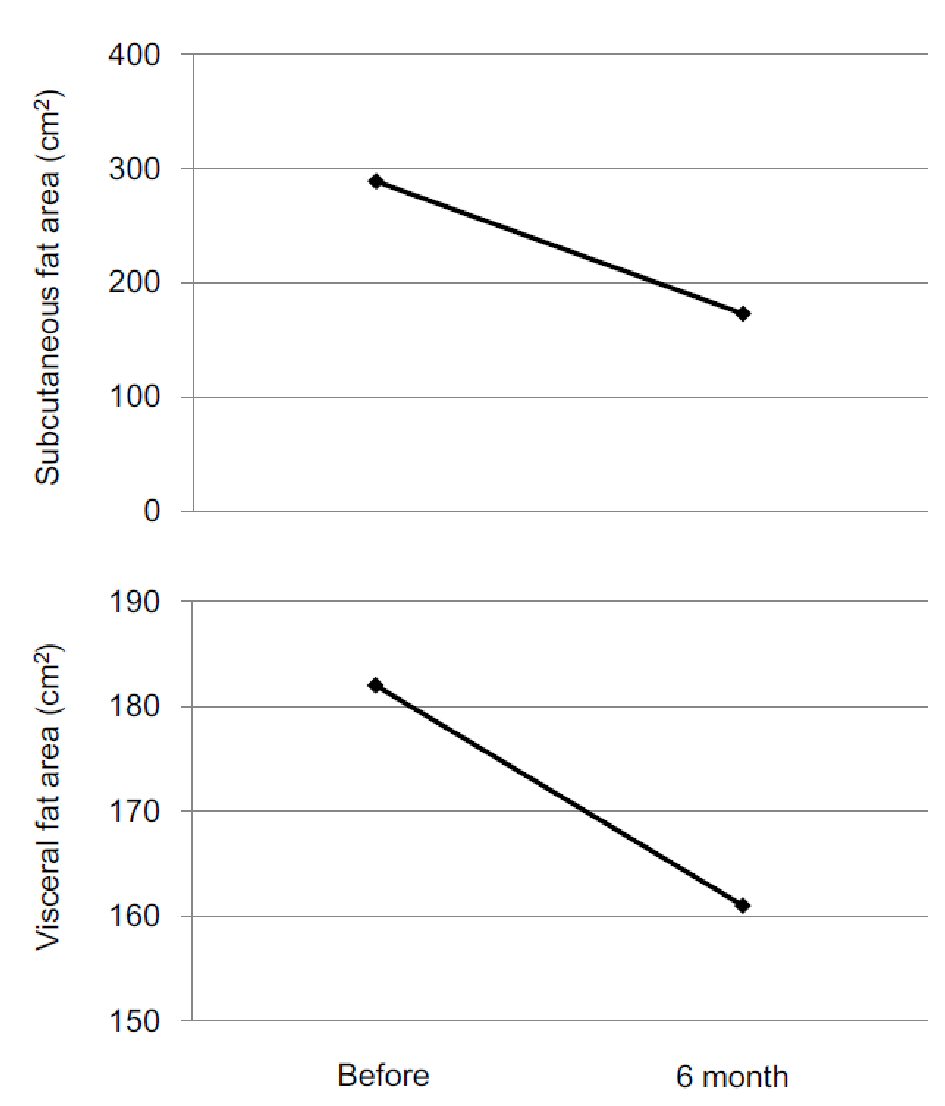 Click for large image | Figure 2. Changes in subcutaneous fat area and visceral fat area after the start of Bofutsushosan use. |
Several mechanisms for anti-obesity effect of BTS were reported. Fructose is absorbed via glucose transporter 5 (GLUT5) in the intestine. Inhibitory effect of BTS on GLUT5 function in vitro was observed [2]. BTS increased mRNA expression levels of leptin, adiponectin, and UCP1 in brown adipose tissues (BAT), and improved insulin resistance, and subsequently reduced serum levels of leptin and triglyceride in parallel with decreased visceral white adipose tissue (WAT) volume and adipocyte size in high-fat diet-fed obese mice [3]. BTS increased gene expression of PGC-1α and UCP1 for energy consumption in BAT and inhibiting inflammation in WAT in obese mice induced by high-fat diet [4]. Reduction of subcutaneous and visceral fat and HbA1c was observed in my patient, which may suggest an existence of the same molecular mechanisms in humans.
