| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Review
Volume 2, Number 2, April 2010, pages 55-61
Biomarkers Predicting Progression of Human Immunodeficiency Virus-Related Disease
Amar Kanekar
Department of Health Studies, 200 Prospect Street, Denike 14 B, East Stroudsburg University of Pennsylvania, East Stroudsburg, PA 18301-2999, USA
Manuscript accepted for publication February 23, 2010
Short title: HIV Progression Biomarkers
doi: https://doi.org/10.4021/jocmr2010.03.255w
| Abstract | ▴Top |
Biomarkers in predicting the progression of HIV infected individuals to a state of HIV disease (AIDS) are studied over more than a decade. Use of surrogate markers in the past for tracking clinical progression of the disease was limited, as little knowledge existed about the disease. The aim of this review was to address various changes in biomarker related studies taking place over the last five years, especially the trend towards use of newer biomarkers and experimentation with novel molecules in a quest for halting HIV disease progression. An open search of PUBMED database was made with search 'key words' such as 'Biomarkers' and 'AIDS (Acquired Immunodeficiency Syndrome)'.The following were the inclusion criteria for articles: a) all articles published in English language, b) years of publication between 2002-2008 and c) articles limited to adult population. This yielded a total of 417 articles. The criteria used for further judging these studies considered a) type of research design, b) number of biomarkers studied, c) validity of the biomarkers, d) techniques to assess the biomarkers and the impact of the studies in furthering biomarker research, e) sample size for the studies and f) article title or abstracts having the following key words 'biomarker' or 'biomarkers' and 'predict progression to AIDS'. A total of 27 abstracts were reviewed and 12 studies met the above criteria. These 12 different studies consisted of three reviews, four cohort designs, three cross-sectional designs, one each of an observational, and an in-vitro design. The various biomarkers emerging as a results were primarily a mix of viral, neural, immunological, HLA (human leukocyte antigen) markers along with lymphocyte counts. Although there have been quite a few advancements in biomarker-related studies, majority of the novel biomarkers discovered need to be further evaluated and replicated in bigger, long-term efficacy trials. Efforts should also be made to discover newer genetic markers of disease progression. Biomarker feedback, a new concept, can be utilized in future studies addressing prevention of HIV infection or halting disease progression.
Keywords: Biomarkers; Progression; Designs; HIV; AIDS; Validity
| Introduction | ▴Top |
Acquired immunodeficiency syndrome or AIDS consists of a constellation of symptoms which are suggestive of end-stages of human immunodeficiency virus (HIV) infection. This syndrome involves loss or decrease in immunity against certain non-threatening illnesses. The HIV infects certain cells of the immune system and can also directly infect brain [1]. Infected individuals are called HIV positive individuals but without AIDS. Most individuals progress from this state towards the disease (AIDS) [2].
The pathogenesis of HIV infection involves series of dynamic interactions between the HIV virus and the host immune cells, which results in a state of continuous immune activation throughout the course of the infection [3]. To assess the biomarkers related to AIDS, we need to understand the spectrum of this disease, i.e., from getting infected with the HIV virus to developing full blown AIDS. A review article which looked at the immunological markers and surrogate markers for predicting clinical progression from HIV infection to AIDS, was able to discuss the importance of markers such as β2 microglobulin [4-8], neopterin [9-11], sIL-2R [12, 13], sCD8 [14, 15], antibodies such as anti-p24 [16, 17], antigp120 [18, 19], anti-p17 [20], anti-gp41 [21], anti-nef [22, 1], anti-sCD4 [23], and anti-leucocyte antibodies [24]. Some of the additional biomarkers studied in the past are antigen markers such as p24 antigens [25, 26], serological markers such as tumor necrosis factor α [27, 28], acid-labile human leukocyte IFN (interferons), 2-5A synthetase, percentage of CD4+ T-cells, absolute CD4+ T-cell numbers, and CD4+/CD8 T-cell ratio. It is clearly seen that there have been a plethora of biomarkers which have been studied which predict the progression of an HIV infected individual to a state of active disease of AIDS. Despite of having a large quantity of surrogate markers for the disease, their clinical use still remains debatable, as they fail to fulfill some of the important criteria like 1) having clear role in the natural history of HIV-induced illness, 2) being detectable in the majority of infected individuals, 3) changing measurably with clinical status in both progression and remission of disease and 4) changing quantifiably after a therapeutic intervention or no change following failure of therapy. Moreover, very few studies have shown the effect of treatment on surrogate markers and long-term survival. There is a great need for validation of these studies in larger trials before surrogate marker measurements would be accepted universally as clinical end-points [3].
