| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Original Article
Volume 10, Number 5, May 2018, pages 405-410
Effects of a Low Advanced Glycation End Products Diet on Insulin Levels: The Feasibility of a Crossover Comparison Test
Shin Sukinoa, Shinsuke Nirengia, Yaeko Kawaguchia, Kazuhiko Kotanib, Kokoro Tsuzakia, Hiroshi Okadaa, Akiko Suganumaa, Naoki Sakanea, c
aDivision of Preventive Medicine, Clinical Research Institute, National Hospital Organization Kyoto Medical Center, Kyoto, Japan
bDivision of Community and Family Medicine, Jichi Medical University, Tochigi, Japan
cCorresponding Author: Naoki Sakane, Division of Preventive Medicine, Clinical Research Institute, National Hospital Organization Kyoto Medical Center, 1-1 Fukakusamukaihata-cho, Fushimi-ku, Kyoto 612-8555, Japan
Manuscript submitted December 17, 2017, accepted January 3, 2018
Short title: Low AGEs Diet on Insulin Levels
doi: https://doi.org/10.14740/jocmr3301w
| Abstract | ▴Top |
Background: Advanced glycation end products (AGEs) are associated with diabetes mellitus. Digested food-derived AGEs have been implicated in the pathogenesis of AGE-related disorders, and restricting diet-derived AGEs improves insulin resistance in animal models. The AGE content in foods changes according to cooking method, and it is higher in baked or oven-fried foods than in those prepared by steaming or simmering. Here, we examined the feasibility of crossover comparison tests for determining how different cooking methods (normal diet vs. low-AGE diet) affect insulin levels in non-diabetic Japanese subjects.
Methods: Five adult men and women (age, 41 ± 7 years; body mass index (BMI), 21.7 ± 2.6 kg/m2) were enrolled. The following dietary regimen was used: days 1 - 3, control meal; day 4, test meal (normal diet vs. low-AGE diet); day 5, washout day; and day 6, test meal. On days 4 and 6, blood samples were collected before and at 2, 4, and 6 h after meals.
Results: Blood levels of N-(carboxymethyl) lysine (CML) increased with dietary intake, but the increase was similar for the normal diet and low-AGE diet groups. Mean plasma glucose, insulin, triglycerides (TG), and CML did not differ significantly between the two groups. The area under the curve (AUC) for insulin levels was lower in the low-AGE diet group (d = 0.8). The sample size calculated from the effect size of the insulin AUC change was 22.
Conclusions: Twenty-two subjects may be needed to investigate the changes in clinical parameters attributable to cooking method in non-diabetic Japanese subjects.
Keywords: Nutrition; Advanced glycation end products; Insulin resistance; Blood glucose level
| Introduction | ▴Top |
Insulin resistance, alone or as part of the metabolic syndrome, has become an increasingly common problem in modern society [1, 2]. Epidemiological studies have shown that increased incidences of insulin resistance and type 2 diabetes are observed in individuals who adopt the modern diet compared with those with the same genetic background who do not [3-5].
Advanced glycation end products (AGEs), which are formed in the Maillard reaction, cause various types of disorders, including diabetes mellitus (DM) [6-8]. The formation and accumulation of AGEs increases with age [1] and progresses at an extremely accelerated rate, particularly in patients who are hyperglycemic [9]. Moreover, the consumption of foods that contain high levels of AGEs [6] may also play an important role in the pathogenesis of AGE-related disorders and DM. Because the amount of AGEs in foods that are baked or fried in the oven is higher than the amount in foods that are steamed or simmered, the modern diet may be AGE-rich [10]. Animal studies have indicated that consuming high levels of dietary AGEs contributes to increased insulin levels, insulin resistance, and defects in first phase insulin secretion, and low-AGE diets were shown to protect against decreasing insulin sensitivity in a mouse model of type 2 diabetes as well as wild-type mice [5, 11, 12]. The mechanism for these effects may involve the secretion of inflammatory cytokines [13] and induction of oxidative stress through the interaction between AGEs and their receptor RAGE [14]. The results of our previous study on non-diabetic subjects indicated that anti-oxidant-rich foods [15, 16] or a short-term low-calorie diet [17] suppresses the accumulation of AGEs. Therefore, a low-AGE diet possibly improves insulin resistance not only in patients with type 2 and type 1 diabetes [13, 18] but also in non-diabetic individuals. However, the effect of a low-AGE diet on insulin levels in non-diabetic individuals is unclear, as the macronutrients were not matched in a controlled diet study [10]. Specifically, the effects of a low-AGE diet on insulin levels in Asian individuals are still not clear, and the sample size necessary for clinical studies has not been calculated.
