| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Review
Volume 10, Number 4, April 2018, pages 294-301
Primary Small Intestinal Angiosarcoma: Epidemiology, Diagnosis and Treatment
Qiang Naia, h, i, Mohammad Ansarib, h, Jing Liuc, Hadi Razjouyand, Stella Paka, Yufei Tiana, Rafay Khanb, Arkady Broderd, Arindam Bagchia, Veena Iyera, Danae Hamoudaa, Mohammad Islamb, Shuvendu Senb, Abdalla Yousifb, Man Hue, f, Yali Loug, Jozsef Duhlb
aUniversity of Toledo Medical Center, Toledo, OH 43614, USA
bRaritan Bay Medical Center, Perth Amboy, NJ 08861, USA
cSchool of Public Health, Shandong University, Jinan 250012, China
dSaint Peter's University Hospital, New Brunswick, NJ 08901, USA
eDepartment of Radiation Oncology and Shandong Province Key Laboratory of Radiation Oncology, Shandong Cancer Hospital Affiliated to Shandong University, Jinan, China
fShandong Academy of Medical Sciences, Jinan 250117, China
gDepartment of Neurology, Brigham and Women's Hospital, 60 Fenwood Road, Boston, MA 02115, USA
hThese authors contributed equally
iCorresponding Author: Qiang Nai, University of Toledo Medical Center, Toledo, OH 43614, USA
Manuscript submitted August 9, 2017, accepted October 9, 2017
Short title: Small Bowel Angiosarcoma
doi: https://doi.org/10.14740/jocmr3153w
| Abstract | ▴Top |
Angiosarcoma is an aggressive mesenchymal sarcoma of endothelial cell origin with high mortality. Its occurrence in the small intestine is exceedingly low. In addition to the rarity of small intestine angiosarcoma, the nonspecific early clinical symptoms obscure the suspicion of such tumors and thereby delay the diagnosis. In a hope to improve the knowledge of this rare but fatal neoplasm, we report one case of angiosarcoma of duodenum and jejunum in a 73-year-old man. Furthermore, we summarize and analyze the common clinical features, tumor markers, treatment, and survival of previous reported cases of this malignancy. Small bowel angiosarcoma occurs more often in men than women (1.6:1). The median age at diagnosis is 68.5 years. The overall median survival time is 150 days; the median survival time in female (300 days) is longer than that of male patients (120 days). Von Willebrand factor (vWF), CD31, CD34, vimentin, and Ulex europaeus agglutinin 1 appear to be the most useful markers for the diagnosis. The majority of the patients underwent surgical resection alone or surgery with subsequent chemotherapy. The patients treated with surgery plus chemotherapy survive longer than those underwent surgical resection only (median 420 days, n = 7 vs. 96.5 days, n = 26, respectively; P = 0.0275). Further studies of more cases are needed for a better understanding of this rare entity, as well as the development of effective strategies for prevention, early diagnosis, and treatment.
Keywords: Angiosarcoma; Small intestine; Diagnosis; Treatment; Survival
| Introduction | ▴Top |
Angiosarcomas are uncommon, aggressive mesenchymal sarcomas of endothelial cell origin with high mortality. It accounts for 1-2 % of soft tissue sarcoma. These tumors can arise from any location in the body due to the ubiquity of blood vessels and lymphatics, but most commonly found in skin and subcutaneous tissues [1]. There are cutaneous, visceral, and soft tissue subtypes of angiosarcomas, among which the visceral angiosarcomas comprise 15% to 47% of such neoplasms and are more difficult to diagnose than other subtypes [2-4]. Angiosarcomas have been reported in the breast, heart, lung, liver, spleen, adrenal glands, ovaries, and rarely in the gastrointestinal tract especially the small intestine, which have only been found in scattered case reports and small series [1, 5-45].
In addition to the rarity of the small intestine angiosarcoma, the nonspecific early clinical findings including nausea, vomiting, abdominal pain, gastrointestinal (GI) bleeding, anemia, fatigue, and weakness may further decrease the suspicion of such tumors and delay the diagnosis. The management of small intestine angiosarcomas would be improved by a better knowledge of such fatal neoplasms. We report a case of angiosracoma in the duodenum and jejunum in a 73-year-old man. In addition, the epidemiology, diagnosis, treatment modalities, and survival of small bowel angiosarcoma of previous reported cases are analyzed.
| Literature Search and Cases | ▴Top |
The previously reported cases before July 2016 of small intestine angiosarcoma were included in this study. The age, gender, symptoms on admission, anatomical locations, tumor markers used for diagnosis, subsequent therapies, and survival time of total of 47 cases are analyzed. There are two cases with indeterminate primary sites, one patient was found to have angiosarcoma in both skin and duodenum [5]; the other had operation on cardiac angiosarcoma 5 years before the diagnosis of the angiosarcoma in jejunum, and the authors thought the jejunal angiosarcoma as recurrent, however, a primary small bowel angiosarcoma is also possible after a 5-year interval since the diagnosis of the cardiac angiosarcoma [41]. The Wilcoxon two-sample test was used to compare the age distribution between male and female genders. The log-rank test was used to compare the survival times between different groups.