Current research in the arena of biomarkers studies related to HIV/AIDS continues to be experimental and bereft of validated biomarkers. There is a shift in the cellular markers of disease progression from lymphocyte predominance to other cellular markers such as monocyte-macrophage system [29]. These preliminary experimental studies need to be followed by more longitudinal studies which discover newer biomarkers which are associative as well as predictive of progression of disease.
The following systematic literature review attempts to look at various studies conducted and published by HIV researchers related to use of biomarkers and its role in association or prediction of HIV infection or AIDS progression.
| Methodology | ▴Top |
An open search of PUBMED database was made with search 'key words' such as 'Biomarkers' and 'AIDS (Acquired Immunodeficiency Syndrome)'. There were 2533 hits with this search. The following were the inclusion criteria for articles: a) all articles published in English language, b) years of publication between 2002-2008 and c) articles limited to the adult population. This yielded a total of 417 articles. The criteria used for further judging these studies considered a) type of research design, b) number of biomarkers studied, c) validity of the biomarkers, d) techniques to assess the biomarkers and the impact of the studies in furthering biomarker research, e) sample size for the studies and f) article title or abstracts having the following key words 'biomarker' or 'biomarkers' and 'predict progression to AIDS'. A total of 27 abstracts were reviewed and 12 studies met the above criteria.
The Figure 1 is a summary of the study flow which states the progress through stages of the systematic review predicting progression of HIV virus related disease.
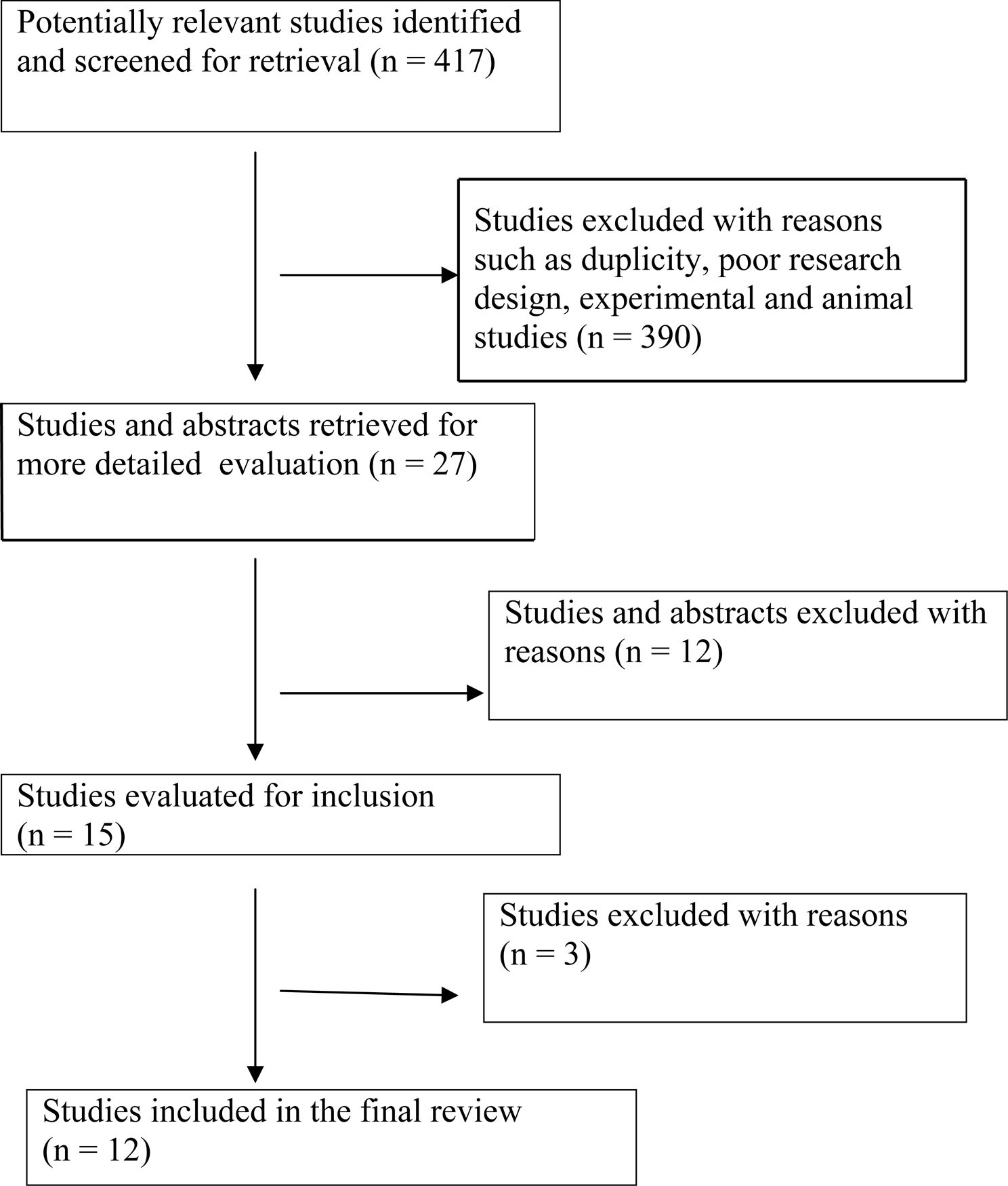 Click for large image | Figure 1. Progress through stages of systematic review of studies predicting progression of HIV virus related disease. |
| Results | ▴Top |