In this pilot study, we compared the effects of different cooking methods on insulin levels in non-diabetic Japanese subjects. The primary aim was to calculate the sample size based on the effects of a low-AGE diet on insulin levels in non-diabetic Japanese subjects.
| Materials and Methods | ▴Top |
Participants
Five non-diabetic subjects (two men and three women with a mean age of 41 ± 7 years and body mass index (BMI) of 21.7 ± 2.6 kg/m2) participated in this study. The exclusion criteria included those who had a history of serious problems involving the liver, kidney, heart, lungs, digestive organs (including gastrectomy), and metabolic systems; those with a history of abnormalities, other serious diseases, or morbidity; those who were hospitalized, smokers, alcohol drinkers (60 g/day alcohol or more), shift workers, or late night workers. Written informed consent was obtained from each patient. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki, as reflected in prior approval by the Ethics Committee of Kyoto Medical Center (approval number: 16-092). This trial was registered with UMIN as 000026413.
We conducted a randomized crossover study over 6 days (Fig. 1). The subjects were given a standardized reference diet on days 1 - 3 and day 5. On days 4 and 6, in the morning (9:00 am), the effects of normal and low-AGEs meals were studied. Day 5 was a washout day [19].
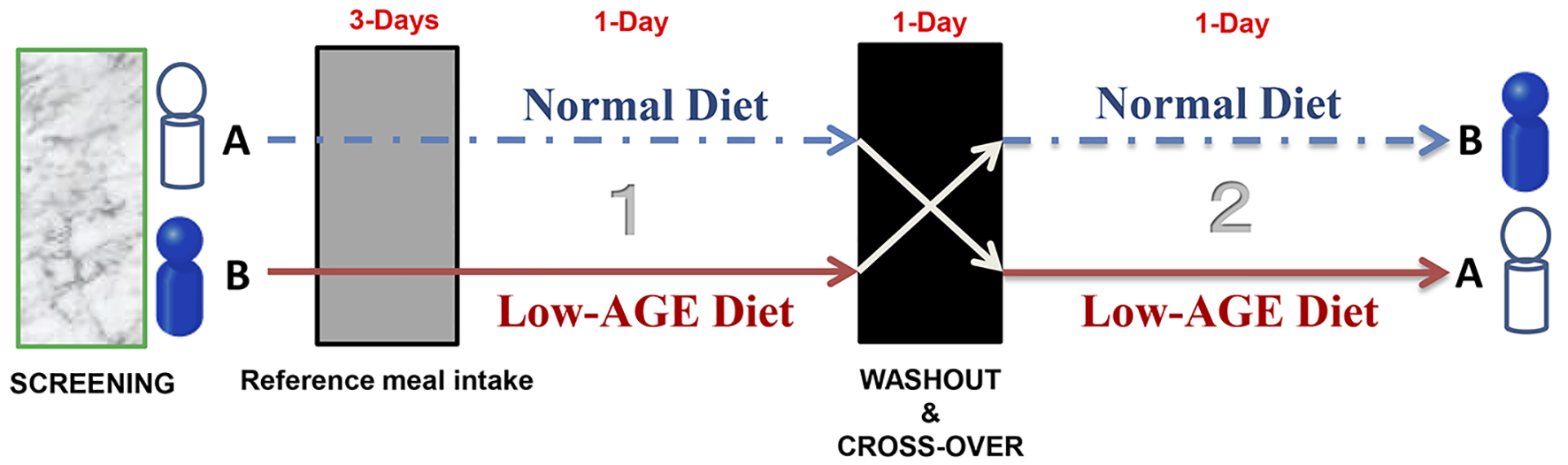 Click for large image | Figure 1. Schematic illustration of the protocol. |
Normal and low-AGE diets
The normal and low-AGE diets were formulated and cooked by a registered dietitian. The normal and low-AGE diets were isocaloric, contained identical ingredients, and differed only by the temperature and time of cooking. Each meal contained bread, vegetables, egg, potatoes, and banana. The components of the normal meals were baked or fried, whereas the components of the low-AGE meals were served raw or boiled or steamed (Table 1).