Case
A 73-year-old man presented with chest pain, dyspnea, melena, weakness, and fatigue for 3 days, and melena for 7 days since the discharge from a prior 3-month-long hospitalization for GI bleeding. No fever, chills, abdominal pain, nausea, or vomiting was reported. During the previous hospitalization, patient had received multiple blood transfusions and undergone endoscopy and push enteroscopy that revealed Los Angeles Grade A erosive esophagitis, small sliding hiatal hernia, two erosions in the bulb, two cratered ulcers with adherent clots in D1 and D2, a fungating mass in D3. The biopsy of the mass showed acute-on-chronic inflammatory cells with granulation tissue. Patient had no prior radiation or polyvinyl exposure and no family history of GI malignancy. He was a former smoker. Vital signs were within normal limits on admission and physical exam showed a non-distended, non-tender abdomen with decreased bowel sounds and no organomegaly.
After admission, the patient received blood transfusions, and underwent multiple enteroscopy, with the biopsy from a bleeding ulcer in D3 revealed poorly differentiated high grade sarcoma. Subsequently, a pylorus-preserving pancreaticoduodenectomy was performed; the pathology demonstrated multiple ulcerated mucosal and submucosal nodules up to 1cm in size of high grade angiosarcoma involving duodenum and jejunum, and metastatic angiosarcoma in 10 of 14 peripancreatic and mesenteric lymph nodes. The positivity for CD34, vimentin, Wilm’s tumor-1, and vWF supported the angiosarcoma diagnosis. Postoperative chest computed tomography (CT) showed masses in both lungs highly suspicious for metastasis that was absent 1 month prior. Patient survived 16 days after surgery on mechanical ventilation before family decided to discontinue treatment.
| Literature Results | ▴Top |
Age and gender distribution
There are 29 male and 18 female included in this study, the male to female ratio is 1.6:1. The ages of 18 female and 28 male patients are obtainable. The ages of female patients range from 25 to 85 years, with median value of 63.5 years; the ages of male patients range from 25 to 87 years, with median of 69.5 years (Fig. 1, Table 1). There was no significant difference in the age distributions between the two genders (n = 28 female vs. 18 male; P = 0.772, Wilcoxon two-sample test).
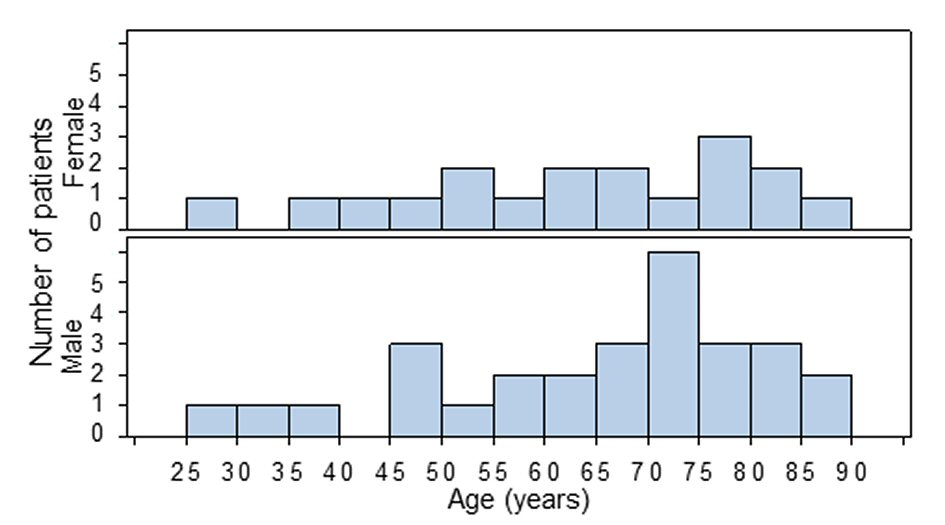 Click for large image | Figure 1. Age distribution in male and female patients of small intestinal angiosarcoma. There is no significant difference in age distribution between male and female genders (P = 0.677, Wilcoxon two-sample test, n = 28 and 18, respectively; the age of one male patient is missing). |
 Click to view | Table 1. Age and Gender Characteristics of Patients With Small Intestinal Angiosarcoma |
The presenting clinical characteristics
The clinical symptoms and signs on initial presentation are nonspecific. The common clinical features (in descending order of frequency) include abdominal pain, anemia, GI bleeding, fatigue/weakness, weight loss, dyspnea, abdominal distension/bowel obstruction, anorexia, nausea/vomiting, acute abdomen, and bowel perforation (Table 2).
 Click to view | Table 2. Symptoms and Signs in Patients With Small Intestine Angiosarcoma |
Risk factors
The development of angiosarcoma has been related to history of radiation exposure, certain environmental toxins or foreign bodies, chronic lymphedema, and genetic factors [3, 4]. There are 17 patients who had previous radiation exposure, among which 16 with therapeutic radiation, one with a 30-year-long exposure to both occupational radiation and polyvinyl chloride. The onset of radiation exposure is available in14 cases, ranging from 3 to 30 years prior to the diagnosis of angiosarcoma, with median latency of 9.5 years. In addition, there was one case with hemodialysis for 21 years (Table 3). Therefore, based on the current data, previous heavy radioactive exposure is likely the most common preventable environmental risk factor for small intestinal angiosarcoma.
 Click to view | Table 3. Risk Factor for Small Intestinal Angiosarcoma in Reported Cases |
Anatomical distribution and stages of small intestinal angiosarcoma
The location of the angiosarcoma in small bowel as reported were (percentage of the 47 cases, in descending order): ileum only (29.8%), jejunum only (27.7%), unspecified small intestine (23.4%), duodenum/jejunum (10.6%), duodenum only (4.3%), jejunum/ileum (2.1%) and duodenum/jejunum/ileum (2.1%) (Table 4). The jejunum and ileum are the most common locations for small intestinal angiosarcoma.
