| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Letter to the Editor
Volume 9, Number 12, December 2017, pages 1029-1031
The Application of Sodium-Glucose Cotransporter 2 Inhibitors to Chronic Kidney Disease Stage 4
Ayako Koguchia, b, Hiroki Adachia, Hidekatsu Yanaia, b, c
aDepartment of Internal Medicine, National Center for Global Health and Medicine, Kohnodai Hospital, Chiba, Japan
bDepartment of Neurology, Center Hospital of the National Center for Global Health and Medicine, Tokyo, Japan
cCorresponding Author: Hidekatsu Yanai, Department of Internal Medicine, National Center for Global Health and Medicine, Kohnodai Hospital, 1-7-1 Kohnodai, Ichikawa, Chiba 272-8516, Japan
Manuscript submitted October 5, 2017, accepted October 12, 2017
Short title: SGLT2i in CKD
doi: https://doi.org/10.14740/jocmr3220w
| To the Editor | ▴Top |
Sodium-glucose cotransporter 2 inhibitors (SGLT2i) are reversible inhibitors of SGLT2, leading to reduction of renal glucose reabsorption and decrease of plasma glucose, in an insulin-independent manner [1]. Possible anti-atherosclerotic effects beyond glucose lowering of SGLT2i can be predicted [2], and we previously showed that SGLT2i improve metabolic parameters including coronary risk factors [3, 4]. Actually, the EMPA-REG OUTCOME trial that examined the effect of empagliflozin in addition to standard of care in patients with type 2 diabetes and established cardiovascular (CV) diseases demonstrated a significant reduction in the incidence of CV death and heart failure hospitalization [5]. Recently, the CANVAS program reported that canagliflozin significantly reduced hospitalization for heart failure as compared with placebo by 33% as well as empagliflozin [6]. Further, the CVD-REAL study, the large multinational study, presented that treatment with SGLT2i was associated with a lower risk of hospitalization for heart failure and death as compared with other glucose lowering drugs [7]. Prevention of development of heart failure by SGLT2i has been attracting a lot of attention [8].
SGLT2 is expressed in the proximal tubule of kidney and mediates reabsorption of approximately 90% of the filtered glucose load [9]; therefore, SGLT2i are not recommended for use in patients with renal insufficiency. Our previous studies demonstrated that estimated glomerular filtration rate (eGFR) has an influence on the improvement in HbA1c [10, 11], and liver function by SGLT2i [10]. However, the EMPA-REG OUTCOME trial showed that empagliflozin significantly reduced incident or worsening of nephropathy, progression to macroalbuminuria, doubling of serum creatinine level accompanied by eGFR of ≤ 45 mL/min/1.73 m2 and initiation of renal replacement therapy [12]. The CANVAS program also demonstrated that canagliflozin reduced progression to macroalbuminuria, and 40% reduction in eGFR, renal replacement therapy, or renal death [6]. Both the EMPA-REG OUTCOME trial and CANVAS program proposed the renal protective effect of SGLT2i. In the EMPA-REG OUTCOME trial, mean eGFR at baseline in low-eGFR group was almost 48 mL/min/1.73 m2, and the rate of patients with urinary albumin-to-creatinine ratio > 300 was 18%. In the CANVAS program, mean eGFR at baseline was 76 mL/min/1.73 m2, and the rate of patients with macroalbuminuria was 7.1%. Effects of SGLT2i on renal function and proteinuria in patients with severe renal insufficiency such as chronic kidney diseases (CKD) stage 4 and nephrotic syndrome remain largely unknown.
Here, we present a 36-year-old man with a long-standing history of type 2 diabetes (21 years) admitted for pneumonia and heart failure. He had a significant prior history of obesity, and CKD stage 4 and nephrotic syndrome due to diabetic nephropathy. Dapagliflozin, one of SGLT2i, successfully reduced his urine protein and body weight, and improved heart failure and exercise tolerance without any adverse effects.
