| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Original Article
Volume 9, Number 9, September 2017, pages 759-764
The Cluster of Abnormalities Related to Metabolic Syndrome Is Associated With Reduced Glomerular Filtration Rate and Raised Albuminuria in Patients With Type 2 Diabetes Mellitus
Miki Kurataa, b, Akiko Takenouchia, Ayaka Tsuboib, c, Satomi Minatob, d, Mika Takeuchia, Kaori Kitaokab, e, Keisuke Fukuoa, b, Tsutomu Kazumib, f, g
aDepartment of Food Sciences and Nutrition, School of Human Environmental Sciences, Mukogawa Women’s University, Nishinomiya, Hyogo, Japan
bResearch Institutes for Nutrition Sciences, Mukogawa Women’s University, Nishinomiya, Hyogo, Japan
cDepartment of Nutrition, Osaka City Juso Hospital, Osaka, Japan
dGraduate School of Human Science and Environment, University of Hyogo, Himeji, Hyogo, Japan
eDepartment of Nutritional Sciences for Well-Being, Faculty of Health Sciences for Welfare, Kansai University of Welfare Sciences, Kashiwara, Osaka, Japan
fDiabetes Division, Department of Medicine, Kohnan Kakogawa Hospital, Kakogawa, Hyogo, Japan
gCorresponding Author: Tsutomu Kazumi, Research Institute for Nutrition Sciences, Mukogawa Women’s University, 6-46, Ikebiraki-cho, Nishinomiya, Hyogo 663-8558, Japan
Manuscript submitted May 25, 2017, accepted June 13, 2017
Short title: MS and CKD in T2DM
doi: https://doi.org/10.14740/jocmr3097w
| Abstract | ▴Top |
Background: As association of metabolic syndrome (MS) with chronic kidney disease (CKD) has not been extensively studied in patients with type 2 diabetes, we addressed these issues.
Methods: Intrapersonal means of 12 measurements of waist circumference, blood pressure and high-density lipoprotein (HDL) cholesterol and those of six measurements of fasting triglycerides during 12 months were calculated in a cohort of 168 previously reported Japanese patients with type 2 diabetes. Based on these means, MS was diagnosed according to the modified National Cholesterol Education Program Adult Treatment Panel III criteria with the Asian definition of abdominal obesity. CKD was defined as the presence of low estimated glomerular filtration rate (eGFR < 60 mL/min/1.73 m2), albuminuria (urinary albumin/creatinine ratio (ACR) ≥ 30 mg/g) or both.
Results: Of 168 patients, 77 patients (46 %) had MS and 67 (40 %) had CKD. As the number of MS components increased from 1 through 5, the prevalence of albuminuria (9%, 38%, 30%, 41%, and 50%, P < 0.001), low eGFR (0%, 10%, 24%, 22%, and 50%, P < 0.001) and consequently, CKD increased (9%, 41%, 48%, 52%, and 75%, P < 0.001). Urinary ACR increased and eGFR decreased as a function of the number of MS components. As compared to patients without MS, prevalence of low eGFR (26% vs. 7%, P = 0.001) and CKD (52% vs. 30%, P = 0.005) was higher in patients with MS but prevalence of albuminuria did not differ (36% vs. 27%, P = 0.2).
Conclusion: In Japanese patients with type 2 diabetes, the cluster of abnormalities related to MS was associated not only with higher prevalence of albuminuria, reduced kidney function and hence the increase in CKD but also with corresponding changes in urinary ACR and eGFR.
Keywords: Metabolic syndrome; Chronic kidney disease; Type 2 diabetes; Albuminuria; Reduced kidney function
| Introduction | ▴Top |
Chronic kidney disease (CKD) [1-4] and metabolic syndrome (MS) [5-7] are each independently associated with cardiovascular disease and are also positively associated with each other. CKD is currently defined as an estimated glomerular filtration rate (eGFR) of less than 60 mL/min/1.73 m2 or a urine albumin-to-creatinine ratio (ACR) of 30 mg/g or higher [2]. There are several definitions for MS being proposed by various international regulatory bodies [8]. International Diabetes Federation, National Heart, Lung and Blood Institute, American Heart Association, World Heart Federation, International Atherosclerosis Society and International Association for the Study of Obesity have proposed a new harmonized definition, which do not consider central obesity as an obligatory component [9].