 Click to view | Table 1. Components of the Test Diets and Cooking Methods |
Measurements
Body composition was measured using a body composition analyzer (InBody 720; Biospace Inc., Seoul, Korea). BMI was calculated based on the measured height and weight.
Blood samples were corrected on days 4 and 6. Serum total protein (TP) and albumin (Alb) were measured by the Biuret method (Serotec Co., Ltd, Hokkaido, Japan) and the bromocresol purple (BCP) method (Serotec Co., Ltd, Hokkaido, Japan), respectively. Glutamate oxaloacetate transaminase (GOT), glutamic pyruvic transaminase (GPT), γ-guanosine triphosphate (γ-GT), total cholesterol (TC), high-density lipoprotein (HDL)-C, low-density lipoprotein (LDL)-C, triglycerides (TG), and HbA1c (Kyowa Medex Co., Ltd, Tokyo, Japan) were determined by enzymatic methods using the automatic biochemical analyzer (JCA-BM8060; JEOL, Ltd, Tokyo, Japan). Plasma glucose concentrations were measured with the hexokinase method (Serotec Co., Ltd, Hokkaido, Japan). Serum insulin and 25-hydroxyvitamin D (25(OH)D) levels were determined by chemiluminescent immunoassay (CLIA) (Siemens Healthineer, Tokyo, Japan). Plasma AGEs N-(carboxymethyl) lysine (CML) was measured using a commercial ELISA kit (CircuLex Co., Ltd, Ina, Nagano, Japan). The blood tests were conducted four times (before and 2 h, 4 h, and 6 h after) on days 4 and 6.
Statistical analysis
Data were expressed as the mean ± SD. A two-way analysis of variance with repeated measures was used to test the interaction and main effect. If there was a significant interaction or main effect, the differences between the baseline and after intervention were analyzed using Student’s t-test. Effect size (ES) was calculated from the mean and SD. ESs refer to a commonly used interpretation as small (d = 0.2), medium (d = 0.5), and large (d ≥ 0.8). Values were considered statistically significant at P < 0.05. The ES and sample size were estimated using the software R 2.13.0 (R Foundation for Statistical Computing, Vienna, Austria). The other statistical analyses were performed using the SPSS program (IBM SPSS Statistics 20.0; IBM Corporation, Armonk, NY, USA).
Ethics
The study protocol was approved by the Ethics Committee of the National Hospital Organization Kyoto Medical Center, and all subjects gave their written informed consents before the start of the study.
| Results | ▴Top |
Clinical characteristics of all subjects are shown in Table 2. All the mean blood parameters were within the normal ranges.
 Click to view | Table 2. Baseline Characteristics |
Figure 2a shows the plasma glucose levels over 6 h. The mean fasting plasma glucose levels before the diet intervention were not significantly different between the normal diet (94.0 ± 1.7 mg/dL) and low-AGE diet (94.8 ± 1.9 mg/dL) groups. Two hours after the diet intervention, plasma glucose slightly increased in the normal diet group (97.4 ± 8.8 mg/dL) and decreased in the low-AGE diet group (93.8 ± 4.9 mg/dL), although the differences between the two groups were not significantly different.