 Click to view | Table 4. Location of Small Intestinal Angiosarcoma |
Among the 47 patients reported, 26 had metastatic disease, three patients with stage III, and four patients with stage II, others indeterminate. The metastatic sites include liver (11/26, 42.3%), lung (11/26, 42.3%), peritoneum (5/26, 19.2%), spleen (4/26, 15.4%), bone (3/26, 11.5%), stomach (3/26, 11.5%), mesentery (3/26, 11.5%), two cases of kidney (2/26, 7.7%), abdominal wall (2/26, 7.7%) and retroperitoneal (2/26, 7.7%); and one case for each of these sites: brain, oropharynx, thoracic cavity, pleura, pancreas, omentum, gall bladder, urinary bladder, appendix and skin (1/26, 3.8%).
Biomarkers for diagnosis
The diagnosis of small bowel angiosarcoma may be very challenging due to the non-specific clinical, radiological and histopathological features as seen in our and previously reported cases [1, 5-45]. Imaging studies including CT, magnetic resonance imaging (MRI), positron emission tomography (PET) scan, and ultrasound can be used to define the extent of abdominal angiosarcoma prior to operation. Endoscopy and enteroscopy help detect the source of bleeding. Although the biopsy done by endoscopy and/or enteroscopy may lead to the appropriate diagnosis, it may be overlooked initially as in our case. Surgical resection and subsequent immunohistochemical studies are therefore required for a definitive diagnosis [3, 4].
Histological characteristics of angiosarcoma may provide useful clues for diagnosis. Angiosarcoma can be classified into different subtypes based on the cytologic appearance: spindle-shaped endothelial cells, epithelioid with large rounded or polygonal cells, and pleomorphic or mixed phenotypes as in most angiosarcoma [21]. Areas of irregular anastomosing vasculature lined by atypical endothelial cells are readily identifiable in histology study. However, the existence of anaplasia in most of angiosarcomas makes it challenging to distinguish such tumors from other undifferentiated ones such as melanoma or carcinoma. Therefore immunohistochemistry is mandatory to establish the definitive diagnosis of angiosarcoma. Expression of endothelial markers CD31, CD34, von Willebrand factor (vWF), Ulex europaeus agglutinin 1 (UEA-1), Friend leukemia integration 1 (Fli-1), endothelin-1, vascular endothelial growth factor (VEGFR), and erythroblast transformation specific related gene (ERG) can help identify angiosarcomas, each with different sensitivity and specificity (Table 5, [2, 46-51]), among which the vWF, UEA-1 and CD31 are considered the most useful for the diagnosis of poorly differentiated cases [1].
 Click to view | Table 5. Sensitivity and Specificity of Some Markers for Angiosarcoma Diagnosis [2, 46-51] |
However, some of these markers may be lost due to progressive tumor dedifferentiation. Otherwise, epithelioid angiosarcoma may express cytokeratins, which makes it difficult to distinguish it from poorly differentiated carcinomas. Therefore, a number of other markers with different tissue specificity including lymphatic endothelial (lymphatic vessel endothelial hyaluronan receptor-1/Lyve-1 or podoplanin/D2-40), smooth muscle (desmin), neural (S100, SOX10), epithelial (keratin), stromal (CD117, DOG1), melanocytic (HMB-45, melan-A), human herpes virus 8 (HHV-8), and mesothelial (calretinin, CK5/6, HBME-1 and WT-1) can be used to distinguish angiosarcoma from other tumors such melanoma [3, 26, 48].
The markers used in the reported cases of small intestinal angiosarcoma are summarized in Table 6. Among which, collagen IV, a marker for basal lamina collagen has proven to be a powerful tool in the identification of endothelial neoplasm in combination with other markers [52]. Overexpression of vimentin, a marker for epithelial-mesenchymal transition, is linked to accelerated tumor growth, tissue invasion, and poor outcome [53].
 Click to view | Table 6. Markers Used for Immunohistochemical Studies in Reported Cases |
The expression of those endothelial markers is heterogeneous in different types of vessels of various organs [49, 54]. Also, unusual expression patterns and loss of endothelial markers are common findings in angiosarcomas [55]. Therefore, a combination of multiple markers may markedly improve the sensitivity and specificity in the diagnosis in challenging angiosarcoma cases (Table 7, [2, 50, 56-58]).
 Click to view | Table 7. Markers Useful for Angiosarcoma Differentiation [2, 50, 56-58] |
Treatment and outcome
The treatment modalities are available in 45 patients with small intestinal angiosarcoma (Table 8), among which 31 patients underwent resection alone (26 cases with obtainable survival times); eight had resection/chemotherapy (seven with survival times); two with surgery/chemotherapy/radiation therapy (both with survival times reported); one patient received surgery, but it is unclear if other treatment modality was added subsequently (with survival time); one patient received chemotherapy only (with survival time); one had chemotherapy/radiation (with survival time), and one patient treated was Argon plasma coagulation (APC, with survival time), the treatment for two patients are unavailable (with survival time). The chemotherapy agents used as single or in combination are anthracycline (three cases), paclitaxel (three cases), dacarbazine (two cases), cisplatin (one case), thalidomide (one case), vincristine (one case), and cyclophosphamide (one case).