A meta-analysis revealed that the presence of MS and the number of MS components were associated with the development of CKD [10]. This meta-analysis used 11 prospective cohort studies in middle-aged people from Asian and Western countries. We have recently shown this association in Japanese elderly women [11]. However, association between MS with CKD has not been extensively studied in patients with type 2 diabetes. One of the reasons may be that most patients with type 2 diabetes mellitus will have MS [9], specifically in Western countries. For example, more than 90% of type 2 diabetes patients in a UK hospital had MS and their body mass index (BMI) averaged 33 kg/m2 [12]. Therefore, we evaluated association between the two conditions in Japanese patients with type 2 diabetes who had a mean BMI < 25 kg/m2 [7, 13].
| Patients and Methods | ▴Top |
We here showed results of 168 patients with type 2 diabetes, whose details have been reported elsewhere [13, 14]. They had been regularly attending the clinic for more than 6 months prior to enrollment and had eight or more monthly visits with anthropometric and blood pressure (BP) measurements and blood samplings during the following 12 months after enrollment. Patients with hepatitis B surface antigen or antibodies against hepatitis C virus were excluded. Those who had aspartate aminotransferase and alanine aminotransferase of 100 U/L and greater, serum creatinine ≥ 2.0 mg/dL were excluded as well. Study protocol was consistent with the Japanese Government’s Ethical Guidelines Regarding Epidemiological Studies in accordance with the Declaration of Helsinki.
For each subject on each monthly visit, waist circumference and body weight were measured. BP was measured by registered nurses on each monthly visit using a sphygmomanometer after patients sat and rested for at least 5 min. Blood was withdrawn at 2 h after breakfast taken at home and after an overnight fasting every other month as previously reported in details [14]. Plasma glucose (PG), serum lipids and lipoproteins, creatinine, hepatic enzymes, uric acid and other blood tests were measured by standard methods using an autoanalyzer. HbA1C values were determined by high performance liquid chromatography. Low-density lipoprotein (LDL) cholesterol was calculated using Friedewald’s formula in blood samples taken after an overnight fasting. Complete blood cell count was analyzed using an automated blood cell counter.
In 153 patients (91%), blood was withdrawn on two occasions as described above. In the remaining 15 patients, blood was obtained after an overnight fasting. In each patient, we calculated a mean of 12 measurements of BMI, waist circumference, total and HDL cholesterol, HbA1c, uric acid, BP, serum protein and hepatic enzymes. Six measurements of fasting and postbreakfast glucose, LDL cholesterol, fasting and postbreakfast triglycerides (TG) averaged in each patient as well. These means were shown in Table 1 and were used for the diagnosis of MS.
 Click to view | Table 1. Clinical Features of 168 Type 2 Diabetes Patients With and Without Metabolic Syndrome |
MS was defined according to the modified criteria of the National Cholesterol Education Program Adult Treatment Panel III guidelines [9]. Abdominal obesity was defined as a waist circumference greater than 85 cm in men and greater than 90 cm in women, according to the Japan Society for the Study of Obesity criteria [15]. Elevated BP was defined as systolic/diastolic BPs of 130/85 mm Hg or greater and/or current use of antihypertensive medicine. Hypertriglyceridemia was defined as a serum fasting TG level of 150 mg/dL and/or current use of fibrates. Low HDL cholesterol level was defined as less than 50 mg/dL in women and as less than 40 mg/dL in men. MS was defined as the presence of three or more components [9]. Patients in the present study had at least one component as all patients had diabetes.
Urinary albumin was measured once during the first 3 - 4 months after enrollment in random urine samples using a turbidimetric immunoassay and expressed as ACR. Serum and urinary creatinine were measured enzymatically and eGFR was determined using the equation recommended by the Japanese Society for Nephrology [16]. Low eGFR was defined as eGFR < 60 mL/min/1.73 m2 and albuminuria was defined as ACR ≥ 30 mg/g [2]. CKD was defined as the presence of low eGFR, albuminuria or both [2]. We used means of eGFR calculated in each patient using 2 - 4 measurements of creatinine during the first 3 - 4 months.
Data were presented as mean ± SE unless otherwise stated. Differences between two groups were analyzed by t-test and frequencies of conditions by Chi-square tests. The association of continuous variables with the number of components of MS and Ptrend was derived using Jonckheeree-Terpstra test. In categorical data analysis, Cochran-Armitage trend analysis was used. Analysis of variance also was used when appropriate. A two-tailed P < 0.05 was considered statistically significant. All calculations were performed with SPSS system 15.0 (SPSS Inc., Chicago, IL).
| Results | ▴Top |
As previously reported [13, 14], patients had relatively good glycemic, lipid and BP control. Of 168 patients, 77 and 67 patients (46% and 40%, respectively) met MS and CKD criteria. Among 67 patients with CKD, 41 patients had albuminuria alone, 14 had low eGFR alone and 12 had both. Among components of MS other than hyperglycemia (all participants had diabetes), elevated BP was the most prevalent (111 patients, 66%; hypertension in 91 patients), followed by abdominal obesity in 63 patients (38%) and then hypertriglyceridemia (38 patients, 23%) and low HDL cholesterol (43 patients, 26%).
