| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Original Article
Volume 10, Number 1, January 2018, pages 41-49
Improvement of Diurnal Blood Pressure Variation by Azilsartan
Keisuke Okamuraa, b, Kazuyuki Shiraia, Tetsu Okudaa, Hidenori Urataa
aDepartment of Cardiovascular Diseases, Fukuoka University Chikushi Hospital, Chikushino, Japan
bCorresponding Author: Keisuke Okamura, Department of Cardiovascular Diseases, Fukuoka University Chikushi Hospital, 1-1-1, Zokumyoin, Chikushino-shi, Fukuoka 818-8502, Japan
Manuscript submitted October 11, 2017, accepted November 7, 2017
Short title: Azilsartan and Diurnal Blood Pressure Variation
doi: https://doi.org/10.14740/jocmr3228w
| Abstract | ▴Top |
Background: Azilsartan is an angiotensin II receptor blocker with a potent antihypertensive effect.
Methods: In a multicenter, prospective, open-label study, 265 patients with poor blood pressure control despite treatment with other angiotensin II receptor blockers were switched to 20 mg/day of azilsartan (patients on standard dosages) or 40 mg/day of azilsartan (patients on high dosages).
Results: Blood pressure was 149/83 mm Hg before switching and was significantly reduced from 1 month after switching until final assessment (132/76 mm Hg, P < 0.001). The pulse rate was 72/min before switching and increased significantly from 3 months after switching until final assessment (74/min, P < 0.005). A significant decrease of home morning systolic and diastolic pressure was observed from 1 and 3 months, respectively. Home morning blood pressure was 143/82 mm Hg before switching and 130/76 mm Hg at final assessment (P < 0.01). The morning-evening difference of systolic blood pressure decreased from 14.6 to 6.6 mm Hg after switching (P = 0.09). The estimated glomerular filtration rate was significantly decreased at 3, 6, and 12 months after switching, and serum uric acid was significantly increased at 12 months. No serious adverse events occurred.
Conclusion: Azilsartan significantly reduced the blood pressure and decreased diurnal variation in patients responding poorly to other angiotensin II receptor blockers.
Keywords: Azilsartan; Angiotensin II receptor blocker; Blood pressure variation
| Introduction | ▴Top |
In Japan, the prevalence of hypertension is the highest among lifestyle-related diseases and it is estimated that approximately 4.3 million people have this condition [1]. Many clinical studies have shown that management of the blood pressure (BP) in patients with hypertension or pre-hypertension is most important for preventing the onset and progression of cardiovascular disease and organ dysfunction.
According to the Hypertension Treatment Guideline 2014 of the Japanese Society of Hypertension, strict 24-h control of BP is recommended [2]. In hypertensive patients with diabetes or chronic kidney disease, the BP target is lower than that in patients with hypertension alone. Moreover, it has been reported that lowering the systolic blood pressure (SBP) to 120 mm Hg improves outcomes [3]. However, the target BP cannot be achieved in many patients, suggesting that more effective antihypertensive agents are needed.
As first-line therapy for hypertension, the following four classes of drugs are recommended: diuretics, calcium channel blockers (CCBs), angiotensin-converting enzyme (ACE) inhibitors, and angiotensin II receptor blockers (ARBs). In Japan, the combination of a CCB and an ARB is frequently prescribed.
Among ARBs, azilsartan has been reported to show higher affinity for the angiotensin II type 1 (AT1) receptor [4] and better tissue penetration [5, 6] compared to current agents, being the first drug to demonstrate statistically significant superiority in head-to-head comparison with another ARB [7].
From the results of basic research, azilsartan is expected to have a stronger antihypertensive effect than other ARBs [8]. Because azilsartan binds strongly to the AT1 receptor and shows slow dissociation from this receptor [4], it is also expected to suppress diurnal variation of BP. However, there have been relatively few clinical investigations of the antihypertensive effect of azilsartan. Because more effective antihypertensive therapy is required, we investigated the role of azilsartan as a treatment option in daily practice through a study performed by a private practitioner’s network in the Chikushi region of Fukuoka Prefecture, Japan.
| Materials and Methods | ▴Top |
Methods
In a multicenter, prospective, open-label observational study, patients with poor BP control (according to the target value in the 2009 Guideline of the Japanese Society of Hypertension at the time of study initiation) despite ARB treatment at standard or higher doses were switched to azilsartan, and the office BP and home BP were investigated before and after switching. The study period was from November 2012 to October 2015. The standard doses of ARBs were defined as follows: 50 mg of losartan, 8 mg of candesartan, 80 mg of valsartan, 40 mg of telmisartan, 20 mg of olmesartan, and 100 mg of irbesartan.
All the patients who took standard dose of common ARBs were switched to azilsartan 20 mg once daily.
If the antihypertensive effect was insufficient after switching to 20 mg of azilsartan, the dose was increased to 40 mg once daily. Patients switching from higher than standard doses of other ARBs received azilsartan at 40 mg once daily.