 Click to view | Table 8. Therapeutic Modalities in Reported Cases |
Among the 26 patients treated with surgery alone and included for survival analysis, 16 had metastatic disease at diagnosis, two with stage III, while the stages of the other eight patients indeterminate. Six out of the seven patients treated with surgery plus chemotherapy were found to have metastatic lesions at diagnosis, while the other one was indeterminate. Furthermore, almost all of the patients with metastatic small bowel angiosarcoma underwent surgical resection with or without additional treatment modalities: surgery alone (18/26, 69.2%), surgery plus chemotherapy (6/26, 23.1%), surgery plus chemotherapy and radiation (1/26, 3.8%), and only one patient was treated with APC (1/26, 3.8%).
The median survival times are obtainable for 41 patients (as reported or from personal communications). The median survival time is 150 days (range 9 to more than 3,720 days) days after diagnosis (Fig. 2a). The median survival times are 300 days for female (n = 15) and 120 days for male patients (n = 26), with no significant difference between the genders (log-rank test: z = 0.17; P = 0.86; Fig. 2b). The overall 1-, 2-, 3-, and 5- year survival rates are 36.6%, 14.6%, 12.2%, and 2.4% respectively. The survival rates are markedly lower than that of the primary soft-tissue sarcomas (5-year survival of 50-60%), and overall angiosarcomas (5-year survival of about 35%) [1].
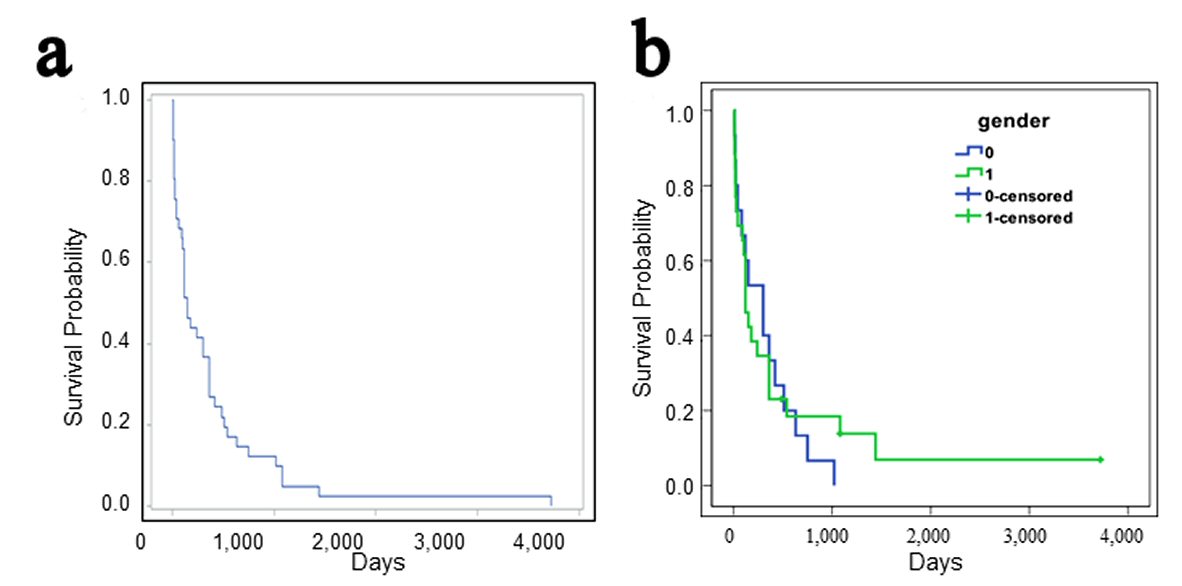 Click for large image | Figure 2. (a) Survival analysis of patients with small intestinal angiosarcoma. (b) There is no gender difference in the survival times between male and female patients (0 = female, 1 = male; Log-rank test, z = 0.17, P = 0.86, n = 15 and 26, respectively). |
The survival times in different treatment groups are further summarized (median survival time) as showed in Figure 3. There is a significant difference in the survival times between patients treated with surgical resection only (range 8 - 3,720 days; median 96.5 days, n = 26) compared with surgery/chemotherapy (range 150 - 1,080 days, median 420 days, n = 7; log-rank test, z = 2.2; P = 0.0275). Due to the small number of cases for other treatment modalities, only range and median survival times are provided here: surgery/chemotherapy/radiation (range 1,020 - 1,440 days, median 1,230 days, n = 2); upfront surgery but with unknown subsequent therapy (1,080 days, n = 1); chemotherapy only (median 120 days, n = 1); chemotherapy/radiation (540 days, n = 1), APC (42 days, n = 1); unspecified treatment (range 120 - 510, median 315 days, n = 2).
