| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Original Article
Volume 8, Number 12, December 2016, pages 899-907
Evaluation of the Antithrombotic Effects of Rivaroxaban and Apixaban Using the Total Thrombus-Formation Analysis System®: In Vitro and Ex Vivo Studies
Hidekazu Sugiharaa, c, Yoshiaki Idemotoa, c, Takashi Kuwanoa, d, Yoshihisa Nagataa, Joji Moriia, Makoto Sugiharaa, Masahiro Ogawaa, Shin-ichiro Miuraa, b, d, Keijiro Sakua, b
aDepartment of Cardiology, Fukuoka University School of Medicine, Fukuoka 814-0180, Japan
bDepartment of Molecular Cardiovascular Therapeutics, Fukuoka University School of Medicine, Fukuoka 814-0180, Japan
cThese authors contributed equally to this work.
dCorresponding Author: Takashi Kuwano, Department of Cardiology, Fukuoka University School of Medicine, 7-45-1 Nanakuma, Jonan-ku, Fukuoka 814-0180, Japan; Shin-ichiro Miura, Department of Cardiology, Fukuoka University School of Medicine, 7-45-1 Nanakuma, Jonan-ku, Fukuoka 814-0180, Japan
Manuscript accepted for publication October 11, 2016
Short title: Effects of Rivaroxaban and Apixaban
doi: http://dx.doi.org/10.14740/jocmr2773w
| Abstract | ▴Top |
Background: The usefulness of the Total Thrombus-Formation Analysis System® (T-TAS®) for monitoring the anticoagulant effects of non-vitamin K oral anticoagulants (NOACs) in clinical practice has been poorly addressed.
Methods: NOACs (rivaroxaban and apixaban) were added to whole blood from healthy subjects in an in vitro study, and their effects on thrombus formation were evaluated by the T-TAS®. We also evaluated antithrombotic effects using ex vivo samples of whole blood from patients given rivaroxaban or apixaban at the respective trough and peak drug concentrations.
Results: T-TAS® could determine anticoagulant effects in whole blood treated with rivaroxaban or apixaban in vitro. The increases in the anticoagulant effects of rivaroxaban and apixaban from the trough to peak concentrations in whole blood were successfully monitored by the T-TAS® using ex vivo samples. The antithrombotic effects of rivaroxaban and apixaban (in terms of factor Xa inhibition) at the peak were strongly linked to those at the trough.
Conclusion: T-TAS® could be a clinically useful tool for monitoring the anticoagulant effects of factor Xa inhibitors, and may represent an accurate quantitative analysis.
Keywords: Factor Xa inhibitors; Antithrombotic capacities; Total Thrombus-Formation Analysis System; Trough; Peak
| Introduction | ▴Top |
Non-vitamin K oral anticoagulants (NOACs), which include dabigatran, rivaroxaban, apixaban and edoxaban, were developed to prevent stroke in atrial fibrillation and deep vein thrombosis [1]. The safety and efficacy of NOACs in these patients have been generally confirmed by recent clinical trials [2-5]. In contrast with warfarin, which has many drug and food interactions, the dose regimens of NOACs are simple and NOACs do not require routine coagulation monitoring. However, there are some circumstances in which the assessment of hemostasis by laboratory testing could be helpful for clinicians to make decisions regarding patient care, such as in the presence of major bleeding or trauma, prior to emergent surgery, suspected overdose or non-compliance. Although chromogenic anti-Xa activity has been shown to be linear over a wide range of rivaroxaban and apixaban levels [6, 7], the turnaround time of this assay is currently too long for its application in clinical settings. Among common coagulant tests, the prolongation of prothrombin time (PT) depends on the concentration of factor Xa (FXa) inhibitors and is not particularly sensitive. In addition, a normal PT does not necessarily exclude clinically relevant drug concentrations [8, 9]. To date, there has been no definitive tool for monitoring the anticoagulant effects of NOACs.
The Total Thrombus-Formation Analysis System® (T-TAS®) is an automated microchip flow chamber system that can quantitatively analyze thrombus formation using whole blood. Thrombus formation in the microchip capillaries is evaluated under flow and static conditions to more closely reflect physiological function. The T-TAS® was initially developed to monitor the efficacy of anticoagulants [10]. Using the T-TAS®, we recently demonstrated that blood samples from patients who had taken NOACs showed less ex vivo fibrin-rich thrombus formation under a constant low rate flow condition [11]. This study supported the usefulness of the T-TAS® for monitoring the anticoagulant effects of NOACs in clinical practice. However, several limitations were noted. First, the antithrombotic effects of NOACs were only evaluated with samples from patients, and a healthy control group was not examined. It is possible that some patient characteristics other than the use of an anticoagulant medication might influence measurements with the T-TAS®. Second, the analysis was only performed with blood samples taken just before the administration of NOACs (at trough dose levels). Thus, it was unclear whether the antithrombotic effects evaluated by the T-TAS® depended on the concentrations of NOACs. The aim of the present study was to resolve these limitations. In this study, rivaroxaban or apixaban was added in vitro to plasma from healthy subjects, and their effects on thrombus formation were evaluated by the T-TAS®. We also tested ex vivo samples from patients given rivaroxaban or apixaban at their trough and peak concentrations.
| Methods | ▴Top |
In vitro experiment with blood from healthy volunteers
For the first part of the study, we obtained whole blood samples from 20 healthy volunteers who had not taken any medications (12 males and eight females; aged 29 - 54 years). All blood samples were numbered in order of registration. To confirm the in vitro antithrombotic effects of rivaroxaban and apixaban at the expected clinical peak drug level, blood samples were prepared with drugs as follows. Rivaroxaban and apixaban were obtained from Toronto Research Chemicals (Toronto, Ontario, Canada). Rivaroxaban and apixaban were each dissolved in 100% dimethylsulfoxide to yield stock solutions with a concentration of 5 mM. Stock solutions were then diluted in blood samples at the time of assay (first 16 samples in the rivaroxaban group and the rest of four samples in the apixaban group). The target final concentration of rivaroxaban in this experiment was 800 μM, which corresponds to the expected peak concentration (Cmax-equivalent) of rivaroxaban in blood after a dose of 15 mg/day [12]. Similarly, blood samples were spiked with apixaban stock solutions. The final concentration of apixaban was 450 μM, which corresponded to the peak concentration of apixaban in blood after a dose of 10 mg/day clinically [13, 14]. We immediately evaluated the antithrombotic effects of these spiked blood samples in comparison with thrombus formation in control blood by the T-TAS®.