The office BP, home BP, and pulse rate (PR) were measured before switching medications and after switching (at 1, 2, 3, 6, and 12 months). Antihypertensive medication was basically administrated after breakfast. The office BP was measured with a standard sphygmomanometer in a sitting position at the outpatient office. The home BP was measured using the each home sphygmomanometer.
In addition, standard laboratory tests, urinalysis, and electrocardiography (ECG) were conducted before switching and after switching (at 3, 6, and 12 months). Some of the patients still took other kind of antihypertensive drugs. The dosages of antihypertensive agents other than ARBs were not changed during the study period. The dose of azilsartan was decided at the discretion of each attending physician.
Patients
Patients attending the outpatient clinic of the Cardiovascular Department at Chikushi Hospital, Fukuoka University and patients of doctors registered with the Chikushi Cardiovascular Disease Clinical Research Network (Chikushi-JRN) were eligible for this study if they were aged 20 years or older, had essential hypertension, were taking a standard or high dose of an ARB, had poor BP control as defined by the target in the 2009 Guideline of the Japanese Society of Hypertension, and gave informed consent to participation.
Exclusion criteria were secondary hypertension, serious vascular complications within the previous 6 months, a history of hypersensitivity to azilsartan, and renal impairment (serum creatinine ≥ 2.0 mg/dL).
Criteria for discontinuation
Patients discontinued the study if BP control was poor after switching to azilsartan, if treatment could not be continued due to excessive hypotension or adverse events, if compliance with medication was poor, or if the attending physician considered that discontinuation was appropriate for other reasons.
Endpoints
The primary endpoint was comparison of the office and home SBP, diastolic BP (DBP), and PR in each patient including drop-outs between before switching medications and after switching medications (at 1, 2, 3, 6, and 12 months and at the final assessment).
Secondary endpoints were the change in the difference of home BP between morning and evening, all adverse events and cardiovascular events throughout the study period, change of BP between before switching medication and the final assessment stratified by the pre-switching ARB dose, and differences of the antihypertensive effect in relation to prior medication and demographic factors in the responders.
In addition, laboratory tests, urinary protein, and ECG findings (Sokolow-Lyon index of left ventricular hypertrophy: SV1 + RV5) were compared between before switching and 3, 6, and 12 months after switching medications.
Sample size
In a phase 3 study of azilsartan, the SBP of the 20 mg group was 2.6 mm Hg lower than the SBP of the group receiving 8 mg of candesartan [7]. Therefore, it was estimated that switching to azilsartan would lead to a 5% increase of patients achieving the target BP. Assuming a power of detection of 90% and level of significance of 0.05, the required sample size was calculated to be 200 patients.
Statistical analysis
Statistical analysis was performed at Fukuoka University using IBM SPSS Statistics 23 software. Differences of variables with a normal distribution were assessed for significance by the t-test. Levene’s test was used if the variance was equal, while Welch’s test was conducted if the variance was unequal. Continuous variables without a normal distribution were analyzed by the Wilcoxon signed rank test. Correlations were determined by Spearman’s rank correlation coefficient analysis. In all analyses, P < 0.05 was considered significant. Results are expressed as the mean with standard deviation (SD), median with interquartile range (IQR), or the frequency (%).
Ethical considerations
This study was performed according to the Declaration of Helsinki. The study protocol was approved by the Institutional Review Committee of Chikushi Hospital, Fukuoka University (approval no.: R12-027), and written informed consent was obtained from each patient before enrollment.
| Results | ▴Top |
Table 1 shows the characteristics of the subjects. A total of 265 patients with a median age of 72 years (IQR: 62 - 81 years) were registered in this study, and 54% were men.
 Click to view | Table 1. Characteristics of the Patients (n = 265) |
Table 2 shows the number of patients switching from each ARB and the dose before switching.
 Click to view | Table 2. ARB Therapy Before Switching to Azilsartan |
Candesartan was the most common ARB before switching and was used by 87 patients, including 77 patients on the standard dose of 8 mg daily. In addition, 58 patients were switched from valsartan and 42 patients were switched from olmesartan.
Table 3 shows changes in the dose of azilsartan during the study period. An initial azilsartan dose of 20 mg was most frequent and was assigned to 214 patients, while 48 patients were switched to 40 mg. At the end of the study, 191 patients were still taking 20 mg daily and 67 patients were receiving 40 mg, including 21 patients with dose escalation to 40 mg.
 Click to view | Table 3. Changes of the Dose of Azilsartan |
There were 59 protocol deviations, which included one fatal event, 12 adverse events, five cases of an insufficient antihypertensive effect, two cases of an excessive antihypertensive effect, and 30 cases of failure to attend a scheduled study visit (drop-out).