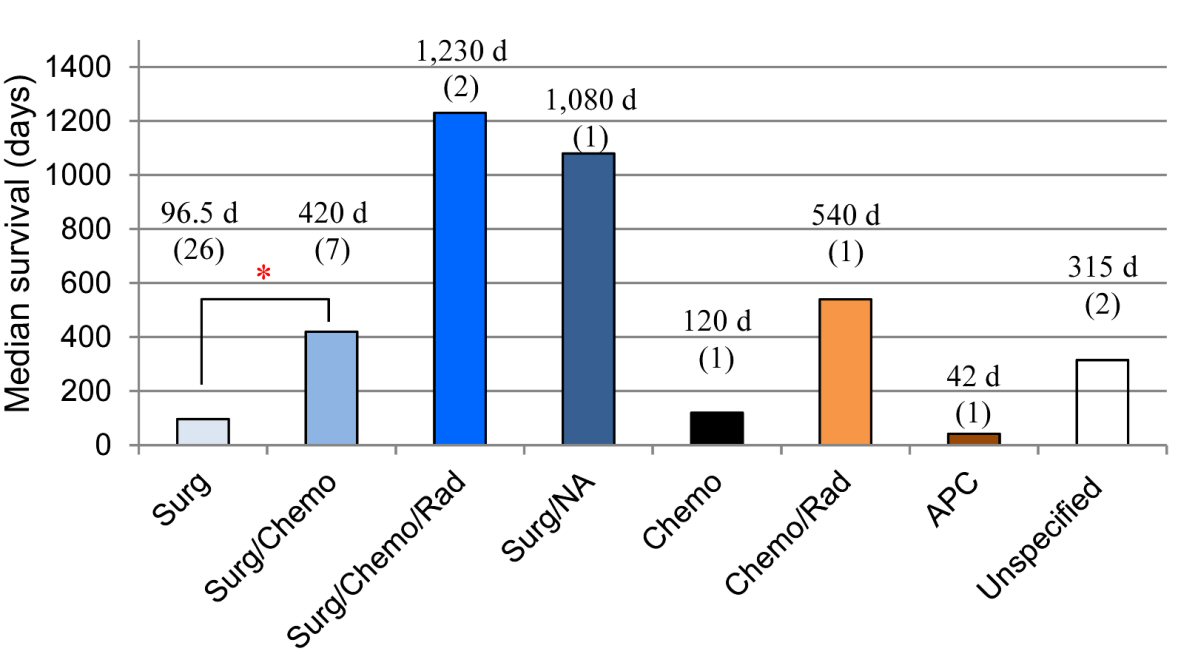 Click for large image | Figure 3. Survival times in different treatment groups. The survival time is longer in patients received resection/chemotherapy compared to that in patients underwent surgery alone (*P = 0.0275; Log-rank test; n = 7 and 26, respectively). The survival times in other treatment groups are not compared due to the limited case numbers. *Compared to treatment with patients treated with surgery only. APC: argon plasma coagulation; Chemo: chemotherapy; NA: the other modality not available; Rad: radiation therapy; Surg: surgery. |
| Discussion | ▴Top |
The incidence, risk factors, symptoms, diagnosis, treatment, and survival time of total of 47 cases of small bowel angiosarcoma reported in English literature are analyzed (including our case). Most of the patients demonstrated nonspecific GI symptoms upon initial presentation and at advanced cancer stages, which might contribute to the late diagnosis and resultant short survival time. The prompt diagnosis not only requires high index of vigilance, but the use of markers such as vWF, UEA-1, and CD31 [1]. The ages at diagnosis range from 25 - 87 years, with median of 68.5 years. Prior radiation exposure was reported in 36% of cases included, with a median latency of 9.5 years, suggesting a causal role of radiation in the carcinogenesis of small bowel angiosarcoma. The overall median survival time is 150 days. Although there is no significant difference in the survival time between the two genders, the median survival time in female seems to be longer than that of male patients (300 vs. 120 days, respectively; P = 0.86). The lack of statistical significance might be related to the small number of cases included.
Due to the rarity of the small intestinal angiosarcoma there remains a lack of randomized clinical trials and recommendations for the treatment. Generally the management includes initial control of anemia and bleeding followed by resection and/or chemotherapy. Surgery is the first line modality for localized resectable tumors, which may also be used for palliative relief in patients with metastatic disease. For localized angiosarcoma, complete radical resection (R0) with wide margins is the primary treatment of choice. However, involved margins (R1 or R2 resection) are common due to diffuse tissue infiltration, and often multifocal characteristics of angiosarcoma, which leads to a worse prognosis, therefore adjuvant radiation therapy may be employed. Radiation therapy alone is largely inadequate, and further radiotherapy is usually avoided for radiation-induced angiosarcomas. The role of neoadjuvant or adjuvant chemotherapy for angiosarcoma is unclear [1]. The adjuvant therapy regimens are generally empiric and based on the protocols for cutaneous angiosarcoma [17, 21, 31]. Neoadjuvant chemotherapy may be considered in patients with adequate functional status, while adjuvant chemotherapy can be continued in patients responding to initial neoadjuvant chemotherapy.
Although the evidence is limited, the metastatic angiosarcoma is primarily treated with cytotoxic chemotherapy. The drugs used for the treatment of angiosarcoma include paclitaxel, docetaxel, vinorelbine, sorafenib, sunitinib, and bevacizumab [59-65]. The recommended treatment for unresectable or stage IV intra-abdominal soft tissue sarcoma is to downstage the tumor first with combination chemotherapy, chemoradiation or radiation therapy, followed by subsequent treatment based on the resectability. If the tumor is resectable based on the imaging assessment, treatment with surgery, or preoperative radiation or chemotherapy then surgery may be used; if unresectable, palliative treatment with chemotherapy, radiation therapy, surgery for symptomatic control, supportive care can be considered, or observation in asymptomatic patients [65]. However, as described above, the vast majority of the patients with metastatic small bowel angiosarcoma underwent surgical resection with or without additional treatment modalities (25/26, 96.2%).