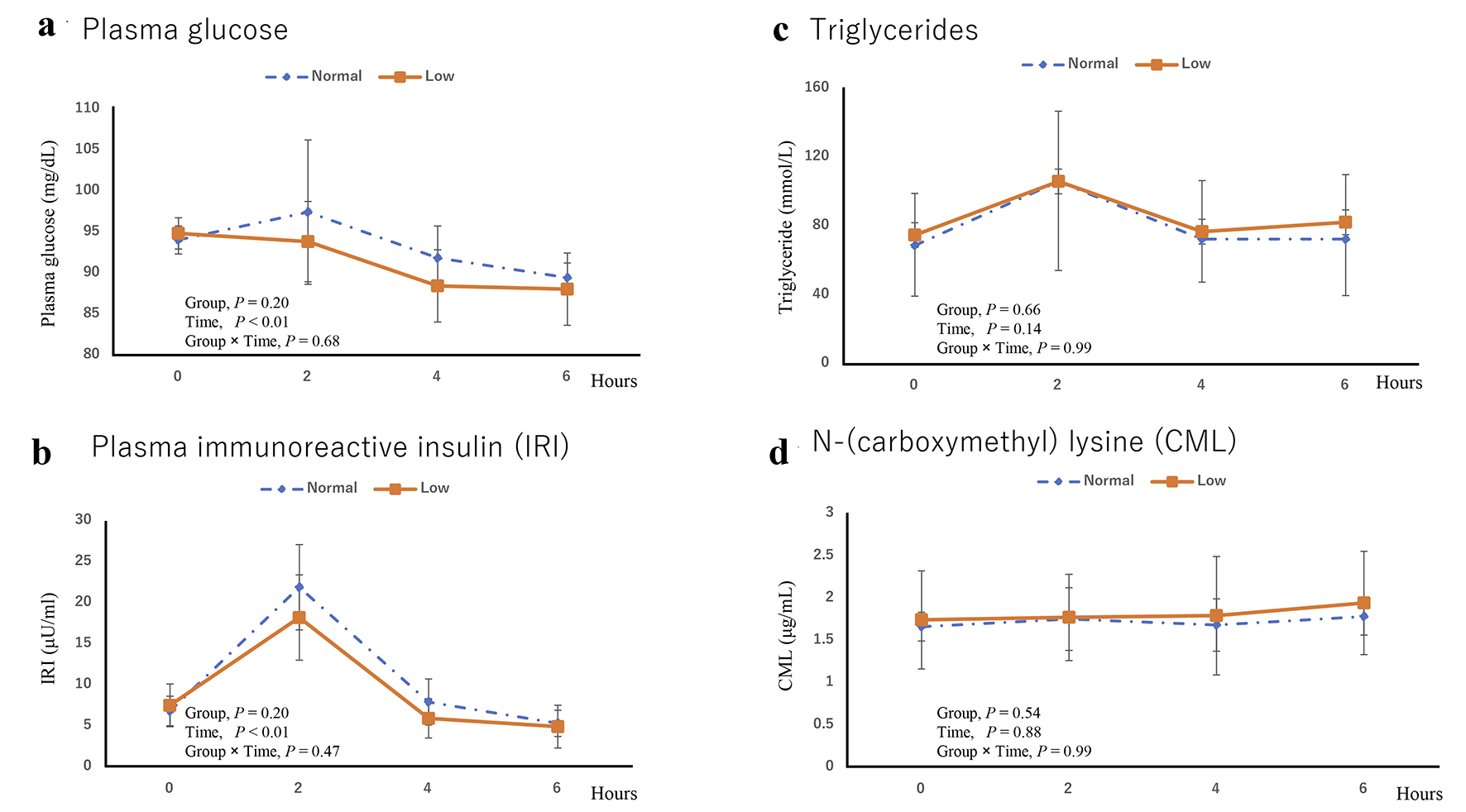 Click for large image | Figure 2. (a) Plasma glucose, (b) plasma immunoreactive insulin (IRI), (c) triglycerides, and (d) Nε-(carboxymethyl) lysine (CML). |
Figure 2b shows the plasma immunoreactive insulin (IRI) levels over 6 h. The mean fasting IRI levels did not significantly differ between the normal diet (6.8 ± 1.8 µU/mL) and low-AGE diet groups (7.5 ± 2.6 µU/mL). Two hours after the diet intervention, the mean IRI levels significantly increased in the normal diet (18.2 ± 5.2 µU/mL) and low-AGE diet (21.9 ± 5.2 µU/mL) groups, but the differences between the two groups were not significant.
The area under the curve (AUC) for insulin levels was lower in the low-AGE group (d = 0.8). The sample size was calculated using ES, 80% power and 5% significance. The sample size calculated from the ES of the insulin AUC change amount was 22 subjects.
Figure 2c shows the TG levels over 6 h. The mean fasting TG levels were not significantly different between the normal diet (68.6 ± 30.2 mmol/L) and low-AGE diet (74.6 ± 29.5 mmol/L) groups. On the other hand, the values at 2 h were significantly higher (P < 0.05) than those measured at baseline, but no significant differences between the two groups were observed.
Figure 2d shows the CML levels over 6 h. The mean fasting plasma CML levels did not significantly differ between the normal diet (1.66 ± 0.17 µg/mL) and low-AGE diet (1.74 ± 0.58 µg/mL) groups. Plasma CML increased 6 h after the intake of the meals (P < 0.05), but the increase was similar between the normal and low-AGE meals.
| Discussion | ▴Top |
This is the first study to investigate the effects of a low-AGE diet on insulin levels in non-diabetic Asian subjects. As a result, although the AUC results for insulin levels did not reach significance between the normal and low-AGE diet groups, the ES was large, whereas the plasma glucose level did not significantly differ between the two groups. Previous studies that examined the effect of AGE foods on insulin levels studied patients with diabetes over several weeks to several months [18]. The AUC result for insulin levels in our study was consistent with the results of these studies. However, the sample size was very small, and it was estimated that 22 subjects would be needed for future studies.