The fatal event was death due to relapse of colorectal cancer, which was assessed as being unrelated to the study drug. There were 14 adverse reactions for which a relationship with switching to azilsartan could not be excluded, including five cases of vertigo/lightheadedness/ataxia, two cases of hypercalcemia, and one case of skin eruption/diarrhea/stomatitis. These events improved after dose reduction or suspension of treatment and were not serious.
Figure 1 displays the changes of office BP and PR. Office SBP (SD) was 149 (11) mm Hg before switching and decreased significantly to 132 (16) mm Hg at the final assessment (P < 0.001, t-test). A significant decrease of the office SBP was maintained from 1 month after switching. Office DBP (SD) was 83 (11) mm Hg before switching and decreased significantly to 76 (12) mm Hg at the final assessment (P < 0.001, t-test), with a significant decrease maintained from 1 month after switching. Office PR (SD) was 72 (11) bpm before switching; it increased significantly by 3 months after switching and was 74 (11) bpm at the final assessment (P < 0.005, t-test).
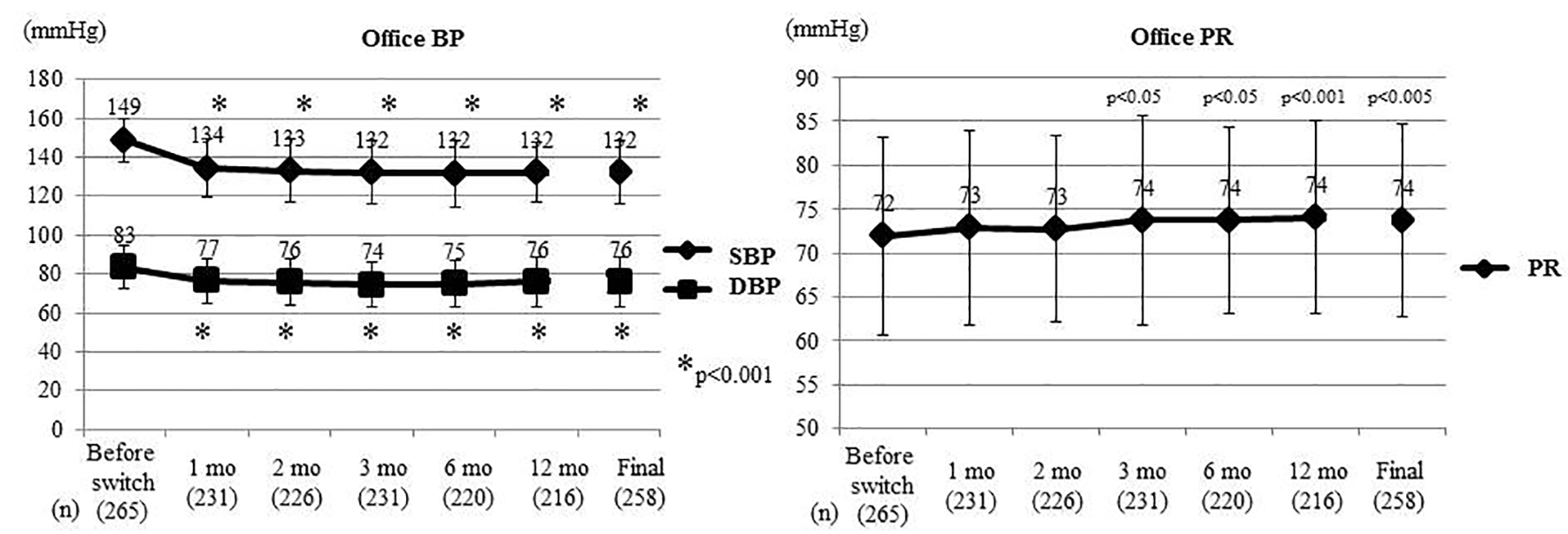 Click for large image | Figure 1. Changes of office BP and PR. At the Final, the last values are shown including patients who experienced deviation. Office BP and PR were compared between before switching and each time point after switching by the t-test. Office SBP and DBP decreased significantly from 1 month after switching, while office PR increased significantly from 3 months. BP: blood pressure; PR: pulse rate; SBP: systolic BP; DBP: diastolic BP; mo: month(s). |
Figure 2 shows the changes of home morning BP and PR in a subset of 36 patients who underwent home BP monitoring. Home morning SBP (SD) was 143 (14) mm Hg before switching and it showed a significant decrease from 1 month after switching, being 130 (14) mm Hg at the final assessment (P < 0.001, t-test). Home morning DBP (SD) was 82 (9) mm Hg before switching and decreased significantly from 3 months to reach 76 (10) mm Hg at the final assessment (P < 0.01, t-test). Home morning PR (SD) was 69 (10) bpm before switching and only showed a significant decrease to 66 (10) bpm (P < 0.05, t-test) at 1 month after switching.