On admission, body height, weight and body mass index were 158.6 cm, 137.9 kg and 54.8 kg/m2, respectively. Fever, tachycardia (heart rate, 118 /min), hypoxemia (SpO2 94% with 4 L oxygen) and tachypnea (respiratory rate, over 20/min) and abnormal findings of chest X-ray (cardiomegaly, pleural effusion and consolidation) suggested the existence of heart failure and pneumonia. Systolic and diastolic blood pressures were 168 and 95 mm Hg, respectively. His eGFR, serum creatinine, and daily urinary protein levels were 14 mL/min/1.73 m2, 4.7 mg/dL and 15.5 g/day, suggesting the existence of CKD stage 4 and nephrotic syndrome [13]. At 3 weeks before admission, plasma glucose and HbA1c levels were 254 mg/dL and 8.3%, respectively.
He had been treated by liraglutide (0.9 mg/day), pioglitazone (15 mg/day), insulin aspart (40, 20 and 24 U before breakfast, lunch and dinner, respectively) and insulin glargine (65 U at bedtime). We stopped the use of pioglitazone because of the development of heart failure, and started to use dapagliflozin (5 mg/day) on day 4. Clinical course is shown in Figure 1. Body weight promptly decreased and body weight loss of over 20 kg was obtained during hospitalization. Serum creatinine gradually decreased after a transient increase, and eGFR also gradually increased after a transient decrease. Urinary protein promptly decreased, and a significant reduction (-10.8 g/day) was obtained during hospitalization.
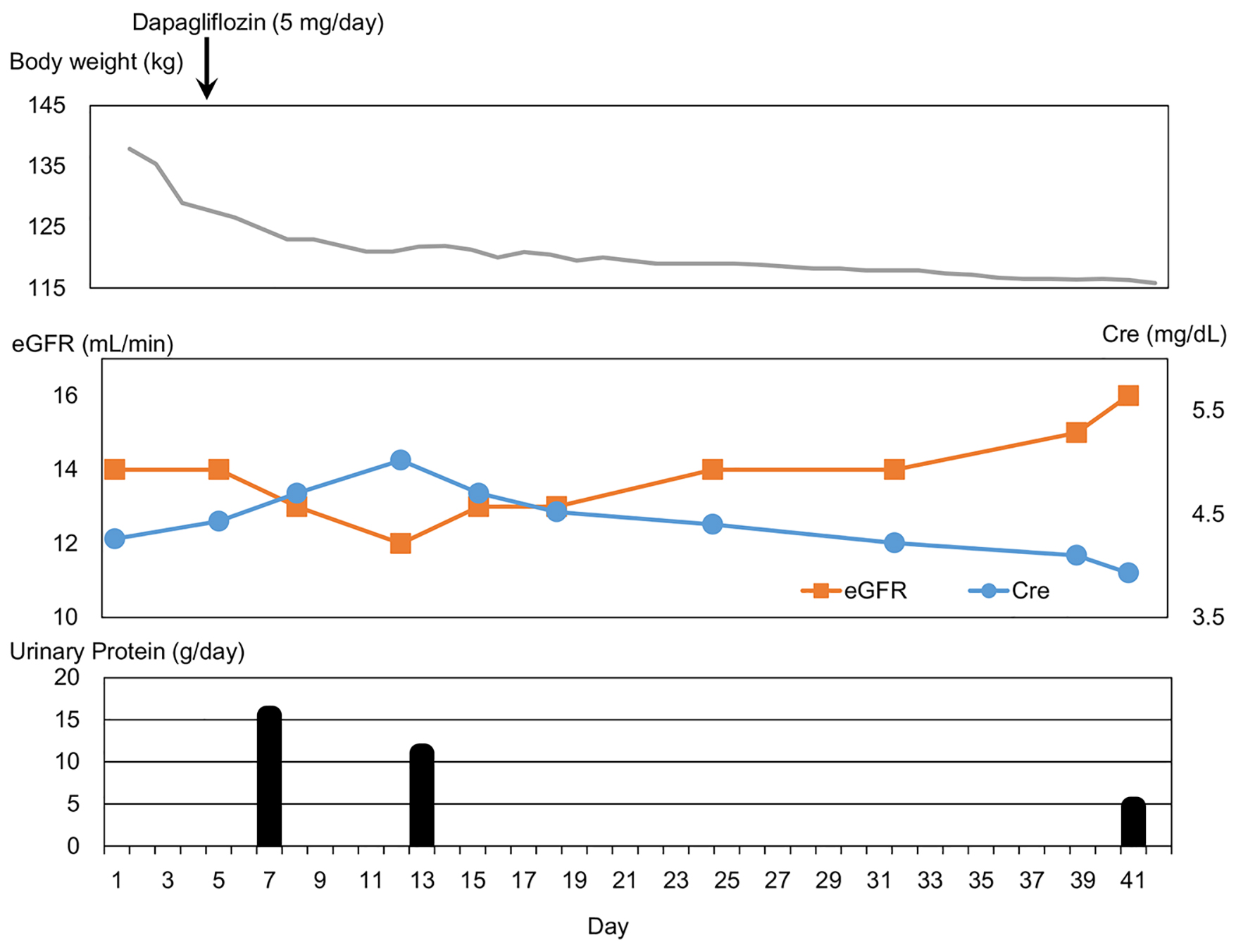 Click for large image | Figure 1. Clinical course. Cre: creatinine; eGFR: estimated glomerular filtration rate. |
Low plasma B-type natriuretic peptide (BNP) levels in obesity due to an increased clearance of active natriuretic peptides by visceral fat was reported [14]. On admission, his plasma BNP level was high (38.6 pg/mL; normal range, 0 - 18.4 pg/mL) and his BNP increased from 7.9 to 38.6 pg/mL in 3 weeks before admission, supporting the development of heart failure. His plasma BNP level decreased to 5.3 pg/mL at 11 weeks after the start of dapagliflozin. At 4 days after the start of dapagliflozin, serum ketone bodies level was high (557 µmol/L; normal range, < 130 µmol/L), and continued to be high (407 µmol/L; normal range, < 130 µmol/L) until 39 days after the start of dapagliflozin. He was discharged from hospital at 40 days after the start of dapagliflozin. The treatment for his diabetes was changed to liraglutide (0.9 mg/day), dapagliflozin (5 mg/day), insulin aspart (46, 10 and 16 U before breakfast, lunch and dinner, respectively) and insulin glargine (41 U at bedtime). Daily insulin dose decreased from 149 to 113 U.