Ex vivo experiment with blood from patients who had taken rivaroxaban or apixaban
In the second part of the study to compare the ex vivo antithrombotic effects of a drug dose at the trough to those of a drug dose at the peak, whole blood samples were obtained from patients at two time points: before and 4 h after drug administration. We enrolled 16 consecutive hospitalized patients who had atrial fibrillation and who had taken either rivaroxaban (n = 6) or apixaban (n = 10) daily for at least 7 days, which was considered to give a steady peak to trough ratio of drug concentrations. Patients who were receiving an antiplatelet drug or anticoagulant drug other than rivaroxaban or apixaban, who were undergoing hemodialysis for renal insufficiency, or who were pregnant were excluded. Six patients were prescribed rivaroxaban and 10 received apixaban. The dose of rivaroxaban was 15 mg once daily, and apixaban was administered at doses of 5 mg twice daily (80% of patients) and 2.5 mg twice daily (20%). To evaluate the effects of the drugs on thrombus formation, all samples were evaluated by the T-TAS® within 1 h after the patient’s blood was drawn by venipuncture. We also evaluated the results of common coagulation tests such as the PT-international normalized ratio (PT-INR) and activated partial thromboplastin time (APTT) at the same two time points.
Evaluation of clinical parameters
The patients’ clinical characteristics and laboratory measurements, including the prevalence of hypertension (HTN), dyslipidemia (DL), diabetes mellitus (DM), medication use, hematocrit, platelet counts and estimated glomerular filtration rate (eGFR), were obtained from medical records. Patients who had a current systolic blood pressure (SBP) ≥ 140 mm Hg and/or diastolic blood pressure (DBP) ≥ 90 mm Hg or who were receiving antihypertensive therapy were considered to have HTN. Patients with low-density lipoprotein cholesterol (LDL-C) ≥ 140 mg/dL, triglycerides (TG) ≥ 150 mg/dL, and/or high-density lipoprotein cholesterol (HDL-C) < 40 mg/dL or who were receiving lipid-lowering therapy were considered to have DL. Patients with random blood glucose (BG) ≥ 200 mg or fasting BG ≥ 126 mg, HbA1c ≥ 6.5% or who were taking a glucose-lowering drug were considered to have DM. Hematological and biochemical laboratory parameters were measured by an auto-analyzer at Fukuoka University Hospital.
Evaluation of thrombogenicity under flow by the T-TAS®
Thrombus formation under flow conditions was evaluated by the T-TAS® (Fujimori Kogyo, Kanagawa, Japan). The T-TAS® is an automated microchip-based flow chamber system that analyzes thrombus formation using two types of single-use microchips, as previously described in detail [11]. Briefly, the capillary of the platelet chip (PL-chip) is coated with type I collagen; platelets from blood samples adhere and aggregate inside the microchip under constant flow conditions. This chip is designed to measure primary hemostasis and it is used for the quantitative evaluation of platelet-specific thrombus formation. The atheroma chip (AR-chip) is coated with type I collagen and tissue thromboplastin and it is designed to measure cumulatively primary and secondary hemostasis. Inside the AR-chip, platelets and the coagulant system are simultaneously activated, which leads to the formation of a fibrin-rich thrombus. These thrombus-forming processes are analyzed by monitoring the changes in internal pressure of the chips. In this study, samples were collected into plastic tubes either containing 3.2% sodium citrate (for AR-chip analysis) or 25 μg/mL hirudin-containing tubes (for PL-chip analysis). Samples were allowed to rest for 1 h after collection, and then loaded in microchips under constant flow rates of 18 and 10 μL/min for the PL-chip and AR-chip, respectively. We analyzed several parameters for each sample. T10 was the time at which the flow pressure increased by 10 kPa from the baseline due to partial occlusion of the capillary, and represented the onset of thrombus formation. T60 and T80 were the times at which the flow pressure increased by 60 and 80 kPa from baseline for the PL-chip and AR chip, respectively. T60 and T80 represented near-occlusion of the capillaries. The area under the flow pressure curve (AUC) was computed to assess thrombogenicity inside the microchips. AR-AUC represented the area under the flow pressure curve for 30 min after the start of perfusion for the AR-chip, and PL-AUC represented the area under the flow pressure curve for 10 min after the start of perfusion for the PL-chip. If a thrombus forms in the capillaries of a microchip early, the internal pressure should rise quickly and the value of AUC should increase.
This study was conducted according to a protocol approved by the Independent Review Board (IRB) of Fukuoka University Hospital (Fukuoka University Hospital IRB: #14-4-04) and registered under UMIN000017228. All subjects gave their written informed consent to participate.
Statistical analysis
The statistical analysis was performed using JMP software, version 12 (SAS Institute, Cary, NC, USA). Values are presented as percentages, means and standard deviation (SD). Categorical and continuous variables were compared between two groups by a Chi-square analysis and unpaired t-test, respectively. For a correlation analysis, the Spearman rank correlation coefficient was used. A value of P < 0.05 was considered significant.
| Results | ▴Top |
In vitro antithrombotic effects of rivaroxaban and apixaban in blood from healthy volunteers
Whole blood samples from healthy volunteers were analyzed for thrombus formation by the T-TAS® with or without drug application. In vitro, rivaroxaban and apixaban significantly suppressed fibrin-rich thrombus formation under flow conditions, whereas these FXa inhibitors did not reduce the rate of platelet thrombus formation (Fig. 1 and Table 1). With regard to AR-chips, rivaroxaban at the estimated clinical peak dose (800 nM, n = 16) significantly suppressed thrombus formation compared to that in control blood samples, as indicated by a lower AR-AUC and prolonged AR-T10 and AR-T80 (AR-AUC for rivaroxaban; 1,605 ± 176 to 831 ± 329 arbitrary units (AU)). Apixaban at a concentration of 450 nM (n = 4) inhibited thrombus formation on AR-chips in a similar fashion (AR-AUC for apixaban; 1,699 ± 42 to 955 ± 141 AU). In contrast, neither rivaroxaban nor apixaban supplementation affected any of the parameters using PL-chips, including PL-AUC, PL-T10 and PL-T60 (Fig. 1 and Table 1). Taken together, these results indicated that in vitro rivaroxaban and apixaban suppressed the coagulant system, but did not affect the function of platelets.
