| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Original Article
Volume 8, Number 6, June 2016, pages 449-460
This article has been retracted. See J Clin Med Res. 2025 Jun 11;17(6):356.
Non-Ergot Dopamine Agonists Do Not Increase the Risk of Heart Failure in Parkinson’s Disease Patients: A Meta-Analysis of Randomized Controlled Trials
Renato De Vecchisa, d, Claudio Cantatrionea, Damiana Mazzeia, Cesare Baldib, Marco Di Maioc
aCardiology Unit, Presidio Sanitario Intermedio “Elena d’Aosta”, ASL Napoli 1 Centro, Napoli, Italy
bHeart Department, Interventional Cardiology, A.O.U. “San Giovanni di Dio e Ruggi d’Aragona”, Salerno, Italy
cDepartment of Cardiology, Second University of Napoli, Monaldi Hospital, Napoli, Italy
dCorresponding Author: Renato De Vecchis, Cardiology Unit, Presidio Sanitario Intermedio “Elena d’Aosta”, ASL Napoli 1 Centro, Napoli, Italy
Manuscript accepted for publication March 31, 2016
Short title: Dopamine Agonists and Heart Failure
doi: http://dx.doi.org/10.14740/jocmr2541e
| Abstract | ▴Top |
Background: In recent years, some observational studies suggested that pramipexole, a non-ergot dopamine agonist (DA) used for the treatment of Parkinson’s disease (PD), may increase the risk of heart failure (HF). However, the limitations inherent in observational studies made it difficult to determine whether the excess of incident HF was related to the drug or to other determinants. Thus, some concerns remained regarding the increased putative HF risk associated with non-ergot DAs as a class or individually.
Methods: In our meta-analysis, primary endpoint was the risk of incident HF in patients with PD treated with non-ergot DAs compared to those treated with monotherapy with levodopa. Secondary outcome measures were all-cause mortality and cardiovascular events. For these purposes, only randomized controlled trials (RCTs) were considered, provided that they offered complete outcome data pertaining to the incident HF, all-cause mortality and risk of cardiovascular events. Systematic searches were performed in the databases of PubMed, Embase and ClinicalTrial.gov up to May 2015. The effect size was estimated using the pooled relative risk (RR) of non-ergot DAs versus placebo on incident HF as well as on all-cause mortality or cardiovascular events.
Results: Six out of 27 RCTs reported at least one case of incident HF; therefore, we included them in the RR estimate, whereas 13 RCTs were included in the meta-analysis for mortality rates and 22 RCTs were included to evaluate cardiovascular events. Treatment with non-ergot DAs did not reveal an increase in the risk of incident HF as compared with the placebo group (pooled RR: 0.95; 95% CI: 0.30 - 2.90; P = 0.893). Similarly, patients treated with non-ergot DAs did not show any significant differences compared to controls with regard to all-cause mortality (pooled RR: 0.617; 95% CI: 0.330 - 1.153; P = 0.13) as well as with regard to cardiovascular events (pooled RR: 1.067; 95% CI: 0.663 - 1.717; P = 0.789).
Conclusions: The use of non-ergot DAs in PD patients was not associated with an increased risk of incident HF, nor was it shown to increase the overall mortality or the risk of cardiovascular events compared to the PD patients taking monotherapy with levodopa alone. However, larger studies are warranted to confirm the cardiovascular safety of non-ergot DAs for PD management.
Keywords: Non-ergot dopamine agonists; Heart failure; Parkinson’s disease; Cardiovascular prevention
| Introduction | ▴Top |
Parkinson’s disease (PD) involves alterations of the extrapyramidal nervous system, which regulates posture and voluntary movements, and is characterized by symptoms such as resting tremors, rigidity and akinesia [1]. It is a neurodegenerative disease caused by degenerative and apoptotic injury of the basal ganglia from an unknown cause [2]. The disease itself is not fatal; however, it produces a progressive deterioration in motor function, which can lead to disability and significantly elevated risks of choking, pneumonia, and falling-related injuries, resulting in a marked reduction in life expectancy [1].
The therapy is primarily based on the administration of levodopa associated with inhibitors of its peripheral degradation such as carbidopa or benserazide [3]. Moreover, for many years, dopamine agonists (DAs) have been incorporated into the therapeutic armamentarium of PD [3]. These drugs, which are commonly subdivided into ergot and non-ergot dopaminergic derivatives, activate the dopamine secretion in the central nervous system with various mechanisms, by acting on dopaminergic neurons located in the substantia nigra, in the corpus striatum and in other extrapyramidal structures in the brain. Levodopa is currently the most effective agent used in the treatment of PD and has been the mainstay of therapy in recent decades. DAs are less effective than levodopa as a treatment regimen; however, they are associated with lower risks of dyskinesia and motor fluctuation. This has led to the wide-scale application of DAs in the early stages of PD in order to postpone the use of levodopa, or as an add-on treatment to reduce levodopa dosages [4, 5].
Evidence suggests that older, ergot-derived DAs, pergolide and cabergoline, induce thickening and dysfunction of the cardiac valves [6-12]. Thus, more recently developed non-ergot-derived DAs, such as pramipexole, ropinirole or rotigotine, are increasingly being used as replacements for ergot-derived DAs in the management of PD. However, in September 2012, the US FDA released a safety communication [13] related to a possible increase in the risk of heart failure (HF) following treatment with pramipexole, i.e. one of the most used non-ergot DAs, based on the pooled data from two phase II and III randomized controlled trials (RCTs) submitted by the manufacturer [13]. In these studies, a larger percentage of patients in the pramipexole group were diagnosed with incident HF compared to the placebo group; however, the difference did not reach statistical significance [13]. In addition, four case-control studies were undertaken to assess the relationship between ischemic events, HF, and the use of ergot and non-ergot DAs; however, the results were inconsistent [14-17]. These observational studies enrolled large numbers of participants for safety evaluations; however, their findings were inherently susceptible to confounding effects propitiated by the severity of PD, comorbidities, unmeasured confounders, confounding by indication, detection bias, and accuracy of HF outcome definition [18].
The present study sought to overcome the potential drawbacks associated with observational studies and the inconclusive findings reported in individual trials by conducting a meta-analysis of RCTs related to the use of non-ergot DAs in patients with PD. Our primary focus was on the risk of newly diagnosed HF in patients with PD who were treated with non-ergot DAs compared to those who were not; secondary outcomes were all-cause mortality and frequency of cardiovascular events.
| Methods | ▴Top |
This study complied with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines [19] with a predefined protocol elaborated by the authors.
Data sources and search strategy
We searched the PubMed, Embase and ClinicalTrials.gov databases from inception to May 2015. Full-text terms and Medical Subject Headings (MeSH) terms used for systematic database searching included PD and DAs. The PubMed search strategy through the PubMed filter was the following: “idiopathic Parkinson’s disease” (MeSH terms) OR “Parkinson’s disease” (all fields) AND (“piribedil” (all fields) OR “rotigotine” (all fields) OR “ropinirole” (all fields) OR “pramipexole” (all fields) OR “dopamine agonists” (MeSH terms) OR “dopamine agonist” (all fields). For this database, the search results were further restricted to RCTs. For the databases of ClinicalTrials.gov, search results were restricted to trials with announced results.
Eligibility criteria
The study inclusion criteria were as follows: 1) RCTs; 2) studies on patients diagnosed with PD in any stage; 3) studies involving patients who received non-ergot DAs, alone or in combination with other anti-Parkinsonian treatments as an intervention, versus a placebo as a control; and 4) trials providing data about the occurrence of incident HF and/or all-cause mortality and/or cardiovascular events. Trials lasting 24 weeks or longer were included to study the long-term effects of non-ergot DAs. We excluded small trials with fewer than 10 participants in each arm of the intervention, delay-start or crossover trials that did not provide information concerning adverse events specifically on the drug exposure period, studies published only in protocols, abstracts, and research in languages other than English.