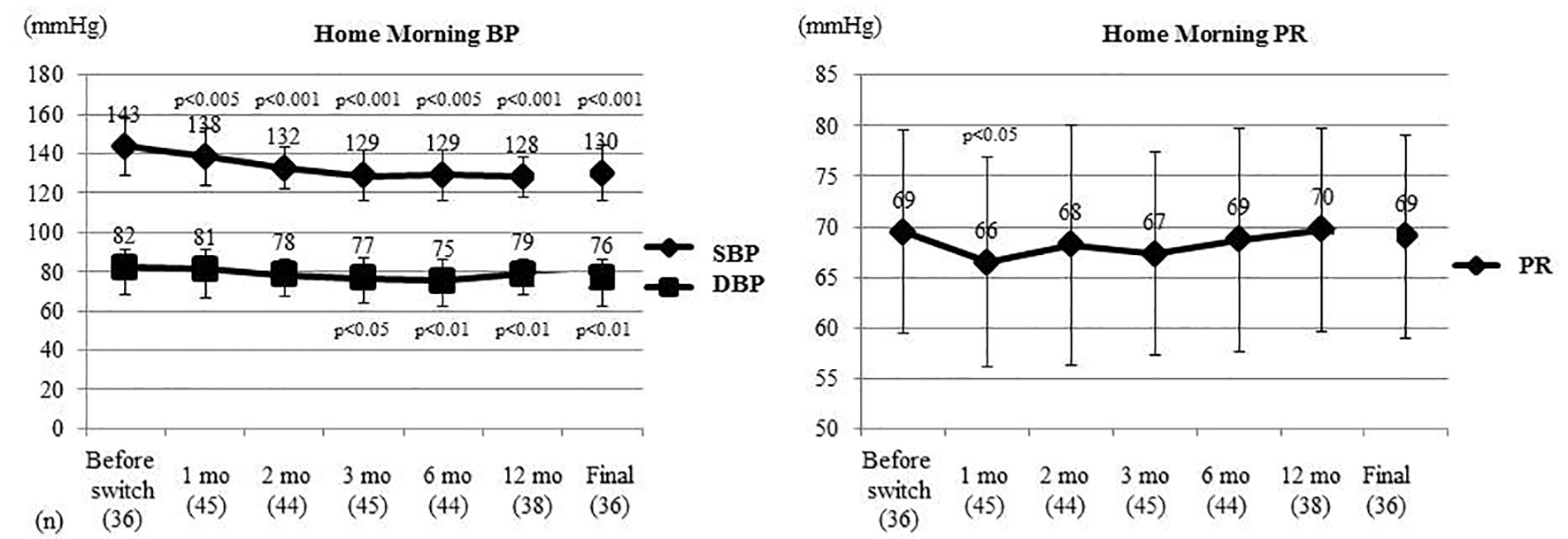 Click for large image | Figure 2. Changes of home morning BP and PR. Home morning BP and PR were compared between before switching and each time point after switching by the t-test. Home morning SBP was significantly decreased from 1 month after switching and home morning DBP was significantly decreased from 3 months. Home morning PR only showed a significant decrease at 1 month. BP: blood pressure; PR: pulse rate; SBP: systolic BP; DBP: diastolic BP; mo: month(s). |
Figure 3 displays the changes of home evening BP and PR in the same subset of patients who underwent home BP monitoring. Home evening SBP (SD) was 128 (13) mm Hg before switching, and it decreased significantly to 121 (15) mm Hg (P < 0.01, t-test) at 3 months after switching and to 122 (15) mm Hg (P < 0.05, t-test) at 6 months. In contrast, the home evening DBP and home evening PR did not show any significant changes.