Patients with MS as compared to those without had a higher prevalence of CKD (Fig. 1). This was due to higher prevalence of low eGFR (Fig. 1) although there was no difference in mean eGFR (Table 1). In contrast, prevalence of albuminuria did not differ between the two groups (Fig. 1) although mean urinary ACR (log-transformed) was higher in patients with MS than those without (Table 1). By definitions, waist circumference, BMI, BP and fasting TG were higher and HDL cholesterol was lower in patients with MS compared to those without (Table 1). However, the two groups did not differ in fasting PG, HbA1c, age, sex, duration of diabetes and therapy for diabetes (Table 1).
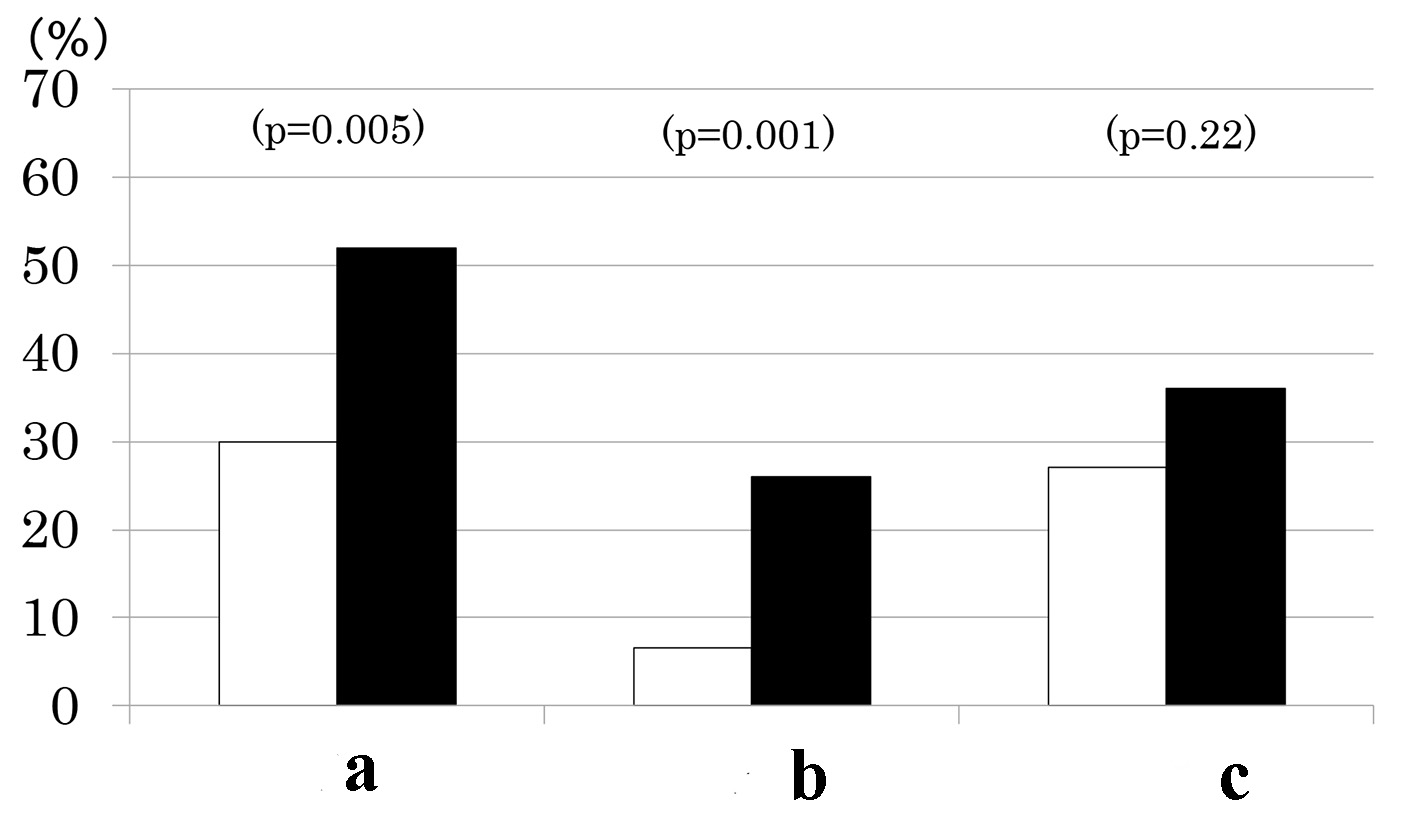 Click for large image | Figure 1. Prevalence of chronic kidney disease (a), low estimated glomerular filtration rate (eGFR < 60 mL/min/1.73 m2) (b) and elevated albuminuria (urinary albumin/creatinine ratio ≥ 30 mg/g) (c) in type 2 diabetes patients with (black columns) and without metabolic syndrome (white columns). |