The addition of chemotherapy to surgery appears to have a survival benefit. The survival time in patients received surgery plus chemotherapy is longer than that in ones underwent surgery only (420 days, n = 7 vs. 96.5 days, n = 26, respectively; P = 0.0019). Although the survival may be affected by the disease stages at diagnosis, it is less likely to explain the difference described above, since a higher percentage of cases received surgery and adjuvant chemotherapy was metastatic (6 out of 7, 85.7%), in contrast to that treated with surgery only (16 out of 26, 61.5%). However, due to the small sample size, the difference in the survival times between those treatment groups may not reflect the true effects of the specific treatment modalities. Further studies are required to determine the optimal treatment strategy for this rare disease entity.
The information provided in this study would help the physicians, especially gastroenterologists, to better understand clinical feature and management of small intestine angiosarcoma and maintain a high level of vigilance, which is essential for the early recognition of this rare but fatal disease. Further studies are needed for the development of better approaches for prevention, early diagnosis, and effective therapy.
| References | ▴Top |
- Young RJ, Brown NJ, Reed MW, Hughes D, Woll PJ. Angiosarcoma. Lancet Oncol. 2010;11(10):983-991.
doi - Hart J, Mandavilli S. Epithelioid angiosarcoma: a brief diagnostic review and differential diagnosis. Arch Pathol Lab Med. 2011;135(2):268-272.
pubmed - Juan CJ, Yu CY, Hsu HH, Chian CP, Huang GS, Fan HC, Chen CY. Visceral and nonvisceral angiosarcomas: Imaging features and clinical correlation. Chinese J Rad. 2000;25(5):183-189.
- Lucas DR. Angiosarcoma, radiation-associated angiosarcoma, and atypical vascular lesion. Arch Pathol Lab Med. 2009;133(11):1804-1809.
pubmed - Al Ali J, Ko HH, Owen D, Steinbrecher UP. Epithelioid angiosarcoma of the small bowel. Gastrointest Endosc. 2006;64(6):1018-1021.
doi pubmed - Al-Daraji W, Husain E, Zelger BG, Zelger B. A practical and comprehensive immunohistochemical approach to the diagnosis of superficial soft tissue tumors. Int J Clin Exp Pathol. 2009;2(2):119-131.
pubmed - Allison KH, Yoder BJ, Bronner MP, Goldblum JR, Rubin BP. Angiosarcoma involving the gastrointestinal tract: a series of primary and metastatic cases. Am J Surg Pathol. 2004;28(3):298-307.
doi pubmed - Aitola P, Poutiainen A, Nordback I. Small-bowel angiosarcoma after pelvic irradiation: a report of two cases. Int J Colorectal Dis. 1999;14(6):308-310.
doi pubmed - Berry GJ, Anderson CJ, Pitts WC, Neitzel GF, Weiss LM. Cytology of angiosarcoma in effusions. Acta Cytol. 1991;35(5):538-542.
pubmed - Butron Vila T, Garcia Villar O, Alonso Garcia S, Bonachia Naranjo O, Perez Espejo G, Lomas Espadas M, Hidalgo Pascual M. Angiosarcoma in the small intestine. Apropos of a particular case. Hepatogastroenterology. 2005;52(64):1139-1142.
pubmed - Chami TN, Ratner LE, Henneberry J, Smith DP, Hill G, Katz PO. Angiosarcoma of the small intestine: a case report and literature review. Am J Gastroenterol. 1994;89(5):797-800.
pubmed - Chen JL, Mok KT, Tseng HH, Wang BW, Liu SI, Chen CW. Duodenal angiosarcoma: an unusual cause of severe gastrointestinal bleeding. J Chin Med Assoc. 2007;70(8):352-355.
doi - Chen KT, Hoffman KD, Hendricks EJ. Angiosarcoma following therapeutic irradiation. Cancer. 1979;44(6):2044-2048.
doi - Cilursu AM. Massive hemorrhage due to angiosarcomatosis diagnosed by intraoperative small bowel endoscopy. Endoscopy. 1991;23(4):245.
doi pubmed - de Mascarenhas-Saraiva MN, da Silva Araujo Lopes LM. Small-bowel tumors diagnosed by wireless capsule endoscopy: report of five cases. Endoscopy. 2003;35(10):865-868.
doi pubmed - Delvaux V, Sciot R, Neuville B, Moerman P, Peeters M, Filez L, Van Beckevoort D, et al. Multifocal epithelioid angiosarcoma of the small intestine. Virchows Arch. 2000;437(1):90-94.
doi pubmed - Zacarias Fohrding L, Macher A, Braunstein S, Knoefel WT, Topp SA. Small intestine bleeding due to multifocal angiosarcoma. World J Gastroenterol. 2012;18(44):6494-6500.
doi pubmed - Fraiman G, Ganti AK, Potti A, Mehdi S. Angiosarcoma of the small intestine: a possible role for thalidomide? Med Oncol. 2003;20(4):397-402.
doi - Grewal JS, Daniel AR, Carson EJ, Catanzaro AT, Shehab TM, Tworek JA. Rapidly progressive metastatic multicentric epithelioid angiosarcoma of the small bowel: a case report and a review of literature. Int J Colorectal Dis. 2008;23(8):745-756.
doi pubmed - Hansen SH, Holck S, Flyger H, Tange UB. Radiation-associated angiosarcoma of the small bowel. A case of multiploidy and a fulminant clinical course. Case report. APMIS. 1996;104(12):891-894.