A summary of each study including the research design used, type of biomarkers studied the main outcomes of the study and relevant conclusions drawn are presented in Table 1. There were three review studies [40, 29, 43] which showed the importance of a) blood and cerebrospinal fluid markers such as CSF HIV 1 RNA and proviral HIV DNA, b) increase in macrophage makers and c) plasma viral HIV RNA, P24 antigen and CD4 cell counts, as biomarkers of progression of HIV infection. Three cross-sectional studies [31-33] looked at C-reactive protein and HLA (Human leukocyte antigens and soluble toll-like receptors as effective biomarkers of disease progression. This clearly shows the importance of immunological markers in the study of disease progression. There were four cohort studies [30, 34-36] which looked at immunoglobulin (IgG levels), neural markers such as sphingomyelin, TLC (total lymphocyte count), hemoglobin, and the CD4 cell counts to determine disease progression.
An observational study [41] used cox proportional hazards regression model to determine association between percent CD4 and disease progression while an in-vitro study [42] looked at regulatory T-cells as predictors of disease worsening. This study also showed a potential of developing new biomarkers by manipulating the regulatory T-cells.
 Click to view | Table 1. Biomarkers Studies Related to HIV Infection or Acquired Immunodeficiency Syndrome (Review Year 2002-2008) |
| Discussion | ▴Top |
Biomarkers related to HIV infection or which predict the progress of HIV infection to AIDS are wide and varied. The trend in last 15 years in conducting biomarker studies has shown a shift from using immunological and surrogate markers such as β2-microglobulin [30, 5], neopterin [9, 10] and anti-leucocyte antibodies [24] to some newer markers such as sTLR2 (soluble toll-like receptors) [31], C-reactive protein [32] and HLA (human leukocyte antigen) markers [33]. There seems to be more research studies which are looking at neuropathogenic biomarkers predicting HIV infection of the central nervous system.