Outcome measures
In this study, the primary outcome was the risk ratio of incident HF, i.e. the ratio of the risk of newly diagnosed cases of HF among PD patients taking non-ergot DAs (exposed group) to the corresponding risk of incident HF found in PD patients free from non-ergot-DAs (non-exposed group). The secondary outcomes were adverse cardiovascular events or mortality. From all eligible trials, we retrieved the number of events pertaining to incident HF, all-cause mortality and cardiovascular events (regardless of severity) from the listings of adverse events in the original articles. Adverse cardiovascular events were defined as a composite outcome of ischemic heart disease (coronary heart disease, myocardial infarction, acute coronary syndrome, and angina), cerebrovascular disease (stroke and transient ischemic attack), HF (including acute cardiac failure and exacerbation of heart insufficiency), valve disorders and arrhythmias/tachycardia (including atrial fibrillation).
Data extraction
All articles identified were screened based on their titles and abstracts (Fig. 1). The eligibility of potentially relevant trials was evaluated based on the full-text articles. If trials produced multiple publications, the most recent publication or the publication with the most complete information was included. For each eligible trial, a definite number of relevant characteristics were searched for: 1) trial characteristics (author, publication year, type of treatment, number of subjects included, study location, study duration, PD stage, and cardiovascular-specific exclusion criteria); 2) patient characteristics at baseline (mean age and proportion of females) and withdrawal rate; 3) number of PD patients with incident heart failure; 4) number of all-cause deaths; and 5) number of PD patients with cardiovascular events. Two investigators (RDV and CC) independently assessed identified references and extracted relevant characteristics and outcomes from the eligible trials. Discrepancies were resolved by aid and consultation of another independent observer (DM).
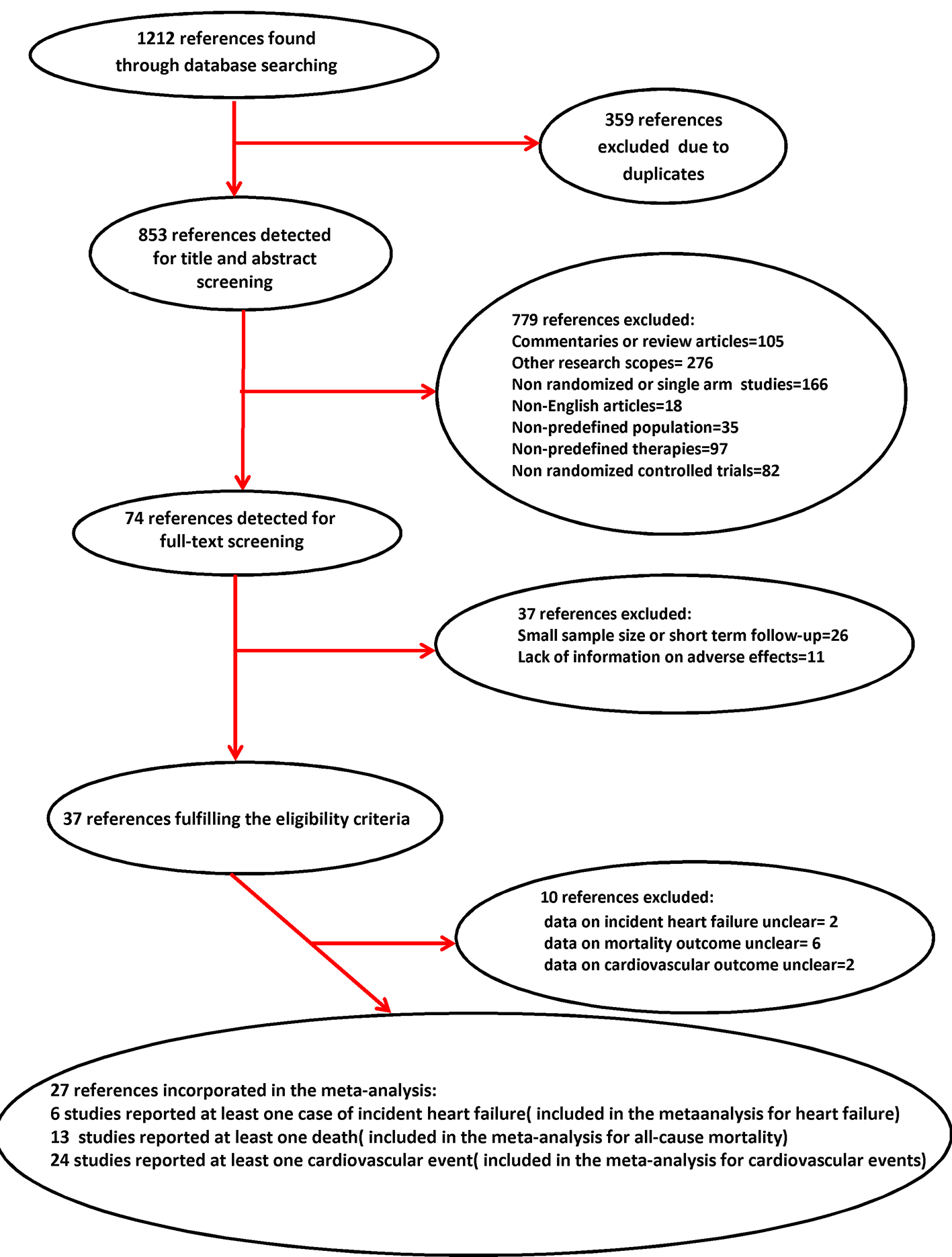 Click for large image | Figure 1. Flow diagram of study selection for meta-analysis. |
Statistical analysis
In this study, we adopted the intention-to-treat analysis strategy. For the comparison of non-ergot DAs versus a placebo, we estimated the risk of incident HF as well as those of mortality from all causes or cardiovascular events, respectively, with the pooled relative risk (RR) and 95% confidence interval (CI). For trials in which multiple groups were administered a single non-ergot DA, the data were summarized into a single overall estimate. In contrast, for trials using more than one non-ergot DA, data were managed as separate trials with the placebo group serving as a common reference. Both fixed and random effects models were used to assess the RR of incident HF (primary outcome) or of mortality or cardiovascular events associated with the use of non-ergot DAs. Sensitivity analyses were performed to deal with sparse outcome data. Statistical heterogeneity was evaluated using the I2 statistic, with a value of 50% or more indicating a substantial level of heterogeneity. To investigate potential publication bias, we visually inspected the contour-enhanced Begg’s funnel plot for any asymmetry. To explore possible effect modifiers pertaining to incident HF associated with the use of non-ergot DAs, we performed a stratified analysis on studies that included PD of various stages (early or advanced) or follow-up periods of different lengths (short term: < 24 weeks or long term: ≥ 24 weeks). We also evaluated the risk of individual non-ergot DAs on the occurrence of newly diagnosed HF. STATA version 9.0 was used for the analysis, and Review Manager® 5.3 was used for plotting risk of bias graphs. A two-sided α value of 0.05 was defined as statistically significant.
| Results | ▴Top |
Twenty-seven studies fulfilled the eligibility criteria for at least one of the outcomes provided for by the meta-analysis protocol (incident HF assumed as a primary outcome, all-cause mortality and cardiovascular events taken as secondary endpoints) [20-46]. Thus, six trials were deemed eligible for HF [24, 27, 32, 35, 36, 41] (Fig. 2), 13 trials were deemed eligible for mortality [21, 24, 27-31, 34, 36-38, 43, 46] (Fig. 3) and 22 trials were judged eligible for cardiovascular events [20-23, 25-28, 30, 32-36, 39-46] in this review (Fig. 4). Patients with orthostatic hypotension were excluded from most of the trials, as it is a common adverse effect of non-ergot DAs.