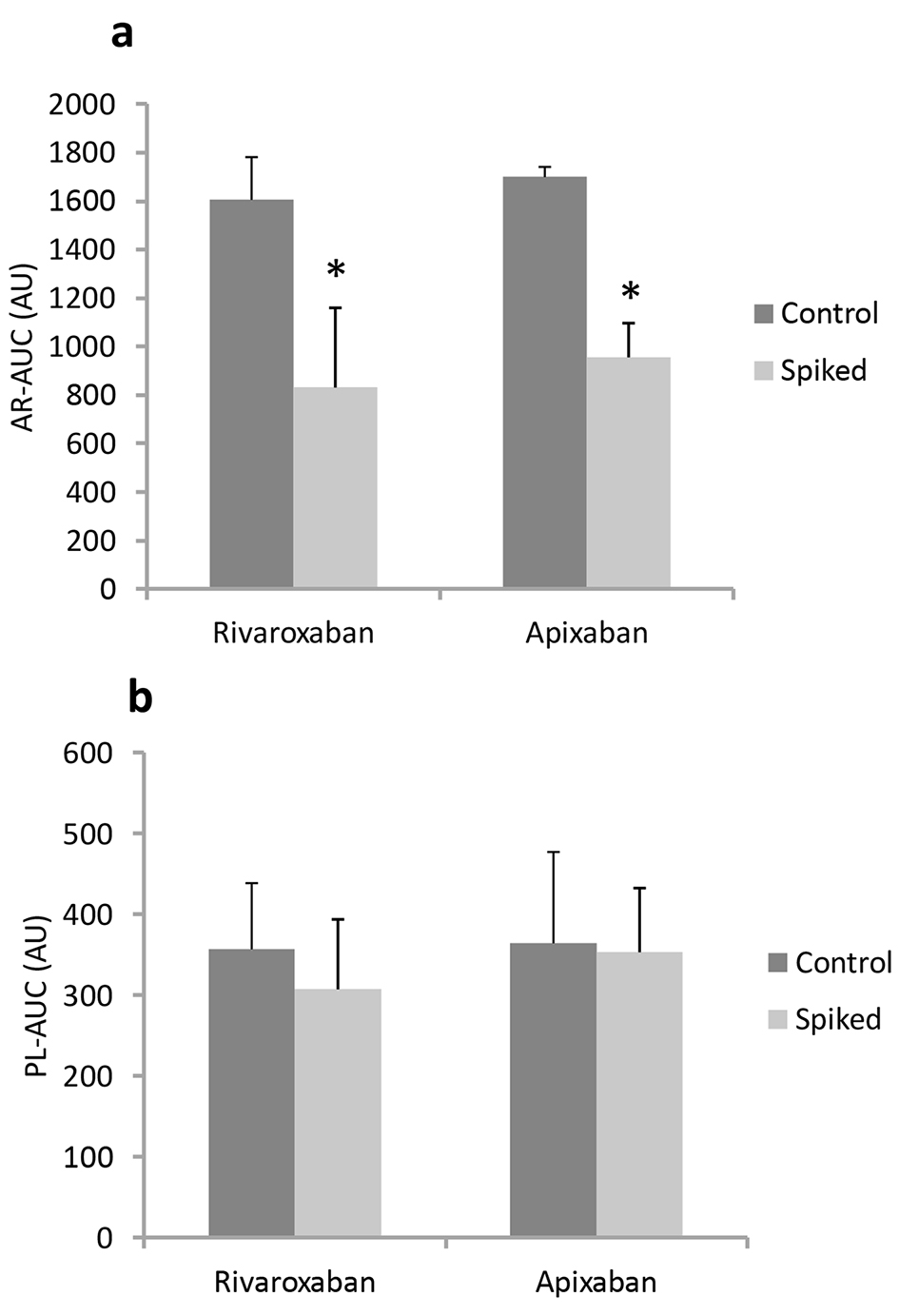 Click for large image | Figure 1. In vitro antithrombotic effects (AR-AUC (a) and PL-AUC (b)) in the absence of (control) or presence of (spiked) rivaroxaban and apixaban in blood from healthy volunteers using AR-chips and PL-chips. *P < 0.05 vs. control. |
 Click to view | Table 1. Changes in T-TAS® Parameters of In Vitro Experiments in the Rivaroxaban and Apixaban |
Baseline patient characteristics
We next tested ex vivo thrombus formation by the T-TAS® using whole blood from patients who had been regularly receiving either rivaroxaban or apixaban. The patient characteristics are shown in Table 2. The apixaban group showed a significantly lower percentage (%) of males and a higher prevalence of DL. There were no significant differences in the other baseline characteristics between the rivaroxaban and apixaban groups.
 Click to view | Table 2. Patient Characteristics in All Patients, the Rivaroxaban and Apixaban Groups |
Antithrombotic effects at the trough and peak concentrations in blood from patients
At the trough, AR-AUC in the rivaroxaban group was significantly higher than that in the apixaban group (1,554 ± 133 vs. 1,193 ± 217 AU, respectively, Fig. 2a). In the rivaroxaban group, the capacity to delay capillary occlusion in AR-chips was significantly enhanced at peak drug levels compared to the trough. AR-AUC was decreased by 23% from trough to peak, and both AR-T10 and AR-T80 were prolonged by 33% (Table 3). Similarly, blood samples collected 4 h after the administration of apixaban had AR-AUC levels 22% lower than those at baseline. AR-T10 and AR-T80 values in the apixaban group also increased by 29% and 20% from the trough to peak, respectively. On the other hand, PL-AUC levels at the trough were similar in the rivaroxaban and apixaban groups (322 ± 79 vs. 252 ± 73 AU, respectively, P = 0.13, Fig. 2b). Moreover, there were no differences in platelet thrombus formation, as reflected by PL-T10, PL-T60 and PL-AUC, between the trough and peak for both groups.