doi pubmed - Huntington JT, Jones C, Liebner DA, Chen JL, Pollock RE. Angiosarcoma: A rare malignancy with protean clinical presentations. J Surg Oncol. 2015;111(8):941-950.
doi pubmed - Hwang TL, Sun CF, Chen MF. Angiosarcoma of the small intestine after radiation therapy: report of a case. J Formos Med Assoc. 1993;92(7):658-661.
pubmed - Khalil MF, Thomas A, Aassad A, Rubin M, Taub RN. Epithelioid angiosarcoma of the small intestine after occupational exposure to radiation and polyvinyl chloride: a case report and review of literature. Sarcoma. 2005;9(3-4):161-164.
doi pubmed - Kelemen K, Yu QQ, Howard L. Small intestinal angiosarcoma leading to perforation and acute abdomen: a case report and review of the literature. Arch Pathol Lab Med. 2004;128(1):95-98.
pubmed - Knop FK, Hansen MB, Meisner S. Small-bowel hemangiosarcoma and capsule endoscopy. Endoscopy. 2003;35(7):637.
doi pubmed - Liu DS, Smith H, Lee MM, Djeric M. Small intestinal angiosarcoma masquerading as an appendiceal abscess. Ann R Coll Surg Engl. 2013;95(1):e22-24.
doi pubmed - Mohammed A, Aliyu HO, Liman AA, Abdullahi K, Abubakar N. Angiosarcoma of the small intestine. Ann Afr Med. 2011;10(3):246-248.
doi pubmed - Maeyashiki C, Nagata N, Uemura N. Angiosarcoma involving solid organs and the gastrointestinal tract with life-threatening bleeding. Case Rep Gastroenterol. 2012;6(3):772-777.
doi pubmed - Nanus DM, Kelsen D, Clark DG. Radiation-induced angiosarcoma. Cancer. 1987;60(4):777-779.
doi - Navarro-Chagoya D, Figueroa-Ruiz M, Lopez-Gomez J, Nava-Leyva H, Alvarez-Ponce CE, Guzman-Sombrero G, Velazquez-Garcia J. Obscure gastrointestinal bleeding due to multifocal intestinal angiosarcoma. Int J Surg Case Rep. 2015;10:169-172.
doi pubmed - Ni Q, Shang D, Peng H, Roy M, Liang G, Bi W, Gao X. Primary angiosarcoma of the small intestine with metastasis to the liver: a case report and review of the literature. World J Surg Oncol. 2013;11:242.
doi pubmed - Ogawa S, Minowa O, Ozaki Y, Kuwatsuru R, Sumi Y, Maehara T. Small bowel intussusception caused by intestinal angiosarcomatosis: usefulness of MR enteroclysis with infusion of water through a nasojejunal catheter. Eur Radiol. 2002;12(3):534-536.
doi pubmed - Ordonez NG, del Junco GW, Ayala AG, Ahmed N. Angiosarcoma of the small intestine: an immunoperoxidase study. Am J Gastroenterol. 1983;78(4):218-221.
pubmed - Policarpio-Nicolas ML, Nicolas MM, Keh P, Laskin WB. Postradiation angiosarcoma of the small intestine: a case report and review of literature. Ann Diagn Pathol. 2006;10(5):301-305.
doi pubmed - Selk A, Wehrli B, Taylor BM. Chylous ascites secondary to small-bowel angiosarcoma. Can J Surg. 2004;47(5):383-384.
pubmed - Siderits R, Poblete F, Saraiya B, Rimmer C, Hazra A, Aye L. Angiosarcoma of small bowel presenting with obstruction: novel observations on a rare diagnostic entity with unique clinical presentation. Case Rep Gastrointest Med. 2012;2012:480135.
doi - Su CC, Jin YT, Chien CH, Yu CY, Lin PW. Postirradiation angiosarcoma of the terminal ileum. Zhonghua Yi Xue Za Zhi (Taipei). 1991;48(2):147-152.
- Suzuki F, Saito A, Ishi K, Koyatsu J, Maruyama T, Suda K. Intra-abdominal angiosarcomatosis after radiotherapy. J Gastroenterol Hepatol. 1999;14(3):289-292.
doi pubmed - Takahashi M, Ohara M, Kimura N, Domen H, Yamabuki T, Komuro K, Tsuchikawa T, et al. Giant primary angiosarcoma of the small intestine showing severe sepsis. World J Gastroenterol. 2014;20(43):16359-16363.
doi pubmed - Taxy JB, Battifora H. Angiosarcoma of the gastrointestinal tract. A report of three cases. Cancer. 1988;62(1):210-216.
doi - Turan M, Karadayi K, Duman M, Ozer H, Arici S, Yildirir C, Kocak O, et al. Small bowel tumors in emergency surgery. Ulus Travma Acil Cerrahi Derg. 2010;16(4):327-333.
pubmed - Usuda H, Naito M. Multicentric angiosarcoma of the gastrointestinal tract. Pathol Int. 1997;47(8):553-556.
doi pubmed - Watanabe K, Hoshi N, Suzuki T, Suzuki T. Epithelioid angiosarcoma of the intestinal tract with endothelin-1-like immunoreactivity. Virchows Arch A Pathol Anat Histopathol. 1993;423(4):309-314.
doi pubmed - Wolov RB, Sato N, Azumi N, Lack EE. Intra-abdominal "angiosarcomatosis" report of two cases after pelvic irradiation. Cancer. 1991;67(9):2275-2279.