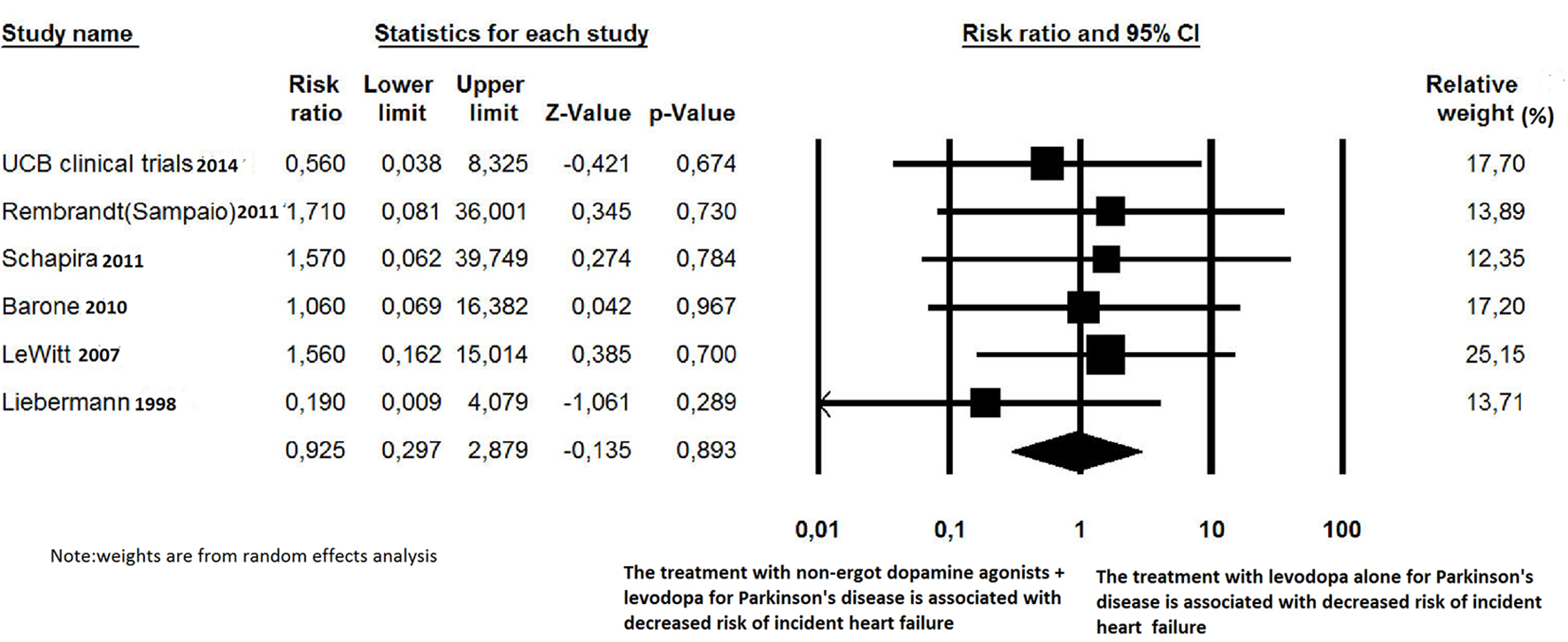 Click for large image | Figure 2. Forest plot of meta-analysis of randomized controlled trials of non-ergot dopamine agonists and the risk of newly diagnosed heart failure in Parkinson’s disease patients. |
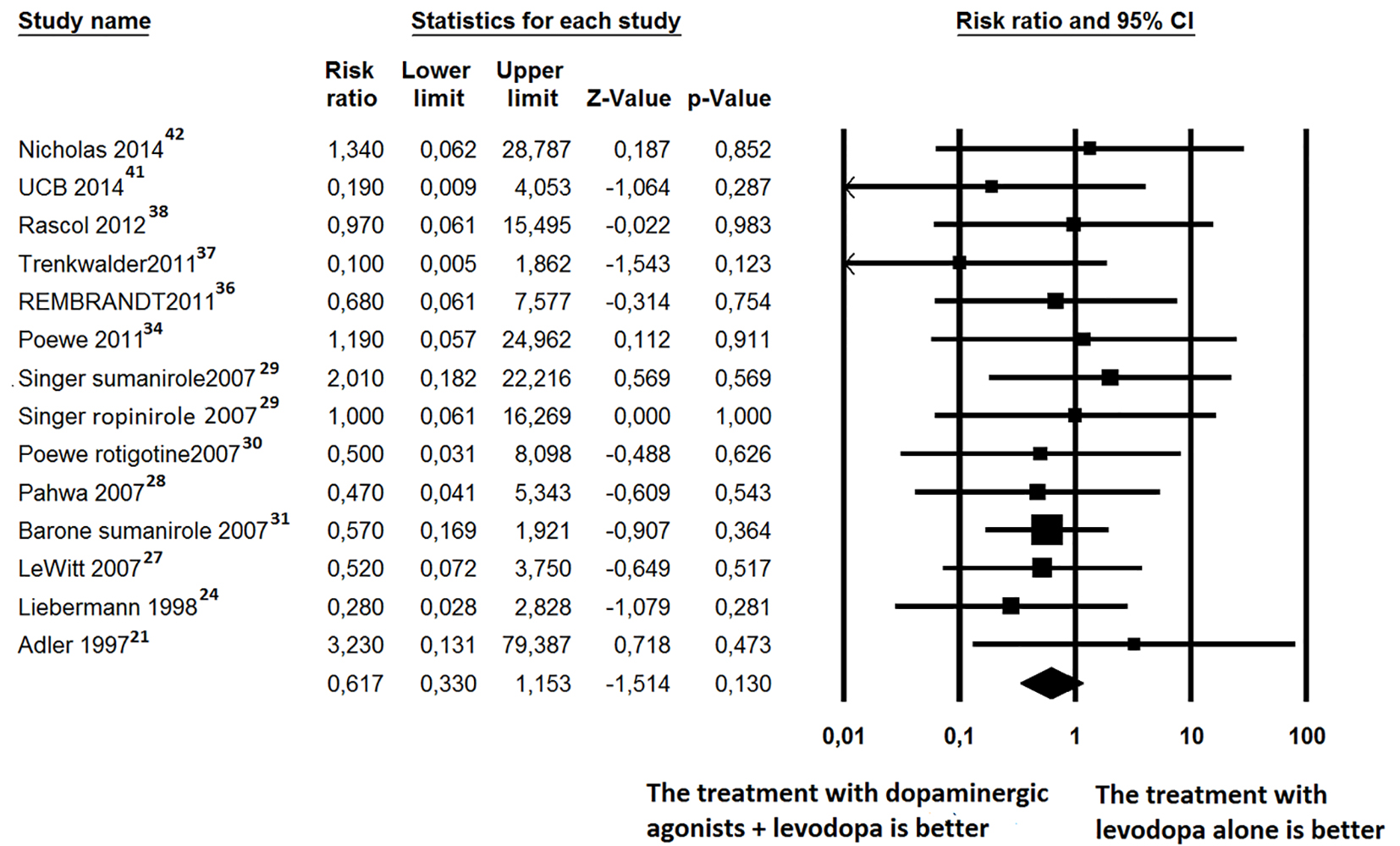 Click for large image | Figure 3. Forest plot of meta-analysis of randomized controlled trials of non-ergot dopamine agonists and the risk of mortality in Parkinson’s disease patients. |
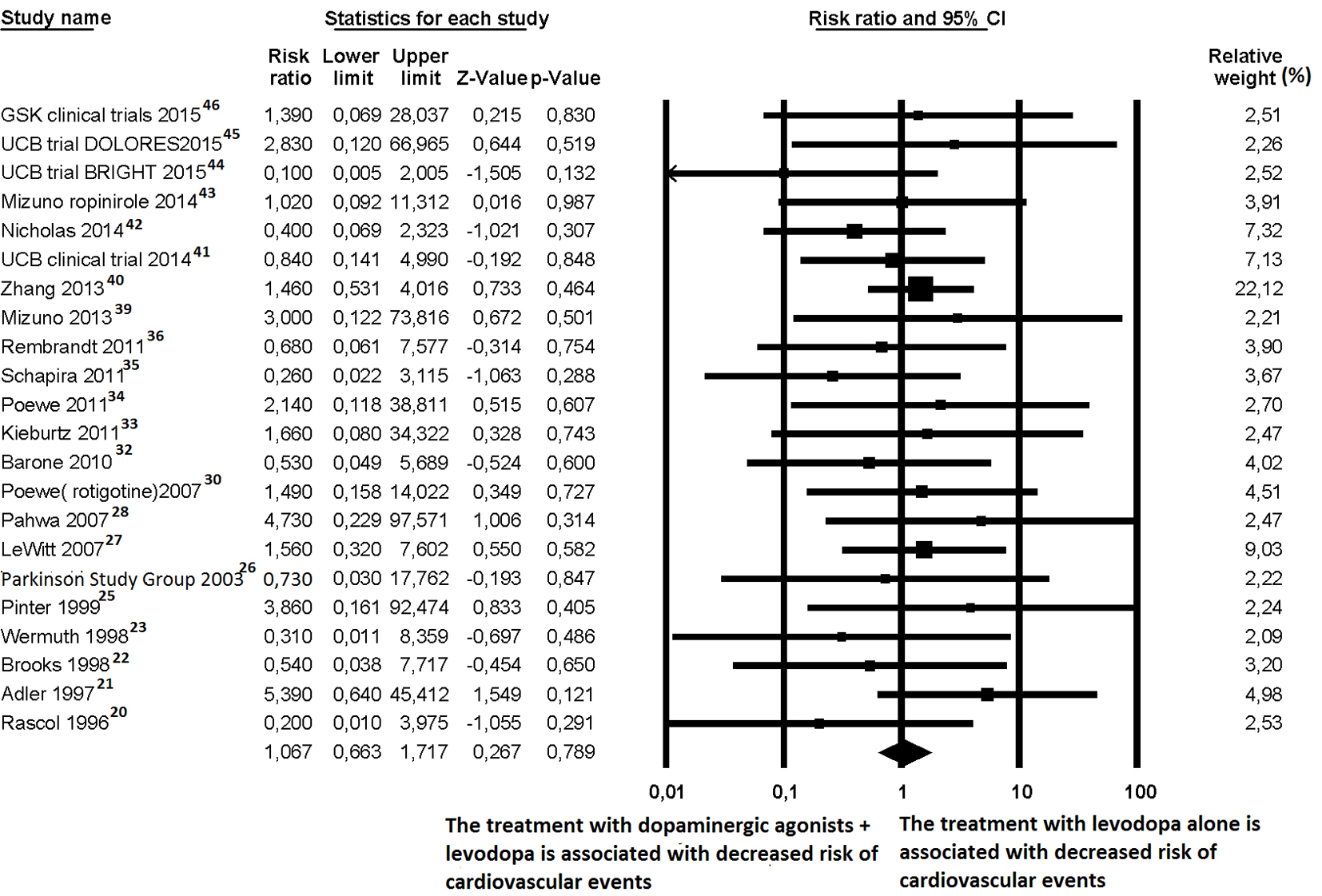 Click for large image | Figure 4. Forest plot of meta-analysis of randomized controlled trials of non-ergot dopamine agonists and the risk of any cardiovascular events in Parkinson’s disease patients. |
Primary outcome
The use of non-ergot DAs and incident HF
For newly diagnosed HF, six out of 27 trials reported at least one case of incident HF and were, therefore, included for RR estimation [24, 27, 32, 35, 36, 41]. Among the 4,560 PD patients treated with non-ergot DAs, only eight cases of HF were reported. Among the 2,359 PD patients in the placebo group, only four cases of HF were reported. Treatment with non-ergot DAs did not reveal an increase in the risk of HF as compared with the placebo group (RR: 0.925; 95% CI: 0.297 - 2.879) (Fig. 2). No significant heterogeneity was