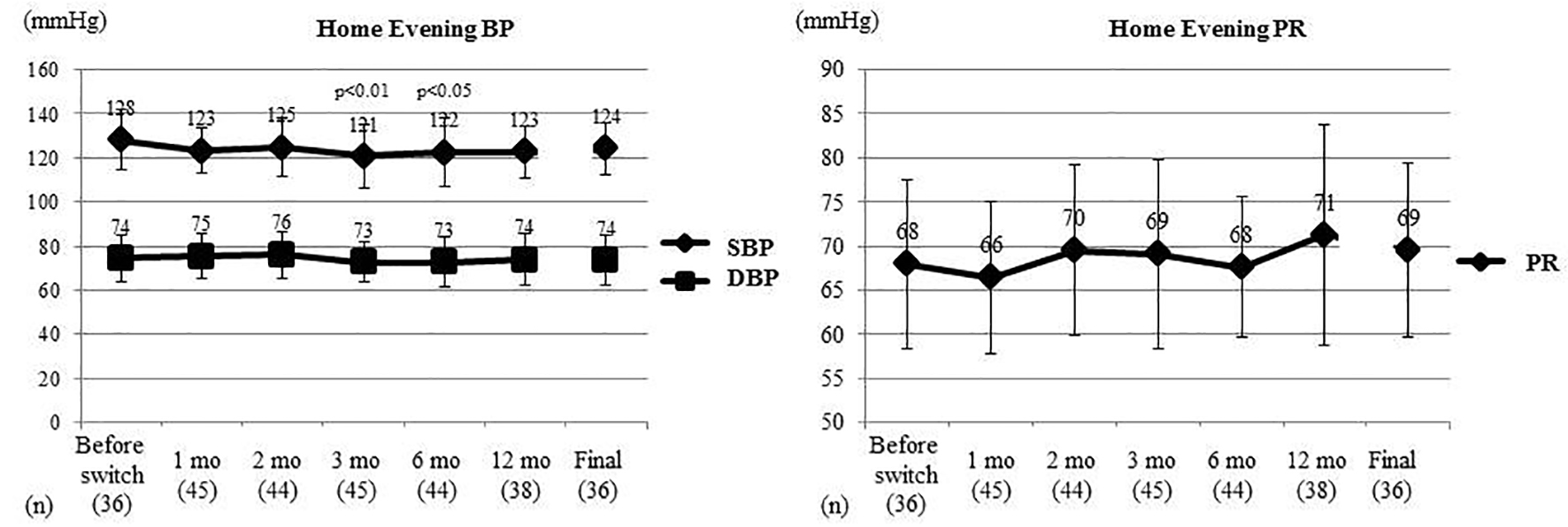 Click for large image | Figure 3. Changes of home evening BP and PR. Home evening BP and PR were compared between before switching and each time point after switching by the t-test. Home evening SBP showed a significant decrease at 3 and 6 months after switching, while home evening DBP and home evening PR did not change significantly. BP: blood pressure; PR: pulse rate; SBP: systolic BP; DBP: diastolic BP; mo: month(s). |
Changes of the office BP and PR in patients taking each dose of the other ARBs are displayed in Table 4. After switching from the standard dose of other ARBs to 20 mg of azilsartan, office SBP decreased significantly in all cases, and office DBP also decreased significantly except in patients switching from irbesartan. Office PR increased significantly after switching from 80 mg of valsartan to 20 mg of azilsartan. In the five patients switching from a high dose of valsartan (160 mg) to 40 mg of azilsartan, both office SBP and DBP were decreased significantly.
 Click to view | Table 4. Changes of Office BP and PR After Switching From the Standard Dose or Higher Dose of Other ARBs |
The changes of the morning-evening difference of SBP, DBP, and PR are shown in Table 5.
 Click to view | Table 5. Morning-Evening Difference of Home SBP, DBP, and PR |
Home evening values of BP and PR were subtracted from the home morning values to calculate the morning-evening differences. It was found that the morning-evening difference of SBP decreased after switching to azilsartan, while the morning-evening difference of DBP did not change significantly. The morning-evening difference of the PR showed a significant increase after switching.
Table 6 displays the changes of laboratory values after switching to azilsartan. Comparison of laboratory data was performed between before switching to azilsartan and 3, 6, and 12 months after switching, and the t-test or the Wilcoxon signed rank test was used for analysis. The estimated glomerular filtration rate (eGFR) showed a significant decrease at 3, 6, and 12 months after switching (P < 0.05, 0.001, and 0.001, respectively), while uric acid was significantly increased at 12 months (P < 0.05). There was no change of urine protein. ECG data showed that SV1 + RV5 was significantly decreased from 2.6 cm before switching to 2.5 cm at 12 months (P < 0.05).
 Click to view | Table 6. Changes of Various Parameters After Switching to Azilsartan |
Correlations between the final BP after switching to azilsartan and various parameters before switching are listed in Table 7. According to Spearman’s correlation analysis, the final antihypertensive effect of azilsartan was stronger in patients with a lower BMI, higher SBP, higher Hb, lower HbA1c, and lower SV1 + RV5 at baseline.
 Click to view | Table 7. Correlations Between the Final Change of Blood Pressure and Various Parameters Before Switching to Azilsartan |
The final decrease of BP after switching from 8 mg of candesartan to 20 mg of azilsartan (which was the most common switching combination) is shown in Table 8 for patients with or without concomitant antihypertensive agents before switching. There were no significant differences of the final antihypertensive effect of azilsartan between patients with or without concomitant diuretics, calcium antagonists, or β-blockers at baseline.
 Click to view | Table 8. Changes of SBP in Patients With or Without Other Concomitant Antihypertensive Agents at Switching From Candesartan (8 mg) to Azilsartan (20 mg) |
| Discussion | ▴Top |
Major findings
This study enrolled 265 patients, among whom 77 patients were switched from the standard dose of candesartan (8 mg) to 20 mg of azilsartan. At the final assessment, 191 patients were using azilsartan at 20 mg daily. The main findings were as follows. 1) Office SBP and DBP were significantly decreased from 1 month after switching and office PR was significantly increased from 3 months. 2) Home morning SBP showed a significant and sustained decrease after switching. 3) The morning-evening difference of SBP was reduced after switching. 4) After switching to azilsartan, the antihypertensive effect was greater in patients with a lower BMI, higher SBP, higher Hb, lower HbA1c, and lower SV1 + RV5 before switching. 5) At 12 months after switching, eGFR was decreased and uric acid was significantly increased, while SV1 + RV5 on the ECG was significantly decreased. 6) There were 59 protocol deviations during the study, but no serious adverse events were attributable to switching to azilsartan.