There is a paucity of data on the effects of AGEs on insulin resistance in humans. Uribarri et al [18] showed that in patients with type 2 diabetes, but not in healthy individuals, insulin resistance (estimated by HOMA-IR) improved after 4 months of dietary AGE restriction. The adverse effects of AGEs on insulin sensitivity could be mediated by increased oxidative stress and inflammation [13, 14, 20]. One study showed that a high-AGE diet increased E-selectin, IL-6, and thiobarbituric acid-reactive substances, whereas a low-AGE diet did not significantly change their levels in acute testing [19]. These effects appear to be mediated by changes in the AGE receptor, RAGE [21-23].
Our study had several limitations. First, a relatively small number of participants were included in the study, and thus, this was a pilot study to determine the appropriate sample size. In addition, since sample size was calculated using a single meal test design, it is unknown whether it can be applied in a long-term study. Although blood CML is considered the main AGE index, it is also necessary to evaluate other indices such as blood methylglyoxal [24] and urinary CML [25]. We also did not evaluate oxidative stress and inflammation markers. Although the diets were isocaloric, the normal diet contained slightly more fat (e.g., the difference was 3 g olive oil) than the low-AGE diet. Therefore, it is unclear whether the adjusted calorie value is representative of the normal diet and low-AGE diet used in this study. Finally, we did not measure the AGEs in the foods in the diets; therefore, CML content in the meals was not known.
Conclusions
In conclusion, the results indicated that 22 subjects may be needed to investigate the differences in clinical parameters due to cooking method in non-diabetic Japanese subjects by a single meal study. Future intervention studies with more than 22 participants should be conducted.
Acknowledgments
This trial was in part supported by JSPS KAKENHI Grant Number 26350178, Hokuto Foundation For Bioscience, and the Japanese Council for Science, Technology and Innovation (CSTI), SIP (Project ID 14533567), “Technologies for creating next-generation agriculture, forestry and fisheries” (funding agency: Bio-oriented Technology Research Advancement Institution, NARO), Kao Research Council for the Study of Healthcare Science.
Competing Interests
The authors declare that they have no competing interests.
| References | ▴Top |
- Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414(6865):782-787.
doi pubmed - Mann N. Dietary lean red meat and human evolution. Eur J Nutr. 2000;39(2):71-79.
doi pubmed - Yu C, Chen Y, Cline GW, Zhang D, Zong H, Wang Y, Bergeron R, et al. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem. 2002;277(52):50230-50236.
doi pubmed - Shafrir E, Ziv E, Mosthaf L. Nutritionally induced insulin resistance and receptor defect leading to beta-cell failure in animal models. Ann N Y Acad Sci. 1999;892:223-246.
doi pubmed - Hofmann SM, Dong HJ, Li Z, Cai W, Altomonte J, Thung SN, Zeng F, et al. Improved insulin sensitivity is associated with restricted intake of dietary glycoxidation products in the db/db mouse. Diabetes. 2002;51(7):2082-2089.
doi pubmed - Uribarri J, Woodruff S, Goodman S, Cai W, Chen X, Pyzik R, Yong A, et al. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J Am Diet Assoc. 2010;110(6):911-916 e912.
- Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813-820.
doi pubmed - Vlassara H, Uribarri J. Advanced glycation end products (AGE) and diabetes: cause, effect, or both? Curr Diab Rep. 2014;14(1):453.
doi pubmed - Hansen BC. The metabolic syndrome X. Ann N Y Acad Sci. 1999;892:1-24.