observed across the studies for the outcome “incident HF”. Due to the small number of trials in each stratum, the stratified analysis was unable to detect effect modifiers or any significant interactions (Figs. 5-7). Some adjunctive information about the risk of incident HF in patients taking non-ergot DAs compared to that exhibited by controls undergoing monotherapy with levodopa alone is summarized in Table 1.
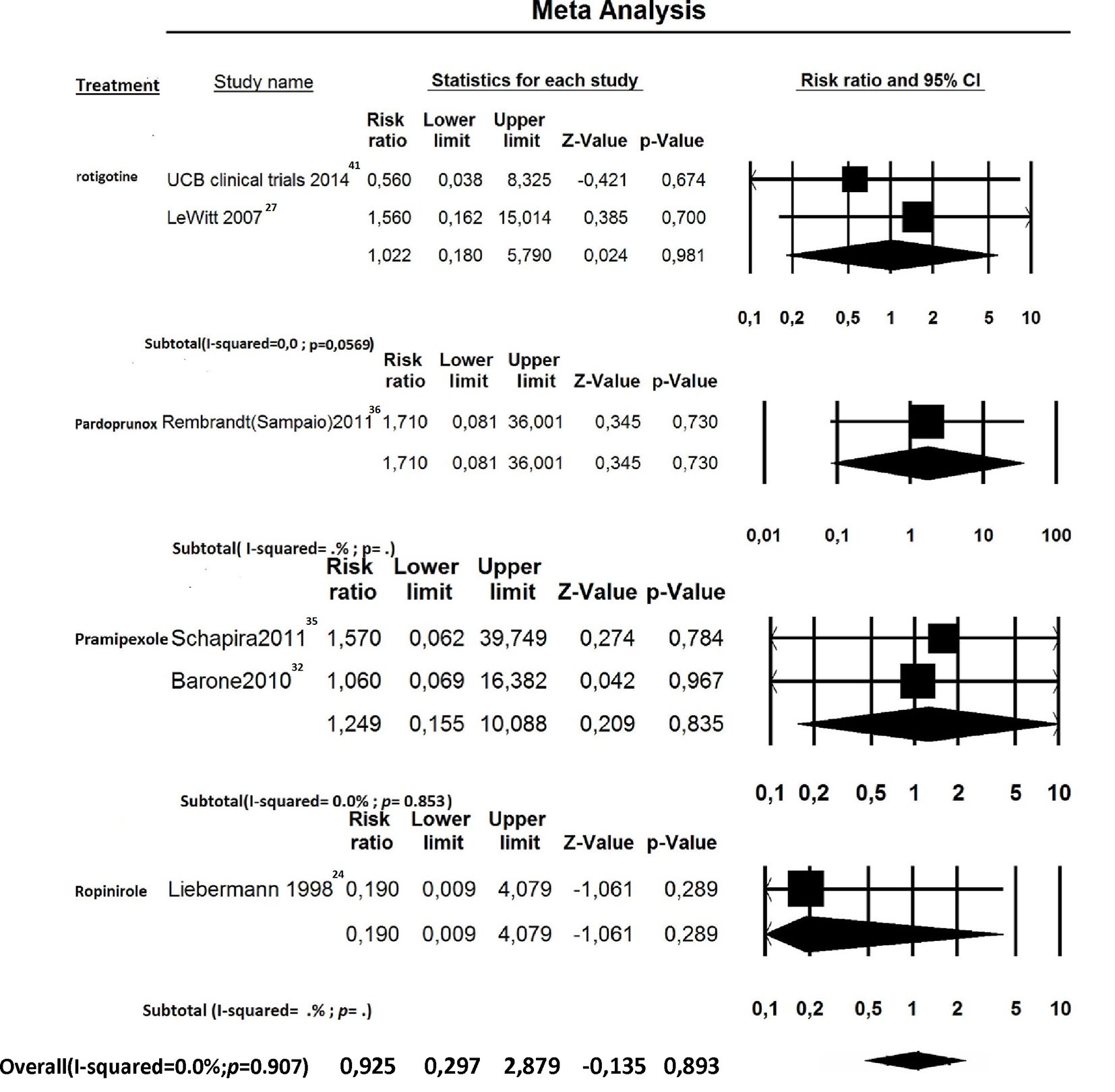 Click for large image | Figure 5. Forest plot of meta-analysis of randomized controlled trials of non-ergot dopamine agonists and the risk of heart failure in Parkinson’s disease patients: stratified by individual drugs. |
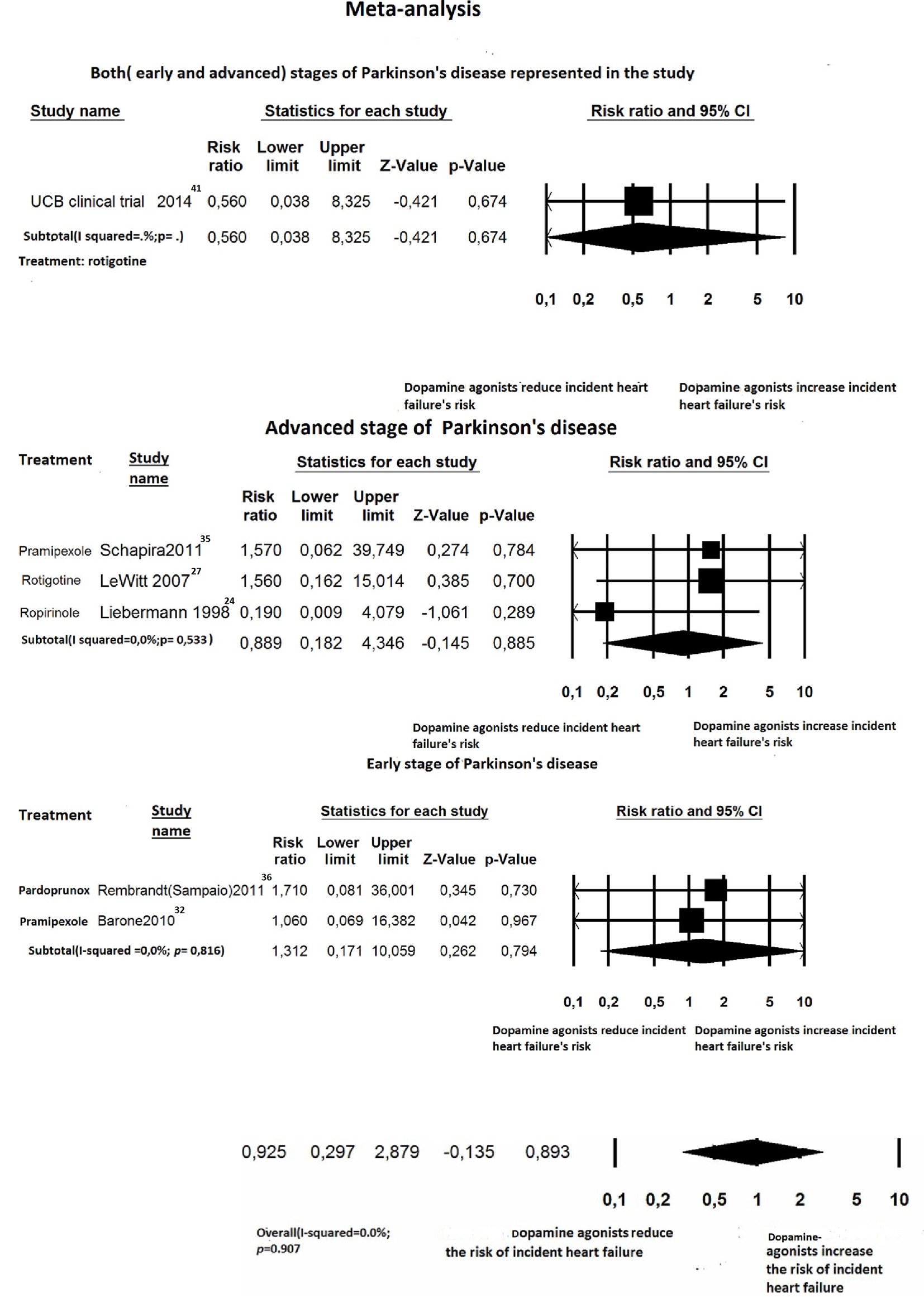 Click for large image | Figure 6. Forest plot of meta-analysis of randomized controlled trials of non-ergot dopamine agonists and the risk of heart failure in Parkinson’s disease patients: stratified by Parkinson’s disease stage. |
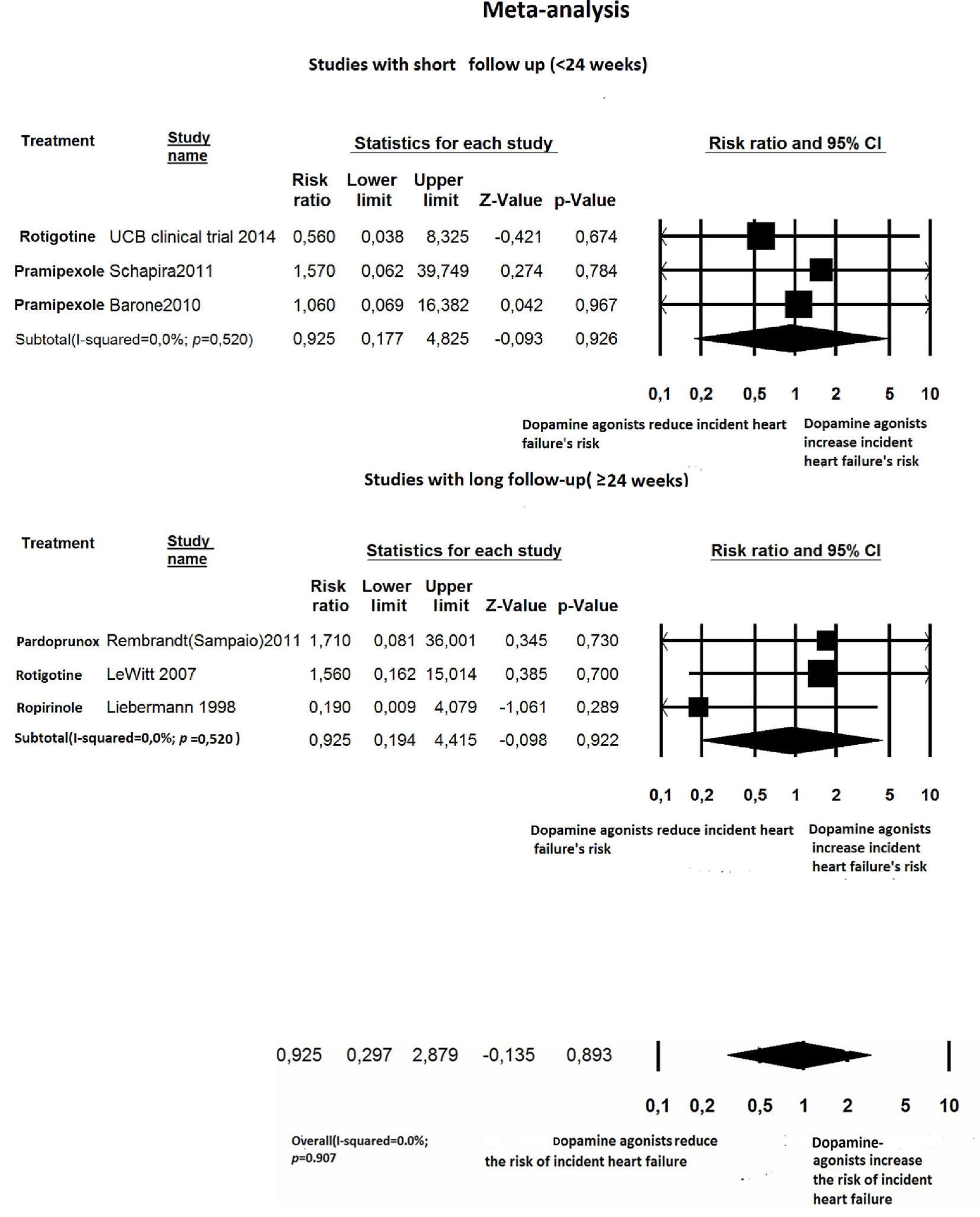 Click for large image | Figure 7. Forest plot of meta-analysis of randomized controlled trials of non-ergot dopamine agonists and the risk of heart failure in Parkinson’s disease patients: stratified by length of follow-up (< 24 weeks: short term; ≥ 24 weeks: long term). |