Number of participants with 1 through 5 MS components was 33 (20%), 58 (35%), 42 (25%), 27 (16%) and 8 (5%), respectively. As the number of components of MS increased, the prevalence of low eGFR (Fig. 2a) and the mean of eGFR decreased (Fig. 2b). Similarly, the prevalence of albuminuria (Fig. 3a) and log ACR increased (Fig. 3b); hence CKD prevalence increased as a function of the number of MS components (Fig. 4). Of 58 patients with two components, 44 patients (76%) had elevated BP and hence mean BP of patients with two components (130 ± 2/73 ± 1 mm Hg) was higher than those with one component (118 ± 1/68 ± 1 mm Hg) and similarly elevated to those with three and four components (131 ± 2/74 ± 1 and 130 ± 2/74 ± 1 mm Hg, respectively) (P < 0.001). Although albuminuria prevalence increased as a function of the number of MS components (Fig. 3a), it was higher in patients with two components than those with three components and was increased to the similar degree in patients with four components (38%, 30% and 41%, respectively). This may be related to no difference in albuminuria prevalence between patients with and without MS (Fig. 1c). There was no difference in age, sex, duration of diabetes and therapy for diabetes among five groups of patients (data not shown).
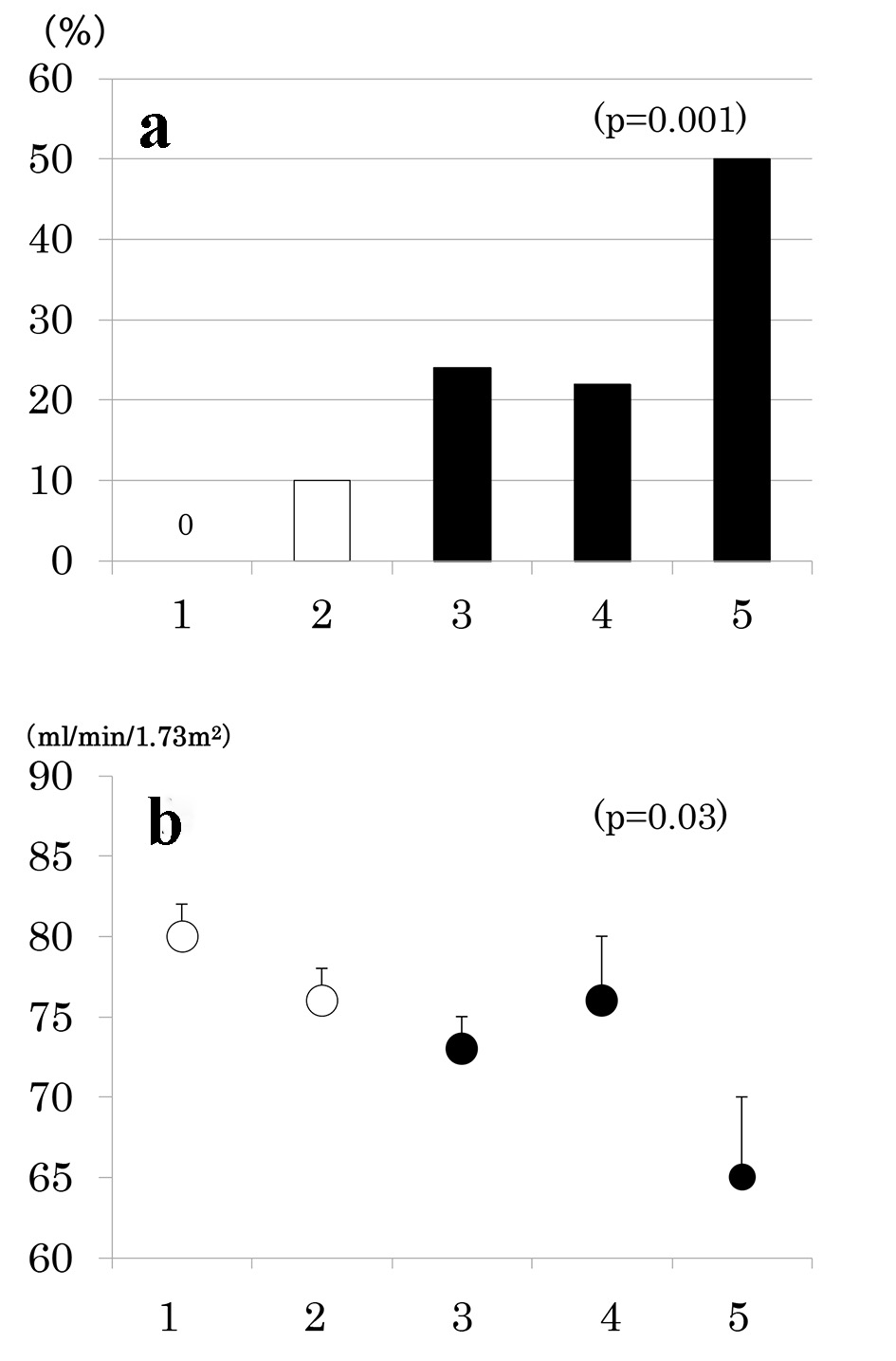 Click for large image | Figure 2. Prevalence of low estimated glomerular filtration rate (eGFR < 60 mL/min/1.73 m2) (a) and mean ± SE of eGFR (b) as a function of the number of components of metabolic syndrome. |
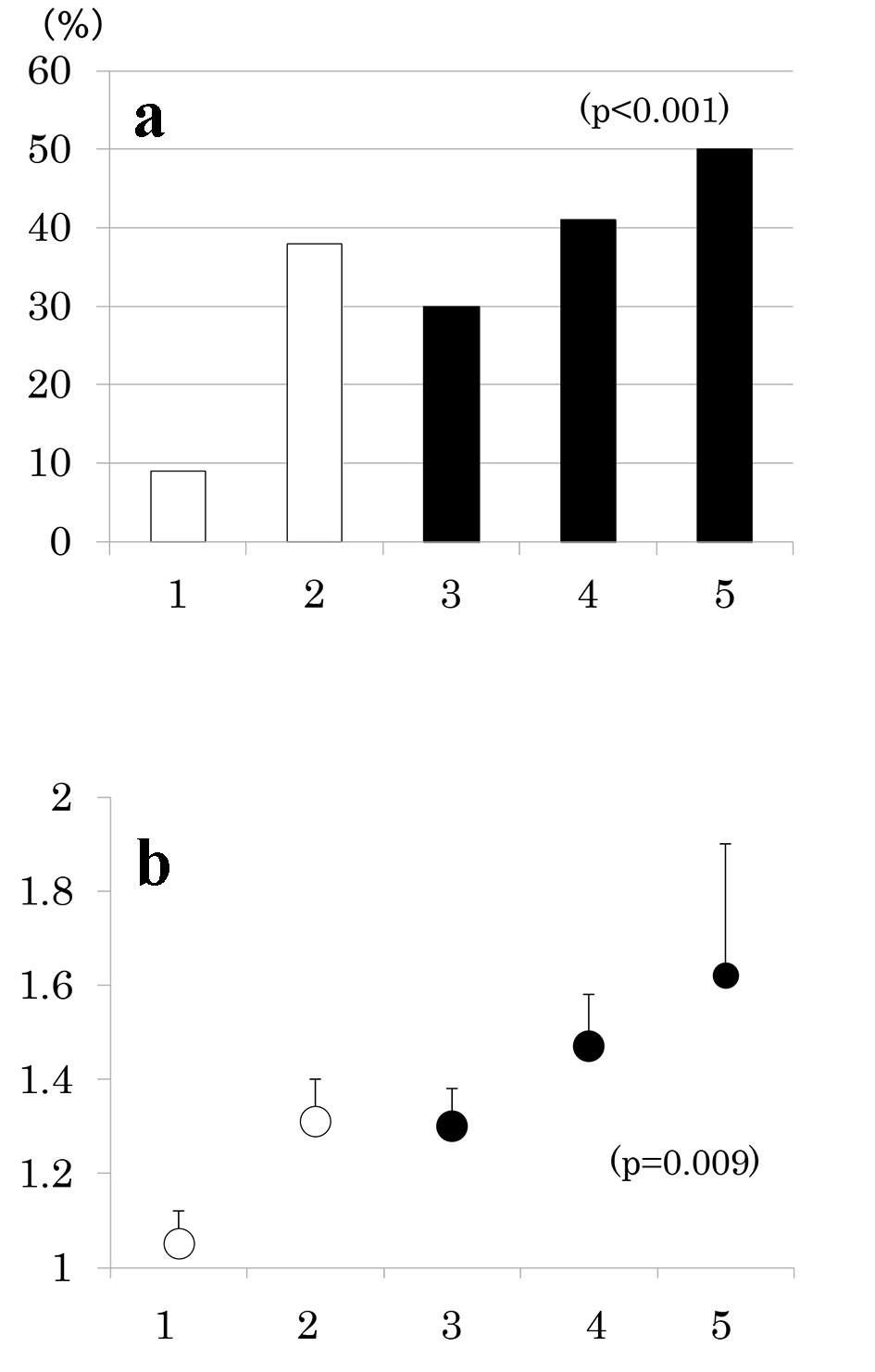 Click for large image | Figure 3. Prevalence of albuminuria (urinary albumin/creatinine ratio (ACR) ≥ 30 mg/g) (a) and mean ± SE of log ACR (b) as a function of the number of components of metabolic syndrome. |
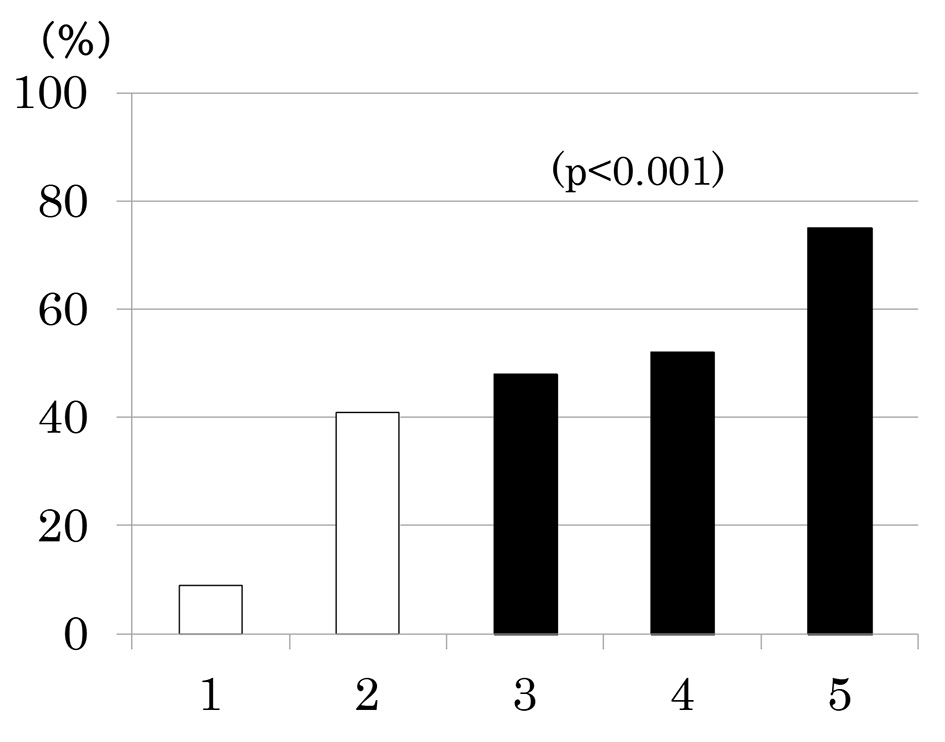 Click for large image | Figure 4. Prevalence of chronic kidney disease (low estimated glomerular filtration rate and/or albuminuria) as a function of the number of components of metabolic syndrome. |
| Discussion | ▴Top |
The present study has demonstrated in Japanese patients with type 2 diabetes with relatively good glycemic, lipid and BP control and preserved kidney function that the cluster of abnormalities related to MS was associated not only with higher prevalence of albuminuria, reduced GFR and hence the increase in CKD but also with corresponding changes in urinary ACR and eGFR. Although MS was not associated with albuminuria it was associated with reduced GFR and hence the increase in CKD. It is noted that in the present study MS was diagnosed using intrapersonal means of 12 measurements of waist circumference, BP and HDL cholesterol and those of six measurements of fasting TG during 12 months. Further, eGFR was estimated using intrapersonal means of 2 - 4 measurements of serum creatinine during 3 - 4 months in each patient and was used for diagnosis of low eGFR and statistical analyses although ACR was measured once.