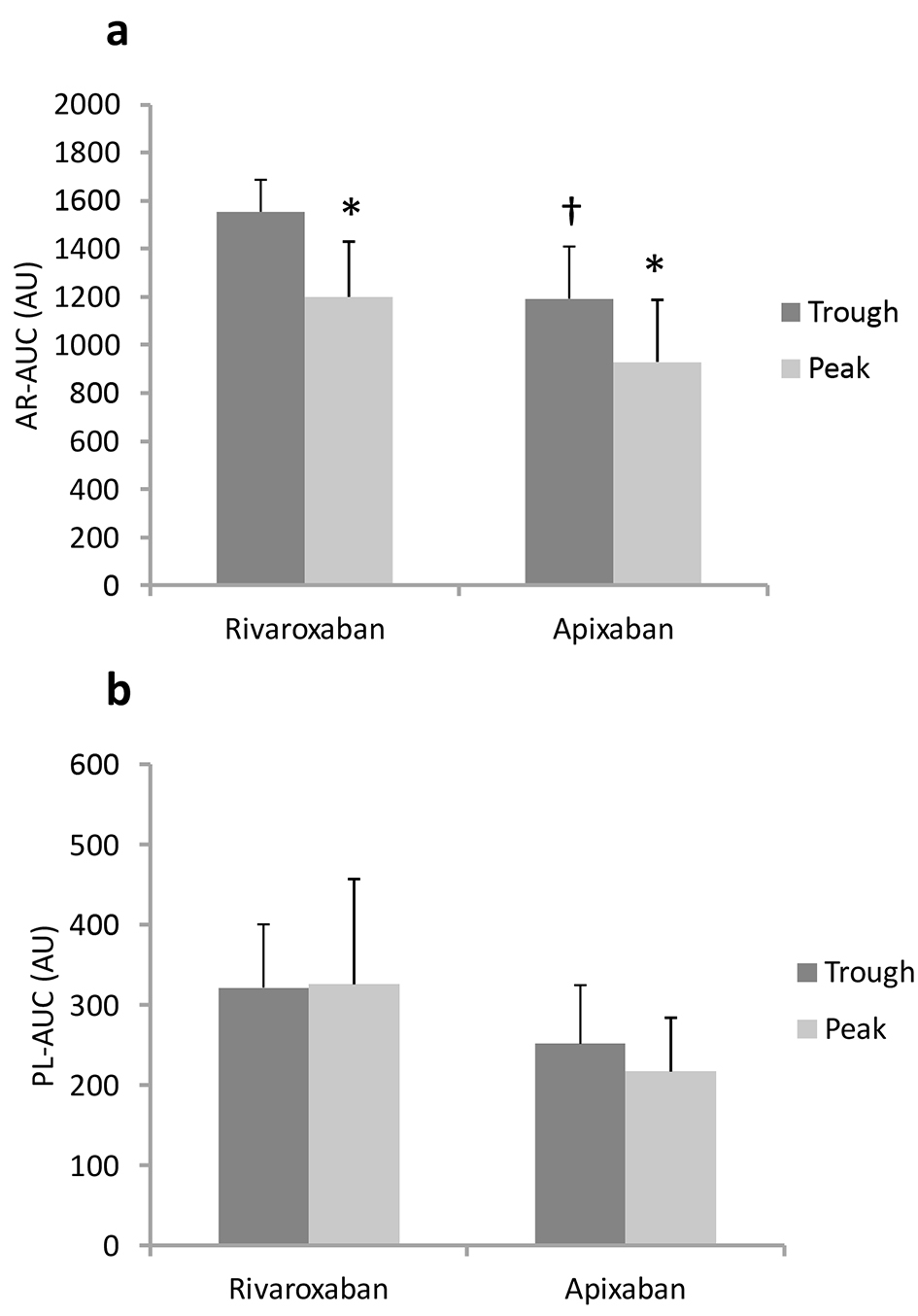 Click for large image | Figure 2. Antithrombotic capacities (AR-AUC (a) and PL-AUC (b)) at trough and peak in blood from patients. †P < 0.05 vs. rivaroxaban at trough. *P < 0.05 vs. trough. |
 Click to view | Table 3. Changes in T-TAS® Parameters, PT-INR and APTT at Trough and Peak in Blood in the Rivaroxaban and Apixaban Groups |
Correlation analysis between AR-AUC and the results of common coagulant tests in all patients
Common coagulation tests (PT-INR and APTT) were performed at the trough and peak time points. At both the trough and peak, there were no significant differences in PT-INR or APTT between the rivaroxaban and apixaban groups (Table 3). PT and APTT were not influenced by the dosing schedule of rivaroxaban or apixaban, and their levels were within the normal range even at the peak drug concentrations. We next performed a correlational analysis to clarify the associations between these common coagulant tests and the anticoagulant parameter (AR-AUC) obtained by the T-TAS. PT-INR in all patients was not correlated with AR-AUC at each time point (Fig. 3a, b). Similarly, no correlations were found between APTT and AR-AUC (Fig. 3c, d).
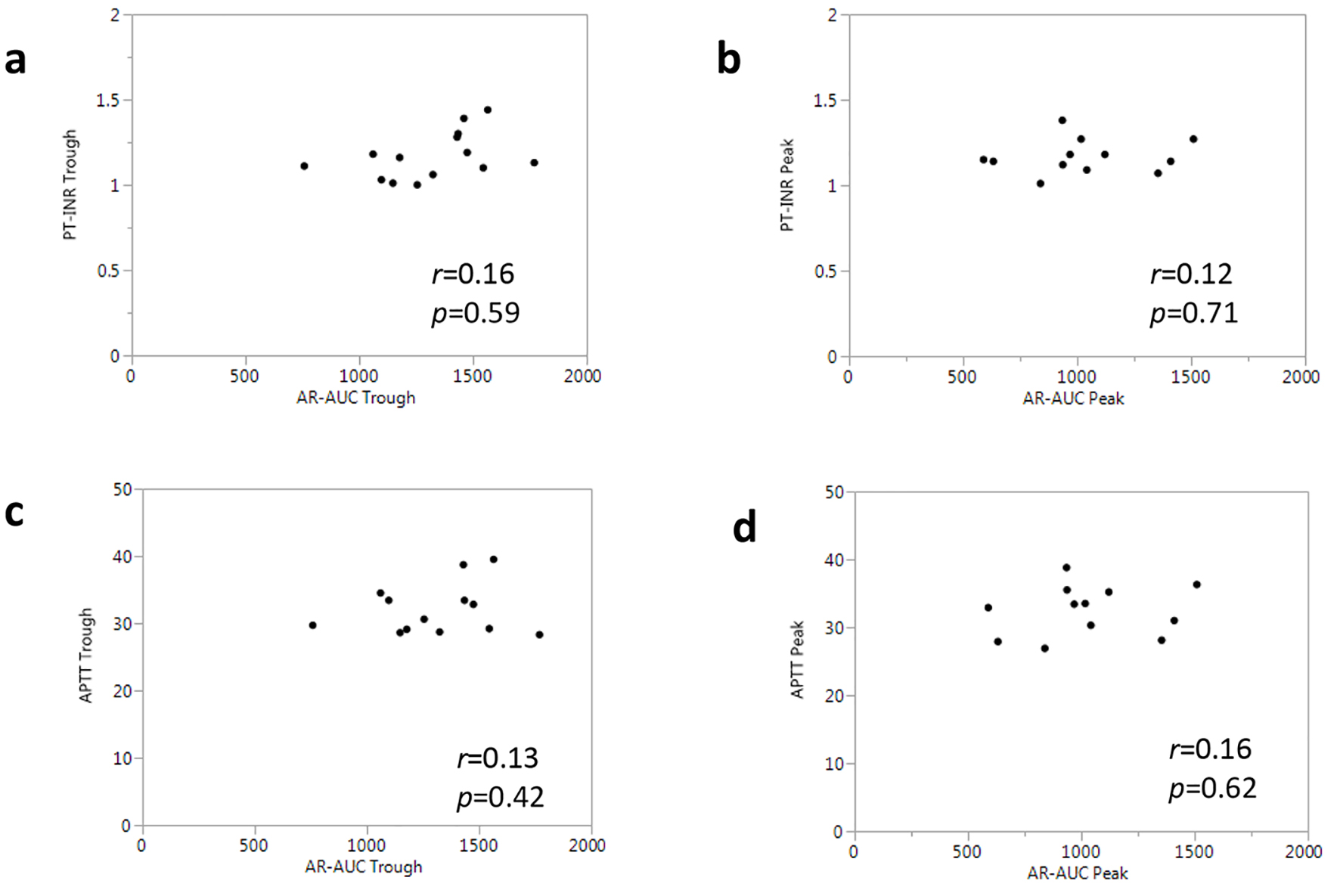 Click for large image | Figure 3. Associations between AR-AUC and common coagulant tests in all patients. |
Associations of the T-TAS parameters at the trough and peak