 Click to view | Table 1. Use of Non-Ergot Dopamine Agonists Versus Placebo and the Risk of Incident Heart Failure in Parkinson’s Disease Patients |
Secondary outcomes
The use of non-ergot DAs and overall mortality
For all-cause mortality, 13 out of 27 trials [21, 24, 27-31, 34, 36-38, 43, 46] reported at least one death and were, therefore, included in the RR estimation (Fig. 3) A total of 5,686 PD patients were enrolled (mean age of 64 years, 42.7% female). Four of the trials [21, 29, 34, 36] recruited patients with early PD, seven of the trials [20, 24, 28, 30, 31, 38, 42] recruited patients with advanced PD, and two of the trials [27, 41] included patients in early and advanced stages. The follow-up time ranged from 12 to 40 weeks. Six of the trials [20, 21, 37, 38, 41, 42] were defined as short-term trials (within 24 weeks). There were 20 deaths among the 3,858 patients who received non-ergot DAs and 21 deaths among the 2,447 patients in the placebo group. Regarding the risk of all-cause mortality, patients treated with non-ergot DAs did not show any significant difference compared to controls (random effects RR: 0.617; 95% CI: 0.330 - 1.153; P = 0.13) (Fig. 3). Moreover, the effects of using non-ergot DAs on mortality were consistent across groups of studies with different lengths of follow-up and across individual drugs.