In a randomized controlled trial (RCT) in 107 hypertensive patients with obesity, participants were randomly assigned to receive either the conventional control therapy or BTS add-on therapy. Daytime systolic blood pressure (BP) variability was decreased in the BTS group (control vs. BTS; the change from baseline in daytime systolic BP variability, 1.0±3.3% vs. -1.0±3.3%; P = 0.006) [5]. In another RCT, 81 Japanese obese women with impaired glucose tolerance (IGT), who had been treated with a low-calorie diet (1,200 kcal/day) and an exercise regimen (300 kcal/day), were randomized to receive either placebo (n = 40) or BTS treatment (n = 41). After 24 weeks treatment, the BTS group lost significantly more body weight and abdominal visceral fat, whereas the placebo group lost body weight and had no significant change in abdominal visceral fat. The BTS group had a lower fasting serum insulin level and a lower level of the homeostasis model assessment of insulin resistance compared with values before treatment, indicating that BTS significantly improved obesity and insulin resistance in obese women with IGT [6]. Yamamoto N, et al reported that BTS effectively attenuated the weight gain observed after receiving anti-psychotic drug, olanzapine [7].
In conclusion, the evidence showing an effectiveness of BTS on metabolic parameters in humans is very limited. To my knowledge, this is the first to report that BTS ameliorated body weight/fat and glucose metabolism in a patient with corticosteroid-induced Cushing’s syndrome while using corticosteroid. This result will be a good news for patients who cannot reduce daily steroid dose and suffer from Cushing’s syndrome.
Conflict of Interest
The author declares that he has no conflict of interest concerning this article.
| References | ▴Top |
- Akaki J, Tachi S, Nakamura N, Arai T, Yamasaki H, Inoue M, Makino T. Promotive effect of Bofutsushosan (Fangfengtongshengsan) on lipid and cholesterol excretion in feces in mice treated with a high-fat diet. J Ethnopharmacol. 2018;220:1-8.
doi pubmed - Gao S, Satsu H, Makino T. Inhibitory effect of bofutsushosan (fang feng tong sheng san) on glucose transporter 5 function in vitro. J Nat Med. 2018;72(2):530-536.
doi pubmed - Kobayashi S, Kawasaki Y, Takahashi T, Maeno H, Nomura M. Mechanisms for the anti-obesity actions of bofutsushosan in high-fat diet-fed obese mice. Chin Med. 2017;12:8.
doi pubmed - Chen YY, Yan Y, Zhao Z, Shi MJ, Zhang YB. Bofutsushosan ameliorates obesity in mice through modulating PGC-1alpha expression in brown adipose tissues and inhibiting inflammation in white adipose tissues. Chin J Nat Med. 2016;14(6):449-456.
pubmed - Azushima K, Tamura K, Haku S, Wakui H, Kanaoka T, Ohsawa M, Uneda K, et al. Effects of the oriental herbal medicine Bofu-tsusho-san in obesity hypertension: a multicenter, randomized, parallel-group controlled trial (ATH-D-14-01021.R2). Atherosclerosis. 2015;240(1):297-304.
doi pubmed - Hioki C, Yoshimoto K, Yoshida T. Efficacy of bofu-tsusho-san, an oriental herbal medicine, in obese Japanese women with impaired glucose tolerance. Clin Exp Pharmacol Physiol. 2004;31(9):614-619.
doi pubmed - Yamamoto N, Inada T. Bofu-tsusho-san effectively attenuates the weight gain observed after receiving olanzapine. Psychiatry Clin Neurosci. 2008;62(6):747.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