Figure 4 shows the results of a correlational analysis for the T-TAS parameters measured with samples from all patients at the trough and peak time points. There was a significantly high correlation between AR-AUC at the trough and AR-AUC at the peak (r = 0.79, P = 0.001, Fig. 4a). The other thrombogenicity parameters obtained from the AR-chip analysis, including AR-T10 and AR-T80, showed an equally strong correlation between the trough and peak (r = 0.72 and r = 0.81, respectively, Fig. 4b, c).
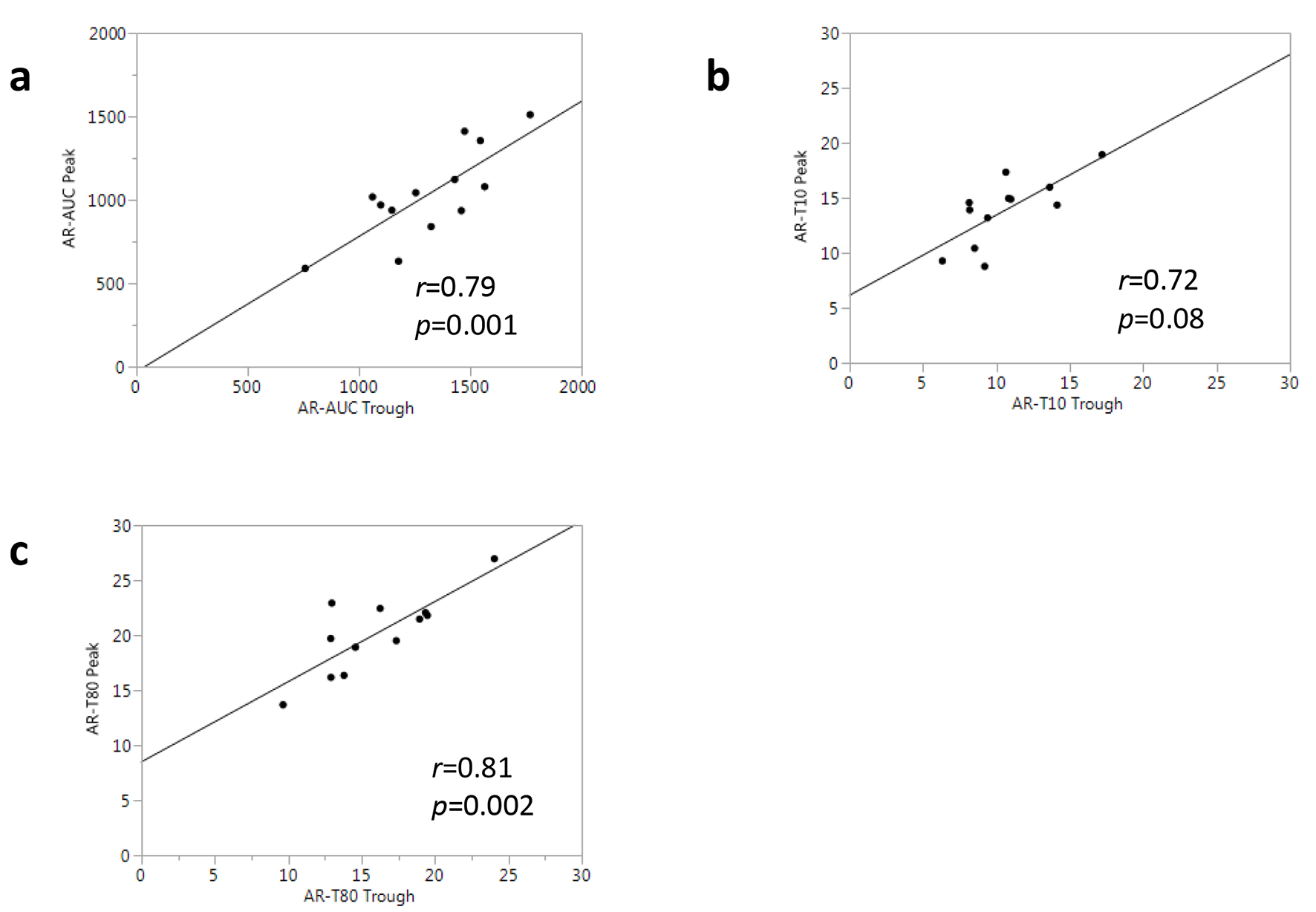 Click for large image | Figure 4. Associations between T-TAS® parameters at trough and peak in all patients. |
T-TAS parameters and complete blood count tests
Blood constituents can influence thrombus formation via the effect of blood rheology [15, 16]. Thus, we measured the hematocrit and platelet counts for all the patients, and examined the associations between these parameters and T-TAS® measurements. There were no differences in the hematocrit or platelet counts between the rivaroxaban and apixaban groups (Table 2). According to the correlation analysis, the hematocrit was not associated with either AR-AUC or PL-AUC at the trough (Fig. 5a, b). In contrast, the platelet count was moderately associated with AR-AUC at the trough (r = 0.57, P = 0.03) (Fig. 5c), whereas it was not significantly correlated with PL-AUC (r = 0.48, P = 0.08) (Fig. 5d).