The use of non-ergot DAs and cardiovascular events
For cardiovascular events, 22 out of the 27 trials [20-23, 25-28, 30, 32-36, 39-46] reported at least one cardiovascular event; thus, they were judged suitable for inclusion in the meta-analysis. Among the 6,734 PD patients, the mean age was 63.8 years and 41.1% were female. Eight trials [21, 22, 24, 28, 34, 36, 39, 46] dealt with patients with early PD, 12 trials [20, 23, 25, 26, 29-33, 35, 42, 43] dealt with advanced PD and two trials [27, 41] included both stages of PD patients. Sixteen trials [20-23, 25, 26, 32, 33, 35, 39, 41-46] were defined as short-term (within 24 weeks) with regard to the duration of follow-up. In the random effects model, patients treated with non-ergot DAs did not show any significant difference with regard to the risk of cardiovascular events compared to patients taking a placebo (random effects RR: 1.067; 95% CI: 0.663 - 1.717; P = 0.789) (Fig. 4).
| Discussion | ▴Top |
The proportion of PD patients who experience HF is higher than among those without PD, probably due to autonomic dysfunction and neurohormonal dysregulation, which have been well demonstrated in PD [47-49]. Thus, careful evaluation regarding cardiovascular safety of anti-Parkinson medications is a crucial issue. Concerns arose from isolated reports that highlighted that non-ergot DAs may precipitate HF, judging from the quite worrying information provided in this regard by several case reports [50] and observational case-control studies [14-17]. In particular, three different case-control studies showed that pramipexole increased the risk of HF. First, Mokhles et al found that this increased risk was especially high during the first months of treatment in PD patients over the age of 80 [15]. Renoux et al [16] also highlighted a higher risk of HF with pramipexole compared to the other DAs used in treating PD and restless legs syndrome. According to these authors [16], in a cohort of 26,814 subjects taking DAs, the incidence rate of HF increased with the current use of a DA; in particular, it was significantly higher for pramipexole and cabergoline, whereas it was not significantly increased by ropinirole. The increased risk for HF associated with pramipexole was, however, not significant, when compared with all DAs taken collectively. Therefore, we can infer that the increased risk of HF is not a class effect, and the ergoline structure is likely not to be implied in this complication, since the deterioration in pump function would occur in both ergot-derived (cabergoline and pergolide) and non-ergot related (pramipexole) DAs. Hsieh and Hsiao [17] studied the risk of DA-related HF among Asian patients. They found an increased, but non-significant, risk with pramipexole proportional to the duration of use. In this study, HF was not found in patients with a history of peripheral edema, with the mechanism most likely being different. In addition to a high affinity for the dopamine D2, D3, and D4 receptors, pramipexole is also an alpha-2-adrenergic receptor agonist [51, 52]. Thus, it is possible that pramipexole directly activates the α2-adrenergic autoreceptors, thereby reducing adrenergic tone and myocardial contractility. Consequently, in September 2012, the US Food and Drug administration warned of a possible increased risk of HF with pramipexole use in PD patients [13]. However, the limitations inherent in observational studies (in particular, detection bias, interference by known or unknown confounding factors, confounding by indication, etc.) make it difficult to determine whether the excess of a given pathologic event, e.g. HF is related to the drug or to other determinants. Therefore, no conclusion was reached by the FDA in this regard, and the warning released in September 2012 was not followed by actions aimed at its marketing limitation or by restrictions concerning the drug’s therapeutic indications. Contrary to some [15-17], but not all, observational studies suggesting that several non-ergot DAs, such as pramipexole, may increase the risk of HF, we did not observe a significant increase in the risk of HF associated with non-ergot DAs, as a class or individually, in an RCT setting. This discrepancy may be because observational studies tend to be more vulnerable to confounding by underlying comorbidities and detection bias, i.e. physicians may preferentially prescribe non-ergot DAs to PD patients with poor cardiac function (so-called confounding by indication) and pay more attention to monitoring the symptoms of HF. As a result, the users of non-ergot DAs would be more likely to be diagnosed with incident HF. Our meta-analysis revealed similar rates of all-cause mortality among PD patients receiving non-ergot DAs compared to those receiving only a placebo (Fig. 3). Furthermore, the use of non-ergot DAs was shown not to be associated with an increased risk of cardiovascular events, including myocardial infarction or ischemic stroke (Fig. 4). Thus, our results seem to affirm the substantial cardiovascular safety of non-ergot DAs, even among PD patients with a higher prevalence of HF and cerebrovascular disease [53, 54].
Implications and inferences of the meta-analysis
Unlike case-control studies, the results obtained in RCTs are less susceptible to residual or unmeasured confounding, e.g. PD severity, comorbidities and a physician’s tendency to use innovative treatments, e.g. non-ergot DAs, especially in patients with a more deteriorated and drug-refractory clinical picture. The limitations of this study primarily reflect the challenges of assessing drug safety in clinical trials. First, only a limited number of trials reported cardiovascular events, which prevented us from conducting further subgroup analyses or meta-regression adjustments to check for the mediation of effects by important characteristics. Second, none of the trials included cardiovascular events as predefined outcomes and these endpoints were not defined uniformly across trials. Therefore, the misclassification of these adverse events is possible. Nonetheless, all of the trials included in this study were double blind, so that any such misclassification is likely to be non-differential, which may bias the results toward the null. Third, some of the studies we included did not provide detailed information related to adverse events, particularly cardiovascular events (possible selective reporting bias). Fourth, we were unable to evaluate confidently the long-term safety of non-ergot DAs due to limitations in the availability of data. Previous clinical trials and observational studies reported that increased risk was observed within months of initiating non-ergot DAs. Finally, we attempted to pool data from multi-center trials that enrolled patients with early stage PD as well as those with advanced stage PD. Thus, our results from the meta-analysis of RCTs may have limited generalizability and may not be applicable to frail PD patients in real practice.