In a community-based sample of elderly men [17], lower insulin sensitivity, assessed using euglycemic clamp, was associated with lower renal function, assessed using cystatin C-based GFR, in cross-sectional and longitudinal analyses. In type 2 diabetic patients [18], there was a significant cross-sectional positive association between insulin sensitivity (by euglycemic hyperinsulinemic clamp) and measured GFR (by EDTA). In addition, the cluster of abnormalities related to MS was strongly associated with reduced GFR [18]. Further, a cross-sectional [19] and a 5-year prospective study [20] of patients with type 2 diabetes of the Hong Kong Diabetes Registry reported that the presence of MS independently associated with the presence and the development of reduced kidney function, respectively. These findings may be consistent with our present observation that both the presence of MS and the number of MS components were associated not only with higher prevalence of reduced eGFR but also with corresponding changes in eGFR in Japanese patients with type 2 diabetes with relatively good glycemic, lipid and BP control and preserved kidney function.
Previous studies [21-23] reported that the presence of MS and the cluster of abnormalities related to MS are associated with higher prevalence of albuminuria and higher levels of urinary ACR in patients with type 2 diabetes mellitus. In the present study, however, although levels of urinary ACR were higher in type 2 diabetes patients with MS compared with those without MS, prevalence of albuminuria did not differ. This may be because type 2 diabetes patients with two components had higher systolic and diastolic BP, with 64% having hypertension and 12% having high normal BP, defined as systolic/diastolic BP; 130 - 139/85 - 89 mm Hg, compared with those with only one MS component.
The strength of the current study is that the diagnosis of low eGFR was based on 2 - 4 creatinine levels measured for 3 - 4 months. In addition, waist circumference, BP, fasting TG and HDL cholesterol were repeatedly measured throughout 12 months. It has been clearly demonstrated that the prevalence of MS is higher in winter than in summer in Japanese male workers in general, in subjects aged greater than or equal to 40 years in particular [24]. This is due to higher serum levels of HDL cholesterol, fasting glucose and systolic and diastolic BP in winter than in summer. In the present study, however, seasonal variations in the diagnosis of MS had been avoided. Major limitations are that study participants were small in number and from a single clinic in Japan. However, the characteristics of our study participants are similar to those reported in a previous large-scale study in Japan [25]. We used eGFR rather than more precise measures of kidney function, like iothalamate clearance. In addition, this cohort of participants consisted of Japanese only, which limits generalizability.
Conclusion
The cluster of abnormalities related to MS was associated not only with higher prevalence of albuminuria, reduced kidney function and hence the increase in CKD but also with corresponding changes in urinary ACR and eGFR in Japanese patients with type 2 diabetes with relatively good glycemic, lipid and BP control and preserved kidney function.
Acknowledgments
We are indebted to all the participants for their dedicated and conscientious collaboration.
Conflicts of Interest
We declare that we have no conflicts of interest.
Author Contributions
MK, AKT, AYT, SM, MT and KK have made substantial contributions to acquisition, analysis and interpretation of data. KF has been involved in drafting the manuscript. TK has been involved in revising it critically for important intellectual content, has given final approval of the version to be published, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors read and approved the final manuscript.
Abbreviations
ACR: albumin/creatinine ratio; BP: blood pressure; CKD: chronic kidney disease; eGFR: estimated glomerular filtration rate; MS: metabolic syndrome; PG: plasma glucose; TG: triglycerides
| References | ▴Top |
- Obrador GT, Pereira BJ, Kausz AT. Chronic kidney disease in the United States: an underrecognized problem. Semin Nephrol. 2002;22(6):441-448.
doi pubmed - National Kidney F. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1-266.
pubmed - Ninomiya T, Kiyohara Y, Kubo M, Tanizaki Y, Doi Y, Okubo K, Wakugawa Y, et al. Chronic kidney disease and cardiovascular disease in a general Japanese population: the Hisayama Study. Kidney Int. 2005;68(1):228-236.
doi pubmed - Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296-1305.
doi pubmed - Isomaa B, Almgren P, Tuomi T, Forsen B, Lahti K, Nissen M, Taskinen MR, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24(4):683-689.
doi pubmed - Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288(21):2709-2716.
doi pubmed - Sone H, Mizuno S, Fujii H, Yoshimura Y, Yamasaki Y, Ishibashi S, Katayama S, et al. Is the diagnosis of metabolic syndrome useful for predicting cardiovascular disease in asian diabetic patients? Analysis from the Japan Diabetes Complications Study. Diabetes Care. 2005;28(6):1463-1471.
doi pubmed - Kassi E, Pervanidou P, Kaltsas G, Chrousos G. Metabolic syndrome: definitions and controversies. BMC Med. 2011;9:48.