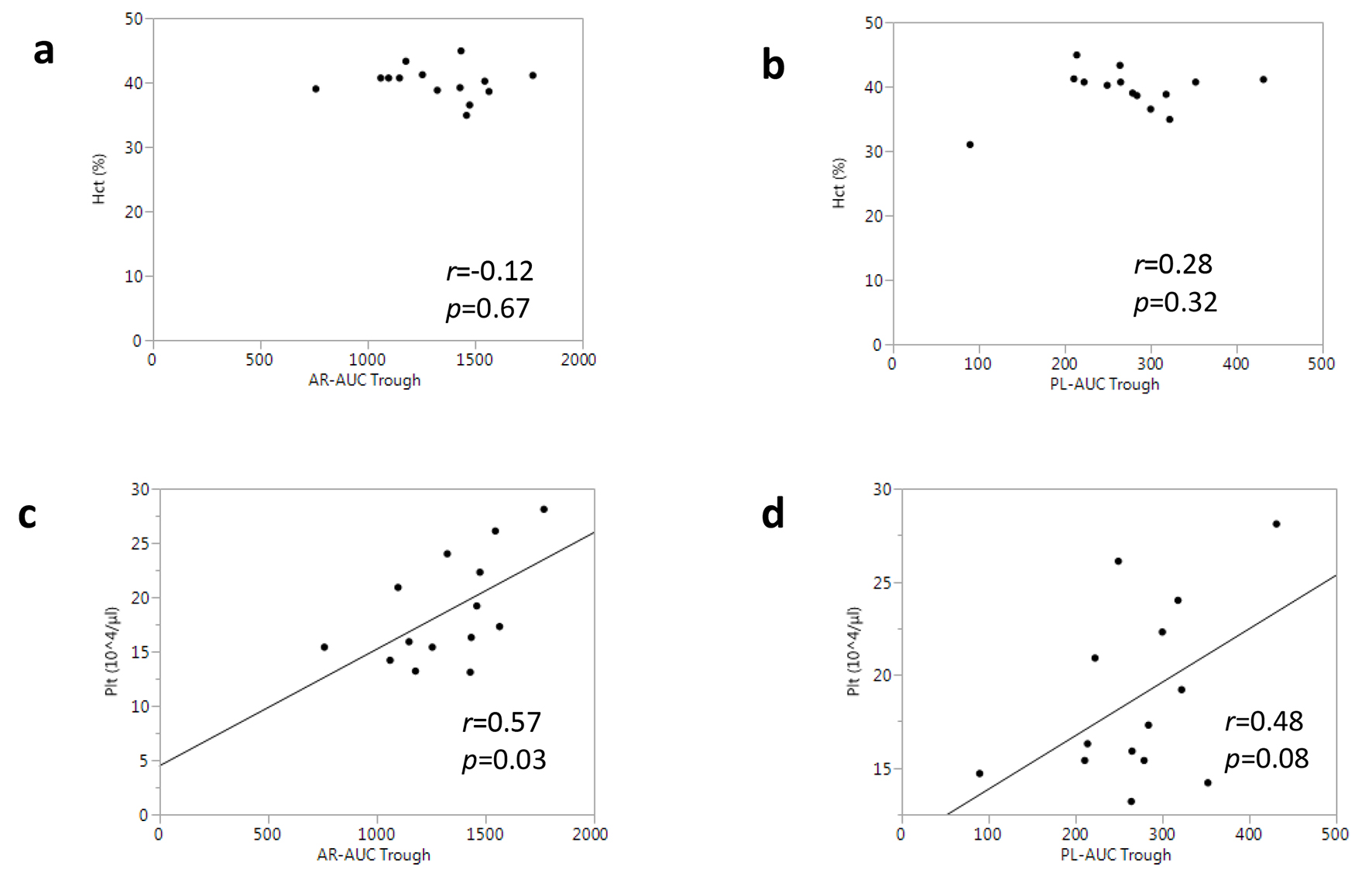 Click for large image | Figure 5. Associations between T-TAS® parameters and complete blood count tests in all patients. |
| Discussion | ▴Top |
In the present study, we determined whether T-TAS® could be a useful tool for monitoring the anticoagulant effects of rivaroxaban and apixaban under various conditions. Our main findings were as follows: 1) The T-TAS® could detect anticoagulant capacities in samples treated in vitro with rivaroxaban or apixaban. 2) The enhancements of the anticoagulant capacities of rivaroxaban and apixaban from concentrations at the trough to peak were successfully monitored by the T-TAS® using ex vivo samples. 3) In the T-TAS® analysis, the antithrombotic capacity of FXa inhibitors at the peak dose was strongly linked to that at the trough.
Recent clinical trials have successfully demonstrated that the T-TAS® could be used to monitor platelets and the coagulation system under various conditions [17-19]. One of the advantages of the T-TAS® assay is that it enables simple point-of-care tests with short turnaround times. The process of thrombus formation is monitored quantitatively in real time and measurements are fully computed for assessment. Another advantage is that flow-chamber testing under various shear conditions with whole blood samples may be more physiological and comprehensive, since this simultaneously evaluates both cellular and soluble elements of hemostasis. On the other hand, the T-TAS® is an indirect assay, which is not specific for FXa concentrations, since the rate of thrombus formation might be affected by patient characteristics other than the dosage of FXa inhibitors. For example, diabetic patients show high platelet reactivity and enhanced coagulation regardless of the administration of antithrombotic agents [20]. Therefore, in this study, whole blood samples from healthy volunteers were first used in a T-TAS® analysis to validate the anticoagulant effects of drugs. At clinically relevant peak concentrations, the addition of rivaroxaban to whole blood samples significantly decreased athero-thrombogenicity as indicated by AR-AUC. In vitro treatment with apixaban also decreased AR-AUC. As expected, AR-AUC levels in the two groups were similar, which confirmed that the T-TAS® is useful for monitoring the anticoagulant effects of rivaroxaban and apixaban. Moreover, the other AR parameters, AR-T10 and AR-T80, were also similar between the rivaroxaban and the apixaban groups. These results suggest that rivaroxaban and apixaban have identical antithrombotic properties throughout thrombus formation under the condition of platelets and coagulation-fibrinolysis factors. Similarly, with the use of blood samples from patients given rivaroxaban or apixaban, our ex vivo experiments demonstrated that AR-AUC levels were significantly decreased from the trough to peak in both groups. Along with AR-AUC levels, AR-T10 and AR-T80 were prolonged in both the rivaroxaban and apixaban groups in a concentration-dependent manner. In contrast to the in vitro experiment, the rivaroxaban group at the trough had less anticoagulant capacity, as indicated by higher AR-AUC levels and shorter AR-T80, than the apixaban group at the trough. According to pharmacokinetic and pharmacodynamic studies, 10 mg rivaroxaban taken orally once daily had a higher peak to trough drug concentration ratio in a steady state, compared to 2.5 mg apixaban taken twice daily [21-23]. Therefore, the decreased blood concentration of rivaroxaban at the trough might result in lower AR-AUC levels due to the different dose regimens in the present study. Based on the findings from our in vitro and ex vivo experiments, we believe that T-TAS® parameters obtained using the AR-chip under a constant shear flow of 600/s are useful for accurate quantitative monitoring of the anticoagulant effects of rivaroxaban and apixaban.