doi - Zemheria E, Engina P, Ozkanlia S, Ozemir IA. Primary angiosarcoma of small intestine presenting with intestinal perforation: a case report. J Med Cases. 2014;5(2):113-115.
doi - Fanburg-Smith JC, Furlong MA, Childers EL. Oral and salivary gland angiosarcoma: a clinicopathologic study of 29 cases. Mod Pathol. 2003;16(3):263-271.
doi pubmed - Folpe AL, Chand EM, Goldblum JR, Weiss SW. Expression of Fli-1, a nuclear transcription factor, distinguishes vascular neoplasms from potential mimics. Am J Surg Pathol. 2001;25(8):1061-1066.
doi pubmed - Miettinen M, Lindenmayer AE, Chaubal A. Endothelial cell markers CD31, CD34, and BNH9 antibody to H- and Y-antigens - evaluation of their specificity and sensitivity in the diagnosis of vascular tumors and comparison with von Willebrand factor. Mod Pathol. 1994;7(1):82-90.
pubmed - Ohsawa M, Naka N, Tomita Y, Kawamori D, Kanno H, Aozasa K. Use of immunohistochemical procedures in diagnosing angiosarcoma. Evaluation of 98 cases. Cancer. 1995;75(12):2867-2874.
doi - Poblet E, Gonzalez-Palacios F, Jimenez FJ. Different immunoreactivity of endothelial markers in well and poorly differentiated areas of angiosarcomas. Virchows Arch. 1996;428(4-5):217-221.
doi - Sullivan HC, Edgar MA, Cohen C, Kovach CK, HooKim K, Reid MD. The utility of ERG, CD31 and CD34 in the cytological diagnosis of angiosarcoma: an analysis of 25 cases. J Clin Pathol. 2015;68(1):44-50.
doi pubmed - Schmidt D, von Hochstetter AR. The use of CD31 and collagen IV as vascular markers. A study of 56 vascular lesions. Pathol Res Pract. 1995;191(5):410-414.
doi - Satelli A, Li S. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell Mol Life Sci. 2011;68(18):3033-3046.
doi pubmed - Pusztaszeri MP, Seelentag W, Bosman FT. Immunohistochemical expression of endothelial markers CD31, CD34, von Willebrand factor, and Fli-1 in normal human tissues. J Histochem Cytochem. 2006;54(4):385-395.
doi pubmed - Rao P, Lahat G, Arnold C, Gavino AC, Lahat S, Hornick JL, Lev D, et al. Angiosarcoma: a tissue microarray study with diagnostic implications. Am J Dermatopathol. 2013;35(4):432-437.
doi pubmed - Gill R, O’Donnell RJ, Horvai A. Utility of immunohistochemistry for endothelial markers in distinguishing epithelioid hemangioendothelioma from carcinoma metastatic to bone. Arch Pathol Lab Med. 2009;133(6):967-972.
pubmed - Kahn HJ, Bailey D, Marks A. Monoclonal antibody D2-40, a new marker of lymphatic endothelium, reacts with Kaposi’s sarcoma and a subset of angiosarcomas. Mod Pathol. 2002;15(4):434-440.
doi pubmed - Lee KB, Lee HS, Lee HE, Park SY, Chung JH, Choe G, Kim WH, Song KY. Immunohistochemical characteristics of Kaposi sarcoma and its mimicries. Korean J Pathol. 2006;40:361-367.
- Penel N, Bui BN, Bay JO, Cupissol D, Ray-Coquard I, Piperno-Neumann S, Kerbrat P, et al. Phase II trial of weekly paclitaxel for unresectable angiosarcoma: the ANGIOTAX Study. J Clin Oncol. 2008;26(32):5269-5274.
doi pubmed - Schlemmer M, Reichardt P, Verweij J, Hartmann JT, Judson I, Thyss A, Hogendoorn PC, et al. Paclitaxel in patients with advanced angiosarcomas of soft tissue: a retrospective study of the EORTC soft tissue and bone sarcoma group. Eur J Cancer. 2008;44(16):2433-2436.
doi pubmed - Maki RG, D’Adamo DR, Keohan ML, Saulle M, Schuetze SM, Undevia SD, Livingston MB, et al. Phase II study of sorafenib in patients with metastatic or recurrent sarcomas. J Clin Oncol. 2009;27(19):3133-3140.
doi pubmed - George S, Merriam P, Maki RG, Van den Abbeele AD, Yap JT, Akhurst T, Harmon DC, et al. Multicenter phase II trial of sunitinib in the treatment of nongastrointestinal stromal tumor sarcomas. J Clin Oncol. 2009;27(19):3154-3160.
doi pubmed - Agulnik M, Yarber JL, Okuno SH, von Mehren M, Jovanovic BD, Brockstein BE, Evens AM, et al. An open-label, multicenter, phase II study of bevacizumab for the treatment of angiosarcoma and epithelioid hemangioendotheliomas. Ann Oncol. 2013;24(1):257-263.
doi pubmed - Nagano T, Yamada Y, Ikeda T, Kanki H, Kamo T, Nishigori C. Docetaxel: a therapeutic option in the treatment of cutaneous angiosarcoma: report of 9 patients. Cancer. 2007;110(3):648-651.
doi pubmed - National Comprehensive Cancer Network. Soft tissue sarcoma. Version 2.1.2017. Copy right© National Comprehensive Cancer Network. Inc., 2017, All right reserved.
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