doi pubmed - Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640-1645.
doi pubmed - Thomas G, Sehgal AR, Kashyap SR, Srinivas TR, Kirwan JP, Navaneethan SD. Metabolic syndrome and kidney disease: a systematic review and meta-analysis. Clin J Am Soc Nephrol. 2011;6(10):2364-2373.
doi pubmed - Kurata M, Tsuboi A, Takeuchi M, Fukuo K, Kazumi T. Association of Metabolic Syndrome with chronic kidney disease in elderly Japanese women: comparison by estimation of glomerular filtration rate from Creatinine, Cystatin C, and both. Metab Syndr Relat Disord. 2016;14(1):40-45.
doi pubmed - Song SH, Hardisty CA. Diagnosing metabolic syndrome in type 2 diabetes: does it matter? QJM. 2008;101(6):487-491.
doi pubmed - Takenouchi A, Tsuboi A, Kurata M, Fukuo K, Kazumi T. Carotid intima-media thickness and visit-to-visit HbA1c variability predict progression of chronic kidney disease in type 2 diabetic patients with preserved kidney function. J Diabetes Res. 2016;2016:3295747.
doi pubmed - Takenouchi A, Tsuboi A, Terazawa-Watanabe M, Kurata M, Fukuo K, Kazumi T. Direct association of visit-to-visit HbA1c variation with annual decline in estimated glomerular filtration rate in patients with type 2 diabetes. J Diabetes Metab Disord. 2015;14:69.
doi pubmed - Oka R, Kobayashi J, Yagi K, Tanii H, Miyamoto S, Asano A, Hagishita T, et al. Reassessment of the cutoff values of waist circumference and visceral fat area for identifying Japanese subjects at risk for the metabolic syndrome. Diabetes Res Clin Pract. 2008;79(3):474-481.
doi pubmed - Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53(6):982-992.
doi pubmed - Nerpin E, Riserus U, Ingelsson E, Sundstrom J, Jobs M, Larsson A, Basu S, et al. Insulin sensitivity measured with euglycemic clamp is independently associated with glomerular filtration rate in a community-based cohort. Diabetes Care. 2008;31(8):1550-1555.
doi pubmed - De Cosmo S, Trevisan R, Minenna A, Vedovato M, Viti R, Santini SA, Dodesini AR, et al. Insulin resistance and the cluster of abnormalities related to the metabolic syndrome are associated with reduced glomerular filtration rate in patients with type 2 diabetes. Diabetes Care. 2006;29(2):432-434.
doi pubmed - Luk AO, Ma RC, So WY, Yang XL, Kong AP, Ozaki R, Ko GT, et al. The NCEP-ATPIII but not the IDF criteria for the metabolic syndrome identify Type 2 diabetic patients at increased risk of chronic kidney disease. Diabet Med. 2008;25(12):1419-1425.
doi pubmed - Luk AO, So WY, Ma RC, Kong AP, Ozaki R, Ng VS, Yu LW, et al. Metabolic syndrome predicts new onset of chronic kidney disease in 5,829 patients with type 2 diabetes: a 5-year prospective analysis of the Hong Kong Diabetes Registry. Diabetes Care. 2008;31(12):2357-2361.
doi pubmed - Esteghamati A, Rashidi A, Khalilzadeh O, Ashraf H, Abbasi M. Metabolic syndrome is independently associated with microalbuminuria in type 2 diabetes. Acta Diabetol. 2010;47(2):125-130.
doi pubmed - Hanai K, Babazono T, Iwamoto Y. Renal manifestations of metabolic syndrome in type 2 diabetes. Diabetes Res Clin Pract. 2008;79(2):318-324.
doi pubmed - Bianchi C, Penno G, Daniele G, Russo E, Giovannitti MG, Del Prato S, Miccoli R. The metabolic syndrome is related to albuminuria in Type 2 diabetes. Diabet Med. 2008;25(12):1412-1418.
doi pubmed - Kamezaki F, Sonoda S, Tomotsune Y, Yunaka H, Otsuji Y. Seasonal variation in metabolic syndrome prevalence. Hypertens Res. 2010;33(6):568-572.
doi pubmed - Sone H, Tanaka S, Iimuro S, Tanaka S, Oida K, Yamasaki Y, Oikawa S, et al. Long-term lifestyle intervention lowers the incidence of stroke in Japanese patients with type 2 diabetes: a nationwide multicentre randomised controlled trial (the Japan Diabetes Complications Study). Diabetologia. 2010;53(3):419-428.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