The antiplatelet effects of FXa inhibitors have recently become a new therapeutic target in patients with cardiovascular disease. The ATLAS ACS 2-TIMI 51 study demonstrated that the addition of rivaroxaban reduced cardiovascular events and mortality in patients with recent acute coronary syndrome [24]. Along with T-TAS® monitoring with an AR-chip, we also sought to evaluate pure platelet thrombus formation using a PL-chip. For PL analysis, blood samples were applied to PL-chips under a shear flow of 1,000/s, which simulates the shear conditions at the arterial wall. As shown in Figure 1b, neither rivaroxaban nor apixaban supplementation in vitro promoted platelet thrombus formation in whole blood samples from healthy subjects. Similarly, data from the ex vivo experiment did not show that the doses of the FXa inhibitors influenced platelets (Fig. 2b). In both the rivaroxaban and apixaban groups, no differences were observed between PL-AUC at the trough and PL-AUC at the peak. Although the rivaroxaban group had less athero-thrombogenicity at the trough in the AR-chip analysis, this group had a similar capacity for platelet thrombus formation at any time point in PL-chip analysis, as compared to the apixaban group. We previously reported that blood samples from patients who were taking NOACs, including dabigatran, rivaroxaban and apixaban, had significantly lower PL-AUC levels at trough than control blood from patients who were not receiving any anticoagulant [11]. Taken together, PL analysis by T-TAS® for patients was influenced by NOACs administration, but it was not suitable for the quantitative assessment of platelet function of NOACs.
It can be difficult to quantify NOAC activity by a common coagulant test, such as PT-INR or APTT, because PT-INR and APTT may be within their normal ranges at trough drug levels [25]. Nevertheless, several studies have reported that FXa inhibitors prolonged PT linearly at high concentrations, and this effect was modestly correlated with anti-FXa activity [19, 26]. In our population, the PT-INR and APTT levels were similar in the rivaroxaban and apixaban groups at all of the time points tested. The PT and APTT from the trough to peak were also not prolonged, which indicated that PT-INR and APTT were not sensitive even at the clinically peak concentrations of rivaroxaban and apixaban. In addition, AR-AUC did not show any correlations with PT-INR or APTT at the trough or peak. Thus, T-TAS® monitoring of anticoagulant capacity is independent of PT-INR and APTT, and T-TAS® measurement may play a different role in anticoagulant monitoring than an anti-FXa activity assay.
A previous report demonstrated that blood constituents, such as the hematocrit and platelet counts, might contribute to the measurements in the T-TAS® [27]. In our ex vivo analysis, the hematocrit and platelet counts in the rivaroxaban group were comparable to those in the apixaban group. A correlation analysis showed that the hematocrit was not related to any T-TAS® parameters including AR-AUC and PL-AUC, whereas platelet counts were significantly associated with AR-AUC (but not with PL-AUC). The strength of the correlation between AR-AUC at the trough and platelet counts was moderate (r = 0.57, P = 0.03), which was consistent with the results in a previous report from another group using samples from healthy individuals (r = 0.51, in ref. 31). Based on these data, while platelet counts may confound T-TAS® measurements with an AR-chip, this interaction may not be influenced by disorders or NOAC medications.
Finally, do we need serial measurements with the T-TAS® to monitor the antithrombotic effects of FXa inhibitors? Also, what AR-parameters would provide the greatest insight into these effects? As shown in Figure 2, all AR parameters at the trough, AR-AUC, AR-T10 and AR-T80, were strongly correlated with those at the peak (r = 0.79, 0.72 and 0.81, respectively). PL-AUC at the trough was also correlated to that at the peak, albeit this may not be suitable for quantification. Thus, we believe that the inter-individual variability in the dose-response relationship between rivaroxaban or apixaban and anticoagulant capacity is not high in the steady state, and hence, serial T-TAS® measurements are not necessary. The endpoints of our T-TAS® analysis with an AR-chip included AR-AUC, AR-T10 and AR-T80, which corresponded to the overall thrombogenicity, the time to the onset of thrombus formation and the time to capillary thrombus occlusion. In our in vitro and ex vivo experiments, treatment with rivaroxaban or apixaban significantly changed all of the AR parameters, and the mean rates of change from the trough to peak were similar among AR-AUC, AR-T10 and AR-T80. In addition to AR-AUC, AR-T10 and AR-T80 at the trough strongly correlated with the values at the peak, and the strength of the correlation was identical among these AR parameters (Fig. 5). Thus, we consider that all AR parameters are useful for monitoring the effects of rivaroxaban and apixaban. None of these parameters appears to offer any special advantage for clinical use. However, the measurement of AR-T10 alone may be acceptable for rapid testing in an emergency setting.
This study has several limitations. First, the study was cross-sectional and included a relatively small number of patients. Second, many of the patients had HTN, DM or DL, which may have influenced the measurements, although the patients did not receive any medications that should have affected thrombus formation, except for rivaroxaban or apixaban. Third, plasma concentrations of NOACs were not measured. Further studies will be needed to clarify the effects of these limitations.
Conclusion
The T-TAS® with an AR-chip was a useful tool for monitoring the anticoagulant effects of FXa inhibitors regardless of the patient characteristics, and may provide accurate quantitative results.
Funding
None.