To our knowledge, this is the first report to show the renal and cardiac protective effects of SGLT2i in a type 2 diabetic patient complicated with CKD stage 4, nephrotic syndrome and heart failure. The addition of dapagliflozin remarkably reduced body weight, serum creatinine level and urinary protein, and increased eGFR. Possible mechanisms for renal protective effects of SGLT2i are shown in Table 1 [5, 15-21]. Increased serum ketone bodies, reduced blood pressure and amelioration of heart failure were observed in this patient. Various factors might contribute to the improvement of renal function.
 Click to view | Table 1. Possible Mechanisms for Renal Protective Effects of SGLT2 Inhibitors |
In this patient, prompt body weight loss, increased exercise tolerance and reduced plasma BNP level were observed after the start of SGLT2i use, suggesting amelioration of heart failure due to SGLT2i. Reduced blood pressure, osmotic diuresis, and induction of utilization of ketone bodies by failing heart, due to SGLT2i, might contribute to amelioration of heart failure [19]. In addition to discontinuation of pioglitazone, reduced daily insulin dose (-25%) and improved renal function, due to SGLT2i, might also contribute to the improvement of heart failure.
We have to mention the limitation of our observation. This is just a case report. To elucidate our hypothesis, further studies, preferably with larger numbers of subjects with CKD stage 4, will be needed.
In conclusion, the application of SGLT2i might be a useful therapeutic option for CKD stage 4.
| References | ▴Top |
- Jabbour SA, Goldstein BJ. Sodium glucose co-transporter 2 inhibitors: blocking renal tubular reabsorption of glucose to improve glycaemic control in patients with diabetes. Int J Clin Pract. 2008;62(8):1279-1284.
doi pubmed - Yanai H, Katsuyama H, Hamasaki H, Adachi H, Moriyama S, Yoshikawa R, Sako A. Sodium-glucose cotransporter 2 inhibitors: possible anti-atherosclerotic effects beyond glucose lowering. J Clin Med Res. 2016;8(1):10-14.
doi pubmed - Katsuyama H, Hamasaki H, Adachi H, Moriyama S, Kawaguchi A, Sako A, Mishima S, et al. Effects of sodium-glucose cotransporter 2 inhibitors on metabolic parameters in patients with type 2 diabetes: a chart-based analysis. J Clin Med Res. 2016;8(3):237-243.