Azilsartan is reported to be highly lipophilic and strongly inhibits angiotensin in target organs [6], so it is expected to exert a rapid and potent antihypertensive effect. In the present study, the office SBP and DBP were significantly decreased from 1 month after switching. In addition, the office BP target achievement rate improved markedly from 1.5% before switching to 41.9% at the final assessment (data not shown).
In non-treatment hypertensive patients, the time for azilsartan to reach its maximum antihypertensive effect was reported to be 7.1 days [9], and its effect showed a stable plateau from the first follow-up visit of the present study (1 month).
The SPRINT study demonstrated that cardiovascular events were decreased by strict management of BP compared to standard therapy, but strict treatment requires multiple antihypertensive drugs [3, 10]. However, if compliance with antihypertensive therapy is poor due to polypharmacy, cardiovascular events are increased [11]. Accordingly, it is considered reasonable to switch to a more potent agent like azilsartan as a strategy for improving the response to antihypertensive agents.
It has been reported that azilsartan inhibits sympathetic activity [12], but the office PR increased significantly from 3 months after switching in the present study and the home morning-evening difference of PR also increased. It is possible that the strong antihypertensive effect of azilsartan led to a reactive increase of the PR, but further assessment is needed.
In a phase 3 study, the decrease of SBP/DBP was greater with azilsartan (20 mg) than candesartan (8 mg) and the difference was 2.6/2.0 mm Hg [7]. When candesartan (8 mg) was switched to azilsartan (20 mg) in this study, the office SBP was reduced from 148 to 129 mm Hg (a decrease of 19 mm Hg). The present investigation was a sequential switching study and not a comparative study, so the difference of the antihypertensive effect might have been larger.
In a prospective study comparing azilsartan (20 mg) with olmesartan (20 mg), the difference of office SBP/DBP was 4.0/2.3 mm Hg in favor of azilsartan [13]. When olmesartan (20 mg) was switched to azilsartan (20 mg) in the current study, office SBP was reduced from 155 to 140 mm Hg (a decrease of 15 mm Hg), so the antihypertensive effect of azilsartan was also more pronounced in this study.
However, the possibility of “big day bias” [14] influencing the office BP at the time of study entry cannot be ruled out. Patients with poor BP control were enrolled in this study by their attending doctors, but some may have been enrolled after only a single unsatisfactory BP reading and BP may have been much lower in such patients after switching. The protocol required enrollment of poorly controlled patients for ethical reasons, and stricter office BP criteria defining poor control would have been desirable, but this was a limitation of the present study. The present study may overestimate the antihypertensive effect of azilsartan.
Measurement of home BP is more effective than office BP for improving prediction of the risk of stroke, and the risk of stroke and cardiovascular events is higher when the home BP is elevated [15, 16]. Thus, office BP may not be as accurate as home BP for assessing the risks associated with hypertension, but measurement of home BP requires the patient’s understanding, cooperation, and patience. In fact, it was only possible to measure home BP in 14% of the participants in the present study. Moreover, home BP is measured by the patient and underreporting or false reporting of BP values may occur [17]. This problem can be overcome if the home BP monitor has a memory for data storage. Also, telemedicine has recently become available in which data are transmitted to medical institutions via phone or the Internet [18], and this method is reported to improve BP control [19].
Although home BP monitoring is considered most objective, it cannot evaluate diurnal changes, and frequent compression of the forearm by the cuff during the night is reported to affect sleep quality and increase BP [20]. Thus, it is difficult to conclude that home BP monitoring is the best approach, and the most accurate method for measurement of BP has not been established. A cuffless sphygmomanometer is currently under development [21], and this may bring about a paradigm change in BP measurement once it is available.
In the present study, the home morning SBP demonstrated a significant and sustained decrease after switching to azilsartan, but home evening SBP was only decreased at 3 and 6 months. The morning-evening SBP difference was reduced after switching to azilsartan.
Fluctuation of the BP is reported to be correlated with cardiovascular events [22]. In patients with a morning surge, there is a large difference between the BP after waking and the lowest pressure during the night, and these patients have an increased risk of cardiovascular events [23]. Because the morning surge of BP is large in Japanese patients [24], cardiovascular events are more likely to occur [25]. In the present study, switching to azilsartan resulted in a smaller morning-evening BP difference compared to that when patients were on the other ARBs, suggesting that azilsartan is superior for suppressing BP variations.