Comparison with previous findings and implications of findings
In our meta-analysis, the risk of overall mortality in the DAs group was similar to that observed in the placebo group. This finding somewhat conflicts with an earlier systematic review and meta-analysis, which reported an RR of 0.45 (95% CI: 0.21 - 0.97) when comparing the all-cause mortality in the patients’ group treated with DAs plus levodopa in comparison with those treated with levodopa alone [55]. The survival benefit associated with the use of DAs was attributed by the authors to the ability of non-ergot DAs to improve motor symptoms and reduce choking and aspiration pneumonia. Also, Ernst et al [56], based on a nested case-control study by analyzing the UK General Practice Research Database, found that the use of pramipexole was associated with a 24% reduction in the risk of pneumonia (RR: 0.76; 95% CI: 0.57 - 1.02).
Considering the discrepancies with regard to the mortality found by comparing our meta-analysis with the one mentioned above, it would be appropriate to defer any definitive judgment on this point. Instead, it could be stated even now that the class of non-ergot DAs is not burdened by increased risk of incident HF or newly diagnosed adverse cardiac events when compared to the conventional therapy with levodopa alone. In any case, additional large-scale population-based studies will be required to confirm the lack of unfavorable repercussions on the heart and cardiovascular system related to the prolonged use of non-ergot DAs in conjunction with levodopa.
Conclusions
In conclusion, the present meta-analysis has substantial implications for clinical practice, since the use of non-ergot DAs for PD patients was proven not to increase the incidence of HF. Likewise, these drugs were shown not to be associated with increased all-cause mortality or increased risk of cardiovascular events. However, further investigations are required to assess the potential benefit and risk concerning the cardiovascular system in PD patients chronically treated with non-ergot DAs. For this purpose, large population-based data, derived from trials specifically aimed at exploring the drug’s cardiovascular safety, should be systematically collected and analyzed.
Conflicts of Interest
The authors have no conflicts of interest to declare concerning the contents of the present article.
Financial Disclosures
None.
| References | ▴Top |
- Jankovic J. Parkinson's disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79(4):368-376.
doi pubmed - Shulman JM, De Jager PL, Feany MB. Parkinson's disease: genetics and pathogenesis. Annu Rev Pathol. 2011;6:193-222.
doi pubmed - Goldenberg MM. Medical management of Parkinson's disease. P T. 2008;33(10):590-606.
- Holloway RG, Shoulson I, Fahn S, Kieburtz K, Lang A, Marek K, McDermott M, et al. Pramipexole vs levodopa as initial treatment for Parkinson disease: a 4-year randomized controlled trial. Arch Neurol. 2004;61(7):1044-1053.
pubmed - Kulisevsky J, Pagonabarraga J. Tolerability and safety of ropinirole versus other dopamine agonists and levodopa in the treatment of Parkinson's disease: meta-analysis of randomized controlled trials. Drug Saf. 2010;33(2):147-161.
doi pubmed - Schade R, Andersohn F, Suissa S, Haverkamp W, Garbe E. Dopamine agonists and the risk of cardiac-valve regurgitation. N Engl J Med. 2007;356(1):29-38.
doi pubmed - Zanettini R, Antonini A, Gatto G, Gentile R, Tesei S, Pezzoli G. Valvular heart disease and the use of dopamine agonists for Parkinson's disease. N Engl J Med. 2007;356(1):39-46.
doi pubmed - Dewey RB, 2nd, Reimold SC, O'Suilleabhain PE. Cardiac valve regurgitation with pergolide compared with nonergot agonists in Parkinson disease. Arch Neurol. 2007;64(3):377-380.
doi pubmed - Corvol JC, Anzouan-Kacou JB, Fauveau E, Bonnet AM, Lebrun-Vignes B, Girault C, Agid Y, et al. Heart valve regurgitation, pergolide use, and parkinson disease: an observational study and meta-analysis. Arch Neurol. 2007;64(12):1721-1726.
doi pubmed - Antonini A, Poewe W. Fibrotic heart-valve reactions to dopamine-agonist treatment in Parkinson's disease. Lancet Neurol. 2007;6(9):826-829.
doi - Trifiro G, Mokhles MM, Dieleman JP, van Soest EM, Verhamme K, Mazzaglia G, Herings R, et al. Risk of cardiac valve regurgitation with dopamine agonist use in Parkinson's disease and hyperprolactinaemia: a multi-country, nested case-control study. Drug Saf. 2012;35(2):159-171.
doi pubmed - De Vecchis R, Esposito C, Ariano C. Cabergoline use and risk of fibrosis and insufficiency of cardiac valves. Meta-analysis of observational studies. Herz. 2013;38(8):868-880.
doi pubmed - Administration. UFaD. FDA drug safety communication: ongoing safety review of Parkinson's drug Mirapex (pramipexole) and possible risk of heart failure. http://www.fda.gov/Drugs/DrugSafety/ucm319779.htm (accessed December 29 2015).
- Arbouw ME, Movig KL, Guchelaar HJ, Neef C, Egberts TC. Dopamine agonists and ischemic complications in Parkinson's disease: a nested case-control study. Eur J Clin Pharmacol. 2012;68(1):83-88.
doi pubmed - Mokhles MM, Trifiro G, Dieleman JP, Haag MD, van Soest EM, Verhamme KM, Mazzaglia G, et al. The risk of new onset heart failure associated with dopamine agonist use in Parkinson's disease. Pharmacol Res. 2012;65(3):358-364.
doi pubmed - Renoux C, Dell'Aniello S, Brophy JM, Suissa S. Dopamine agonist use and the risk of heart failure. Pharmacoepidemiol Drug Saf. 2012;21(1):34-41.
doi pubmed - Hsieh PH, Hsiao FY. Risk of heart failure associated with dopamine agonists: a nested case-control study. Drugs Aging. 2013;30(9):739-745.
doi pubmed - Perez-Lloret S, Rey MV, Crispo J, Krewski D, Lapeyre-Mestre M, Montastruc JL, Rascol O. Risk of heart failure following treatment with dopamine agonists in Parkinson's disease patients. Expert Opin Drug Saf. 2014;13(3):351-360.
doi pubmed - Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
doi pubmed - Rascol O, Lees AJ, Senard JM, Pirtosek Z, Montastruc JL, Fuell D. Ropinirole in the treatment of levodopa-induced motor fluctuations in patients with Parkinson's disease. Clin Neuropharmacol. 1996;19(3):234-245.
doi pubmed - Adler CH, Sethi KD, Hauser RA, Davis TL, Hammerstad JP, Bertoni J, Taylor RL, et al. Ropinirole for the treatment of early Parkinson's disease. The Ropinirole Study Group. Neurology. 1997;49(2):393-399.
doi pubmed - Brooks DJ, Abbott RJ, Lees AJ, Martignoni E, Philcox DV, Rascol O, Roos RA, et al. A placebo-controlled evaluation of ropinirole, a novel D2 agonist, as sole dopaminergic therapy in Parkinson's disease. Clin Neuropharmacol. 1998;21(2):101-107.
pubmed - Wermuth L. A double-blind, placebo-controlled, randomized, multi-center study of pramipexole in advanced Parkinson's disease. Eur J Neurol. 1998;5(3):235-242.
doi pubmed - Lieberman A, Olanow CW, Sethi K, Swanson P, Waters CH, Fahn S, Hurtig H, et al. A multicenter trial of ropinirole as adjunct treatment for Parkinson's disease. Ropinirole Study Group. Neurology. 1998;51(4):1057-1062.
doi pubmed - Pinter MM, Pogarell O, Oertel WH. Efficacy, safety, and tolerance of the non-ergoline dopamine agonist pramipexole in the treatment of advanced Parkinson's disease: a double blind, placebo controlled, randomised, multicentre study. J Neurol Neurosurg Psychiatry. 1999;66(4):436-441.
doi pubmed - A controlled trial of rotigotine monotherapy in early Parkinson's disease. Arch Neurol. 2003;60(12):1721-1728.