doi pubmed - Yanai H, Hakoshima M, Adachi H, Kawaguchi A, Waragai Y, Harigae T, Masui Y, et al. Effects of six kinds of sodium-glucose cotransporter 2 inhibitors on metabolic parameters, and summarized effect and its correlations with baseline data. J Clin Med Res. 2017;9(7):605-612.
doi pubmed - Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128.
doi pubmed - Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644-657.
doi pubmed - Kosiborod M, Cavender MA, Fu AZ, Wilding JP, Khunti K, Holl RW, Norhammar A, et al. Lower risk of heart failure and death in patients initiated on sodium-glucose cotransporter-2 inhibitors versus other glucose-lowering drugs: The CVD-REAL study (comparative effectiveness of cardiovascular outcomes in new users of sodium-glucose cotransporter-2 inhibitors). Circulation. 2017;136(3):249-259.
doi pubmed - Yanai H. Sodium-glucose cotransporter 2 inhibitors for heart failure. J Endocrinol Metab. 2017;7(3):75-76.
doi - Vallon V, Platt KA, Cunard R, Schroth J, Whaley J, Thomson SC, Koepsell H, et al. SGLT2 mediates glucose reabsorption in the early proximal tubule. J Am Soc Nephrol. 2011;22(1):104-112.
doi pubmed - Katsuyama H, Yanai H. An influence of the estimated glomerular filtration rate on improvement in metabolic parameters by sodium-glucose cotransporter 2 inhibitors. J Clin Med Res. 2016;8(6):486-488.
doi pubmed - Yanai H, Hakoshima M, Adachi H. What properties of sodium-glucose cotransporter 2 inhibitors determine the improvement in hemoglobin A1c and body weight? J Clin Med Res. 2017;9(5):446-448.
doi pubmed - Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, Johansen OE, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(4):323-334.
doi pubmed - National Kidney F. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1-266.
pubmed - Clerico A, Giannoni A, Vittorini S, Emdin M. The paradox of low BNP levels in obesity. Heart Fail Rev. 2012;17(1):81-96.
doi pubmed - Chilton R, Tikkanen I, Cannon CP, Crowe S, Woerle HJ, Broedl UC, Johansen OE. Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes Metab. 2015;17(12):1180-1193.
doi pubmed - Cherney DZ, Perkins BA, Soleymanlou N, Har R, Fagan N, Johansen OE, Woerle HJ, et al. The effect of empagliflozin on arterial stiffness and heart rate variability in subjects with uncomplicated type 1 diabetes mellitus. Cardiovasc Diabetol. 2014;13:28.
doi pubmed - Vallon V, Gerasimova M, Rose MA, Masuda T, Satriano J, Mayoux E, Koepsell H, et al. SGLT2 inhibitor empagliflozin reduces renal growth and albuminuria in proportion to hyperglycemia and prevents glomerular hyperfiltration in diabetic Akita mice. Am J Physiol Renal Physiol. 2014;306(2):F194-204.
doi pubmed - Skrtic M, Yang GK, Perkins BA, Soleymanlou N, Lytvyn Y, von Eynatten M, Woerle HJ, et al. Characterisation of glomerular haemodynamic responses to SGLT2 inhibition in patients with type 1 diabetes and renal hyperfiltration. Diabetologia. 2014;57(12):2599-2602.
doi pubmed - Mudaliar S, Alloju S, Henry RR. Can a shift in fuel energetics explain the beneficial cardiorenal outcomes in the EMPA-REG OUTCOME study? A Unifying Hypothesis. Diabetes Care. 2016;39(7):1115-1122.
doi pubmed - Sano M, Takei M, Shiraishi Y, Suzuki Y. Increased hematocrit during sodium-glucose cotransporter 2 inhibitor therapy indicates recovery of tubulointerstitial function in diabetic kidneys. J Clin Med Res. 2016;8(12):844-847.
doi pubmed - Yanai H, Katsuyayama H. A possible mechanism for renoprotective effect of sodium-glucose cotransporter 2 inhibitor: elevation of erythropoietin production. J Clin Med Res. 2017;9(2):178-179.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