After switching to azilsartan, laboratory tests revealed a significant decrease of eGFR and increase of uric acid at 12 months, although no change of urine protein was observed. These findings suggest that care must be taken in relation to potential adverse effects of azilsartan on renal function or related to hyperuricemia. Also, the ECG showed a significant decrease of SV1 + RV5 at 12 months, which was considered to result from reduced left ventricular load due to the significantly stronger antihypertensive effect of azilsartan.
This study showed that the antihypertensive effect of azilsartan was stronger in patients with a lower BMI, higher SBP, higher Hb, lower HbA1c, and lower SV1 + RV5 at baseline. In patients who are lean and do not have high left ventricular potentials, arteriosclerosis has not progressed and it may be easier to achieve a useful reduction of BP. Furthermore, the decrease of SBP was proportional to the BP at initiation of treatment and excessive hypotension was not noted, suggesting that azilsartan is safe.
Because BP increases at low ambient temperatures and during winter [26, 27], we investigated whether there was a difference in the antihypertensive effect of azilsartan based on the season when treatment was initiated, but no significant difference was observed (data not shown).
Many combination medications containing an ARB and CCB or diuretic have recently been marketed, each with distinct characteristics [28, 29]. Therefore, we evaluated the effects of azilsartan in patients with or without concomitant diuretics, CCBs, and β-blockers, but no differences were found between patients with or without concomitant antihypertensive drugs of these classes. Thus, our investigation of other antihypertensive agents that could achieve a stronger antihypertensive effect when co-administered with azilsartan did not identify any such drug, suggesting that concomitant drugs may be selected according to the characteristics of each patient.
Limitations
This was a single-arm open-label study, so we cannot rule out a possible placebo effect. In addition, only a small subset of the patients underwent assessment of home BP and PR. Furthermore, the possible influence of big day bias on the BP at study initiation cannot be ruled out. Finally, the dosage was changed in some patients after switching to azilsartan.
Conclusion
Azilsartan is the most recent ARB to be introduced to the market. It has rapid onset of action and a strong antihypertensive effect. The results of the present study suggest that azilsartan is an effective antihypertensive agent for patients with a poor response to other ARBs and that it can also suppress diurnal variation of BP.
We could not avoid the possibility of big day bias at initiation of this study, which demonstrates the limitations of clinical investigations carried out by practicing doctors based on office BP and emphasizes the importance of measuring home BP. However, home BP measurement is currently associated with various issues, so development of new devices and methods to measure the real BP in daily life is required.
Acknowledgments
We thank Mrs. Nao Totake for her excellent technical assistance. We also thank members of Chikushi-JRN for recruiting patients to the study.
Conflict of Interest
The authors have received financial support from Takeda Pharmaceutical Co., Ltd.
| References | ▴Top |
- Miura K, Nagai M, Ohkubo T. Epidemiology of hypertension in Japan: where are we now? Circ J. 2013;77(9):2226-2231.
doi pubmed - Oparil S. Updated guidelines for management of high blood pressure in Japan. Hypertens Res. 2014;37(6):484-487.
doi pubmed - Group SR, Wright JT, Jr., Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373(22):2103-2116.
doi pubmed - Ojima M, Igata H, Tanaka M, Sakamoto H, Kuroita T, Kohara Y, Kubo K, et al. In vitro antagonistic properties of a new angiotensin type 1 receptor blocker, azilsartan, in receptor binding and function studies. J Pharmacol Exp Ther. 2011;336(3):801-808.
doi pubmed - Takai S, Jin D, Sakonjo H, Takubo T, Nakanishi T. Significance of the vascular concentration of angiotensin II-receptor blockers on the mechanism of lowering blood pressure in spontaneously hypertensive rats. J Pharmacol Sci. 2013;123(4):371-379.
doi pubmed - Kohara Y, Kubo K, Imamiya E, Wada T, Inada Y, Naka T. Synthesis and angiotensin II receptor antagonistic activities of benzimidazole derivatives bearing acidic heterocycles as novel tetrazole bioisosteres. J Med Chem. 1996;39(26):5228-5235.
doi pubmed - Rakugi H, Enya K, Sugiura K, Ikeda Y. Comparison of the efficacy and safety of azilsartan with that of candesartan cilexetil in Japanese patients with grade I-II essential hypertension: a randomized, double-blind clinical study. Hypertens Res. 2012;35(5):552-558.
doi pubmed - Kurtz TW, Kajiya T. Differential pharmacology and benefit/risk of azilsartan compared to other sartans. Vasc Health Risk Manag. 2012;8:133-143.
doi pubmed - Satoh M, Haga T, Hosaka M, Obara T, Metoki H, Murakami T, Kikuya M, et al. The velocity of antihypertensive effects of seven angiotensin II receptor blockers determined by home blood pressure measurements. J Hypertens. 2016;34(6):1218-1223.
doi pubmed - Williamson JD, Supiano MA, Applegate WB, Berlowitz DR, Campbell RC, Chertow GM, Fine LJ, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged >/=75 years: a randomized clinical trial. JAMA. 2016;315(24):2673-2682.
doi pubmed - Mazzaglia G, Ambrosioni E, Alacqua M, Filippi A, Sessa E, Immordino V, Borghi C, et al. Adherence to antihypertensive medications and cardiovascular morbidity among newly diagnosed hypertensive patients. Circulation. 2009;120(16):1598-1605.
doi pubmed - Sueta D, Mitsuyama S. Exploring the potential of a novel ARB - New era for antihypertensive therapy - Expectation of azilsartan based on basic research: Improvement of diurnal fluctuation abnormality of blood pressure. Ketsuatsu. 2012;19:1109-1114 (in Japanese).