- Ames JM. Determination of N epsilon-(carboxymethyl)lysine in foods and related systems. Ann N Y Acad Sci. 2008;1126:20-24.
doi pubmed - Sandu O, Song K, Cai W, Zheng F, Uribarri J, Vlassara H. Insulin resistance and type 2 diabetes in high-fat-fed mice are linked to high glycotoxin intake. Diabetes. 2005;54(8):2314-2319.
doi pubmed - Cai W, Ramdas M, Zhu L, Chen X, Striker GE, Vlassara H. Oral advanced glycation endproducts (AGEs) promote insulin resistance and diabetes by depleting the antioxidant defenses AGE receptor-1 and sirtuin 1. Proc Natl Acad Sci U S A. 2012;109(39):15888-15893.
doi pubmed - Vlassara H, Cai W, Crandall J, Goldberg T, Oberstein R, Dardaine V, Peppa M, et al. Inflammatory mediators are induced by dietary glycotoxins, a major risk factor for diabetic angiopathy. Proc Natl Acad Sci U S A. 2002;99(24):15596-15601.
doi pubmed - Yamagishi S, Nakamura K, Matsui T. Potential utility of telmisartan, an angiotensin II type 1 receptor blocker with peroxisome proliferator-activated receptor-gamma (PPAR-gamma)-modulating activity for the treatment of cardiometabolic disorders. Curr Mol Med. 2007;7(5):463-469.
doi pubmed - Sukino S, Kotani K, Nirengi S, Gugliucci A, Caccavello R, Tsuzaki K, Kawaguchi Y, et al. Dietary intake of vitamin D is related to blood levels of advanced glycation end products during a weight loss program in obese women. J Biomed. 2016;1:1-4.
doi - Kawaguchi Y, Nirengi S, Kotani K, Somei J, Kawamoto T, Tsuzaki K, Yonei Y, et al. Mushroom intake and advanced glycation end products in the skin among community-dwelling elderly subjects: preliminary data. J Biomed. 2017;2:8-11.
doi - Gugliucci A, Kotani K, Taing J, Matsuoka Y, Sano Y, Yoshimura M, Egawa K, et al. Short-term low calorie diet intervention reduces serum advanced glycation end products in healthy overweight or obese adults. Ann Nutr Metab. 2009;54(3):197-201.
doi pubmed - Uribarri J, Cai W, Ramdas M, Goodman S, Pyzik R, Chen X, Zhu L, et al. Restriction of advanced glycation end products improves insulin resistance in human type 2 diabetes: potential role of AGER1 and SIRT1. Diabetes Care. 2011;34(7):1610-1616.
doi pubmed - Negrean M, Stirban A, Stratmann B, Gawlowski T, Horstmann T, Gotting C, Kleesiek K, et al. Effects of low- and high-advanced glycation endproduct meals on macro- and microvascular endothelial function and oxidative stress in patients with type 2 diabetes mellitus. Am J Clin Nutr. 2007;85(5):1236-1243.
doi pubmed - Unoki H, Bujo H, Yamagishi S, Takeuchi M, Imaizumi T, Saito Y. Advanced glycation end products attenuate cellular insulin sensitivity by increasing the generation of intracellular reactive oxygen species in adipocytes. Diabetes Res Clin Pract. 2007;76(2):236-244.
doi pubmed - Coughlan MT, Yap FY, Tong DC, Andrikopoulos S, Gasser A, Thallas-Bonke V, Webster DE, et al. Advanced glycation end products are direct modulators of beta-cell function. Diabetes. 2011;60(10):2523-2532.
doi pubmed - Zhao Z, Zhao C, Zhang XH, Zheng F, Cai W, Vlassara H, Ma ZA. Advanced glycation end products inhibit glucose-stimulated insulin secretion through nitric oxide-dependent inhibition of cytochrome c oxidase and adenosine triphosphate synthesis. Endocrinology. 2009;150(6):2569-2576.
doi pubmed - Forbes JM, Soderlund J, Yap FY, Knip M, Andrikopoulos S, Ilonen J, Simell O, et al. Receptor for advanced glycation end-products (RAGE) provides a link between genetic susceptibility and environmental factors in type 1 diabetes. Diabetologia. 2011;54(5):1032-1042.
doi pubmed - Uribarri J, Cai W, Peppa M, Goodman S, Ferrucci L, Striker G, Vlassara H. Circulating glycotoxins and dietary advanced glycation endproducts: two links to inflammatory response, oxidative stress, and aging. J Gerontol A Biol Sci Med Sci. 2007;62(4):427-433.
doi pubmed - Clarke RE, Dordevic AL, Tan SM, Ryan L, Coughlan MT. Dietary advanced glycation end products and risk factors for chronic disease: a systematic review of randomised controlled trials. Nutrients. 2016;8(3):125.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