Conflicts of Interest
KS is a Chief Director and SM is a Director of NPO Clinical and Applied Science, Fukuoka, Japan. KS has an Endowed “Department of Molecular Cardiovascular Therapeutics” supported by MSD, Co. LTD. SM belongs to the Department of Molecular Cardiovascular Therapeutics, which is supported by MSD, Co. LTD. SM’s spouse is an employee of Bayer Yakuhin Ltd. KS and SM received a grant from Bayer Yakuhin Ltd.
| References | ▴Top |
- Gross PL, Weitz JI. New anticoagulants for treatment of venous thromboembolism. Arterioscler Thromb Vasc Biol. 2008;28(3):380-386.
doi pubmed - Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139-1151.
doi pubmed - Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883-891.
doi pubmed - Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981-992.
doi pubmed - Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093-2104.
doi pubmed - Samama MM, Amiral J, Guinet C, Le Flem L, Seghatchian J. Monitoring plasma levels of factor Xa inhibitors: how, why and when? Expert Rev Hematol. 2013;6(2):155-164.
doi pubmed - Douxfils J, Tamigniau A, Chatelain B, Chatelain C, Wallemacq P, Dogne JM, Mullier F. Comparison of calibrated chromogenic anti-Xa assay and PT tests with LC-MS/MS for the therapeutic monitoring of patients treated with rivaroxaban. Thromb Haemost. 2013;110(4):723-731.
doi pubmed - Jackson LR, 2nd, Becker RC. Novel oral anticoagulants: pharmacology, coagulation measures, and considerations for reversal. J Thromb Thrombolysis. 2014;37(3):380-391.
doi pubmed - Blann AD, Lip GY. Laboratory monitoring of the non-vitamin K oral anticoagulants. J Am Coll Cardiol. 2014;64(11):1140-1142.
doi pubmed - Hosokawa K, Ohnishi T, Kondo T, Fukasawa M, Koide T, Maruyama I, Tanaka KA. A novel automated microchip flow-chamber system to quantitatively evaluate thrombus formation and antithrombotic agents under blood flow conditions. J Thromb Haemost. 2011;9(10):2029-2037.
doi pubmed - Idemoto Y, Miura SI, Norimatsu K, Suematsu Y, Hitaka Y, Shiga Y, Morii J, et al. Evaluation of the antithrombotic abilities of non-vitamin K antagonist oral anticoagulants using the Total Thrombus-formation Analysis System(R). Heart Vessels. 2016.
doi pubmed - Mueck W, Borris LC, Dahl OE, Haas S, Huisman MV, Kakkar AK, Kalebo P, et al. Population pharmacokinetics and pharmacodynamics of once- and twice-daily rivaroxaban for the prevention of venous thromboembolism in patients undergoing total hip replacement. Thromb Haemost. 2008;100(3):453-461.
doi - Frost C, Wang J, Nepal S, Schuster A, Barrett YC, Mosqueda-Garcia R, Reeves RA, et al. Apixaban, an oral, direct factor Xa inhibitor: single dose safety, pharmacokinetics, pharmacodynamics and food effect in healthy subjects. Br J Clin Pharmacol. 2013;75(2):476-487.
doi pubmed - Bonar R, Favaloro EJ, Mohammed S, Ahuja M, Pasalic L, Sioufi J, Marsden K. The effect of the direct factor Xa inhibitors apixaban and rivaroxaban on haemostasis tests: a comprehensive assessment using in vitro and ex vivo samples. Pathology. 2016;48(1):60-71.
doi pubmed - Turitto VT, Weiss HJ. Red blood cells: their dual role in thrombus formation. Science. 1980;207(4430):541-543.
doi - Turitto VT, Hall CL. Mechanical factors affecting hemostasis and thrombosis. Thromb Res. 1998;92(6 Suppl 2):S25-31.
doi - Ogiwara K, Nogami K, Hosokawa K, Ohnishi T, Matsumoto T, Shima M. Comprehensive evaluation of haemostatic function in von Willebrand disease patients using a microchip-based flow chamber system. Haemophilia. 2015;21(1):71-80.
doi pubmed - Ito M, Kaikita K, Sueta D, Ishii M, Oimatsu Y, Arima Y, Iwashita S, et al. Total Thrombus-Formation Analysis System (T-TAS) Can Predict Periprocedural Bleeding Events in Patients Undergoing Catheter Ablation for Atrial Fibrillation. J Am Heart Assoc. 2016;5(1).
doi - Samama MM, Martinoli JL, LeFlem L, Guinet C, Plu-Bureau G, Depasse F, Perzborn E. Assessment of laboratory assays to measure rivaroxaban - an oral, direct factor Xa inhibitor. Thromb Haemost. 2010;103(4):815-825.
doi pubmed - Grant PJ. Diabetes mellitus as a prothrombotic condition. J Intern Med. 2007;262(2):157-172.
doi pubmed - Mueck W, Schwers S, Stampfuss J. Rivaroxaban and other novel oral anticoagulants: pharmacokinetics in healthy subjects, specific patient populations and relevance of coagulation monitoring. Thromb J. 2013;11(1):10.
doi pubmed - Frost C, Nepal S, Wang J, Schuster A, Byon W, Boyd RA, Yu Z, et al. Safety, pharmacokinetics and pharmacodynamics of multiple oral doses of apixaban, a factor Xa inhibitor, in healthy subjects. Br J Clin Pharmacol. 2013;76(5):776-786.
doi pubmed - Frost C, Song Y, Barrett YC, Wang J, Pursley J, Boyd RA, LaCreta F. A randomized direct comparison of the pharmacokinetics and pharmacodynamics of apixaban and rivaroxaban. Clin Pharmacol. 2014;6:179-187.
doi - Mega JL, Braunwald E, Wiviott SD, Bassand JP, Bhatt DL, Bode C, Burton P, et al. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med. 2012;366(1):9-19.
doi pubmed - Cuker A, Siegal DM, Crowther MA, Garcia DA. Laboratory measurement of the anticoagulant activity of the non-vitamin K oral anticoagulants. J Am Coll Cardiol. 2014;64(11):1128-1139.
doi pubmed - Tripodi A, Chantarangkul V, Guinet C, Samama MM. The International Normalized Ratio calibrated for rivaroxaban has the potential to normalize prothrombin time results for rivaroxaban-treated patients: results of an in vitro study. J Thromb Haemost. 2011;9(1):226-228.
doi pubmed - Yamaguchi Y, Moriki T, Igari A, Matsubara Y, Ohnishi T, Hosokawa K, Murata M. Studies of a microchip flow-chamber system to characterize whole blood thrombogenicity in healthy individuals. Thromb Res. 2013;132(2):263-270.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