- Perez A, Cao C. Azilsartan in patients with mild to moderate hypertension using clinic and ambulatory blood pressure measurements. J Clin Hypertens (Greenwich). 2017;19(1):82-89.
doi pubmed - Howard JP, Francis DP. Overcoming the three biases obscuring the science of renal denervation in humans: big-day bias, check-once-more bias and I-will-take-it-now bias. Trends Cardiovasc Med. 2015;25(2):116-118.
doi pubmed - Ohkubo T, Asayama K, Kikuya M, Metoki H, Hoshi H, Hashimoto J, Totsune K, et al. How many times should blood pressure be measured at home for better prediction of stroke risk? Ten-year follow-up results from the Ohasama study. J Hypertens. 2004;22(6):1099-1104.
doi pubmed - Asayama K, Ohkubo T, Metoki H, Obara T, Inoue R, Kikuya M, Thijs L, et al. Cardiovascular outcomes in the first trial of antihypertensive therapy guided by self-measured home blood pressure. Hypertens Res. 2012;35(11):1102-1110.
doi pubmed - Mengden T, Hernandez Medina RM, Beltran B, Alvarez E, Kraft K, Vetter H. Reliability of reporting self-measured blood pressure values by hypertensive patients. Am J Hypertens. 1998;11(12):1413-1417.
doi - Parati G, Pickering TG. Home blood-pressure monitoring: US and European consensus. Lancet. 2009;373(9667):876-878.
doi - McManus RJ, Mant J, Bray EP, Holder R, Jones MI, Greenfield S, Kaambwa B, et al. Telemonitoring and self-management in the control of hypertension (TASMINH2): a randomised controlled trial. Lancet. 2010;376(9736):163-172.
doi - Degaute JP, van de Borne P, Kerkhofs M, Dramaix M, Linkowski P. Does non-invasive ambulatory blood pressure monitoring disturb sleep? J Hypertens. 1992;10(8):879-885.
pubmed - Boubouchairopoulou N, Kollias A, Chiu B, Chen B, Lagou S, Anestis P, Stergiou GS. A novel cuffless device for self-measurement of blood pressure: concept, performance and clinical validation. J Hum Hypertens. 2017;31(7):479-482.
doi pubmed - Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JE, Dahlof B, Sever PS, et al. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375(9718):895-905.
doi - Li Y, Thijs L, Hansen TW, Kikuya M, Boggia J, Richart T, Metoki H, et al. Prognostic value of the morning blood pressure surge in 5645 subjects from 8 populations. Hypertension. 2010;55(4):1040-1048.
doi pubmed - Hoshide S, Kario K, de la Sierra A, Bilo G, Schillaci G, Banegas JR, Gorostidi M, et al. Ethnic differences in the degree of morning blood pressure surge and in its determinants between Japanese and European hypertensive subjects: data from the ARTEMIS study. Hypertension. 2015;66(4):750-756.
doi pubmed - McMullan CJ, Yano Y, Bakris GL, Kario K, Phillips RA, Forman JP. Racial impact of diurnal variations in blood pressure on cardiovascular events in chronic kidney disease. J Am Soc Hypertens. 2015;9(4):299-306.
doi pubmed - Youn JC, Rim SJ, Park S, Ko YG, Kang SM, Choi D, Ha JW, et al. Arterial stiffness is related to augmented seasonal variation of blood pressure in hypertensive patients. Blood Press. 2007;16(6):375-380.
doi pubmed - Alperovitch A, Lacombe JM, Hanon O, Dartigues JF, Ritchie K, Ducimetiere P, Tzourio C. Relationship between blood pressure and outdoor temperature in a large sample of elderly individuals: the Three-City study. Arch Intern Med. 2009;169(1):75-80.
doi pubmed - Okamura K, Okuda T, Kumagai N, Mitsutake R, Urata H, CHAT-E i. Antihypertensive effect and safety of a candesartan/hydrochlorothiazide combination in patients with uncontrolled hypertension. Therapeutic Research. 2015;36(5):439-448.
- Okamura K, Shirai K, Totake N, Okuda T, Urata H. Prospective direct comparison of antihypertensive effect and safety between high-dose amlodipine or indapamide in hypertensive patients uncontrolled by standard doses of angiotensin receptor blockers and amlodipine. Clin Exp Hypertens. 2017:1-8.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