doi pubmed - LeWitt PA, Lyons KE, Pahwa R. Advanced Parkinson disease treated with rotigotine transdermal system: PREFER Study. Neurology. 2007;68(16):1262-1267.
doi pubmed - Pahwa R, Stacy MA, Factor SA, Lyons KE, Stocchi F, Hersh BP, Elmer LW, et al. Ropinirole 24-hour prolonged release: randomized, controlled study in advanced Parkinson disease. Neurology. 2007;68(14):1108-1115.
doi pubmed - Singer C, Lamb J, Ellis A, Layton G. A comparison of sumanirole versus placebo or ropinirole for the treatment of patients with early Parkinson's disease. Mov Disord. 2007;22(4):476-482.
doi pubmed - Poewe WH, Rascol O, Quinn N, Tolosa E, Oertel WH, Martignoni E, Rupp M, et al. Efficacy of pramipexole and transdermal rotigotine in advanced Parkinson's disease: a double-blind, double-dummy, randomised controlled trial. Lancet Neurol. 2007;6(6):513-520.
doi - Barone P, Lamb J, Ellis A, Clarke Z. Sumanirole versus placebo or ropinirole for the adjunctive treatment of patients with advanced Parkinson's disease. Mov Disord. 2007;22(4):483-489.
doi pubmed - Barone P, Poewe W, Albrecht S, Debieuvre C, Massey D, Rascol O, Tolosa E, et al. Pramipexole for the treatment of depressive symptoms in patients with Parkinson's disease: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2010;9(6):573-580.
doi - Kieburtz K. Twice-daily, low-dose pramipexole in early Parkinson's disease: a randomized, placebo-controlled trial. Mov Disord. 2011;26(1):37-44.
doi pubmed - Poewe W, Rascol O, Barone P, Hauser RA, Mizuno Y, Haaksma M, Salin L, et al. Extended-release pramipexole in early Parkinson disease: a 33-week randomized controlled trial. Neurology. 2011;77(8):759-766.
doi pubmed - Schapira AH, Barone P, Hauser RA, Mizuno Y, Rascol O, Busse M, Salin L, et al. Extended-release pramipexole in advanced Parkinson disease: a randomized controlled trial. Neurology. 2011;77(8):767-774.
doi pubmed - Sampaio C, Bronzova J, Hauser RA, Lang AE, Rascol O, van de Witte SV, Theeuwes AA. Pardoprunox in early Parkinson's disease: results from 2 large, randomized double-blind trials. Mov Disord. 2011;26(8):1464-1476.
doi pubmed - Trenkwalder C, Kies B, Rudzinska M, Fine J, Nikl J, Honczarenko K, Dioszeghy P, et al. Rotigotine effects on early morning motor function and sleep in Parkinson's disease: a double-blind, randomized, placebo-controlled study (RECOVER). Mov Disord. 2011;26(1):90-99.
doi pubmed - Rascol O, Bronzova J, Hauser RA, Lang AE, Sampaio C, Theeuwes A, van de Witte SV. Pardoprunox as adjunct therapy to levodopa in patients with Parkinson's disease experiencing motor fluctuations: results of a double-blind, randomized, placebo-controlled, trial. Parkinsonism Relat Disord. 2012;18(4):370-376.
doi pubmed - Mizuno Y, Nomoto M, Kondo T, Hasegawa K, Murata M, Takeuchi M, Ikeda J, et al. Transdermal rotigotine in early stage Parkinson's disease: a randomized, double-blind, placebo-controlled trial. Mov Disord. 2013;28(10):1447-1450.
doi pubmed - Zhang Z, Wang J, Zhang X, Chen S, Wang Z, Zhang B, Liu C, et al. The efficacy and safety of ropinirole prolonged release tablets as adjunctive therapy in Chinese subjects with advanced Parkinson's disease: a multicenter, double-blind, randomized, placebo-controlled study. Parkinsonism Relat Disord. 2013;19(11):1022-1026.
doi pubmed - UCB Clinical Trial. Placebo-controlled Study in Patients With Parkinson's Disease to Evaluate the Effect of Rotigotine on Non-motor Symptoms. 2014. http://www.ucb.com/rd/clinical-study-information/neupro-rotigotine.
- Nicholas AP, Borgohain R, Chana P, Surmann E, Thompson EL, Bauer L, Whitesides J, et al. A randomized study of rotigotine dose response on 'off' time in advanced Parkinson's disease. J Parkinsons Dis. 2014;4(3):361-373.
pubmed - Mizuno Y, Nomoto M, Hasegawa K, Hattori N, Kondo T, Murata M, Takeuchi M, et al. Rotigotine vs ropinirole in advanced stage Parkinson's disease: a double-blind study. Parkinsonism Relat Disord. 2014;20(12):1388-1393.
doi pubmed - UCB Clinical Trial. Trial to Evaluate The Efficacy Of Rotigotine on Parkinson's Disease-Associated Motor Symptoms And Apathy (BRIGHT). 2015. http://www.ucb.com/rd/clinical-study-information/neupro-rotigotine (accessed December 18 2015).
- UCB Clinical Trial. Study to Evaluate the Efficacy of Rotigotine on Parkinson's Disease-Associated Pain (DOLORES). Secondary Study to Evaluate the Efficacy of Rotigotine on Parkinson's Disease-Associated Pain (DOLORES). 2015. http://www.ucb.com/rd/clinical-study-information/neupro-rotigotine.
- GSK clinical trials. A Fixed Dose, Dose Response Study for Ropinirole Prolonged Release in Patients With Early Stage Parkinson's Disease (TANDEM-662). 2015. http://www.gsk- clinicalstudyregister.com/study/111662#rs.
- Goldstein DS. Dysautonomia in Parkinson's disease: neurocardiological abnormalities. Lancet Neurol. 2003;2(11):669-676.
doi - Haensch CA, Lerch H, Jorg J, Isenmann S. Cardiac denervation occurs independent of orthostatic hypotension and impaired heart rate variability in Parkinson's disease. Parkinsonism Relat Disord. 2009;15(2):134-137.
doi pubmed - Ziemssen T, Reichmann H. Cardiovascular autonomic dysfunction in Parkinson's disease. J Neurol Sci. 2010;289(1-2):74-80.
doi pubmed - Apraxine M, Pasquet A, Jeanjean A. Pramipexole-Induced reversible heart failure. Mov Dis Clin Pract. 2014;1(4):381-382.
doi - Piercey MF, Hoffmann WE, Smith MW, Hyslop DK. Inhibition of dopamine neuron firing by pramipexole, a dopamine D3 receptor-preferring agonist: comparison to other dopamine receptor agonists. Eur J Pharmacol. 1996;312(1):35-44.
doi - Chernoloz O, El Mansari M, Blier P. Sustained administration of pramipexole modifies the spontaneous firing of dopamine, norepinephrine, and serotonin neurons in the rat brain. Neuropsychopharmacology. 2009;34(3):651-661.
doi pubmed - Gorell JM, Johnson CC, Rybicki BA. Parkinson's disease and its comorbid disorders: an analysis of Michigan mortality data, 1970 to 1990. Neurology. 1994;44(10):1865-1868.
doi pubmed - Zesiewicz TA, Strom JA, Borenstein AR, Hauser RA, Cimino CR, Fontanet HL, Cintron GB, et al. Heart failure in Parkinson's disease: analysis of the United States medicare current beneficiary survey. Parkinsonism Relat Disord. 2004;10(7):417-420.
doi pubmed - Stowe R, Ives N, Clarke CE, Deane K, Wheatley K, Gray R, Handley K, et al. Evaluation of the efficacy and safety of adjuvant treatment to levodopa therapy in Parkinson s disease patients with motor complications. Cochrane Database Syst Rev. 2010;7:CD007166.
- Ernst P, Renoux C, Dell'Aniello S, Suissa S. Pramipexole use and the risk of pneumonia. BMC Neurol. 2012;12:113.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