The biological basis for some of the newer markers (such as quinolinic acids, chemokines, and matrix metalloproteinases) in neuropathogenic HIV disease is surrogates as well as causal agents [29]. Looking at other important biomarkers, β-2 microglobulin and neopterin indicate the degree of immunological activation, CD4+ T-helper cells which have prognostic and diagnostic importance and HIV-specific antibodies whose decline is crucial in indicating prognosis of the disease [3]. The role of C-reactive protein in HIV pathogenesis is yet unclear [30] and rise in sphingomyelin, ceramides is due to oxidative stress which in turn is due to loss of cellular homeostasis in HIV infection [34].
There is a mix of cross-sectional, cohort and review studies which attempt to study biomarkers predicting progression of the disease. Some of these studies were associative studies and need replication in bigger trials. Validation of these various biomarkers is the need of the hour as some of the studies reviewed were experimental. The assessment of biomarkers were for predicting and establishing diagnosis of acquired immunodeficiency syndrome, understanding the pathogenic process of disease progression and developing newer genetic and surrogate markers for understanding the disease process. Surrogate marker usage in the past and at present continues, as they have a potential for reducing the cohort size required to conduct such studies and the duration of each trial.
Although biomarkers are useful in predicting the severity and progression of the disease, they should always be correlated with clinical disease progression. In developing world where access to advanced biomarker detection techniques is less, markers such as total lymphocyte count and hemoglobin concentration are useful in monitoring disease progression as shown in a prospective cohort study [35]. The past decade has seen extraordinary advancements in HIV therapy and with the advent of viral protease inhibitors in 1995, has invited multi-drug combination protocols such as highly-active anti-retroviral therapy (HAART). The effect of the HAART in changing the immunological profile of HIV infected individuals showed a decrease in absolute CD4, memory CD4 and nave CD4 lymphocytes while a prospective study showed no difference in time to AIDS progression when CD4 cells were used as the biomarkers. Although CD4 cells continue to be used as one of the best predictive biomarkers for disease progression, a suggestion of using HIV RNA is made from prognostic point of view [36].
The uses of genetic biomarkers for predicting progression of the disease shows mixed evidence. An international meta-analysis, which tried to assess the role of CCR5 delta 32 and CCR2-641 alleles on disease progression among pediatric population, showed no protection over long term [37] while another meta-analysis carried out in United States, Europe and Australia among adult population showed decreased risk of progression to AIDS and deaths [38]. Hence, it seems we need more studies to be fully convinced of the protective effects of these alleles in predicting HIV progression.
One of the ways of acquiring HIV infection is from indulging in unsafe sexual practices. It would be helpful for us as researchers, to know if biomarkers and biomarker feedback can be effective in generating healthy behavior change as a primary prevention strategy. Biomarker feedback has been used to study various behaviors such as smoking cessation, dietary change and increased physical activity with mixed results [39]. It can either make an individual motivated and getting ready for a behavioral change, or cause immense psychological distress and adverse behavioral changes. There is a need of additional research studies in these areas.
| Conclusions | ▴Top |
In conclusion, the last decade has shown some advances in biomarker definitions and techniques for biomarker assays. These need to be validated over long-term by replicating studies in larger trials and with different population groups. Some of the biomarkers discovered are still in their nascent stages and may prove to be useful in future.
| References | ▴Top |
- Barhaoui E, Benjouad A, Sabatier J, Allain J, Laurian Y, Montaignier L, Gluckman JC.. Relevance of anti-nef antibody detection as an early serological marker of human immunodeficiency virus infection. Blood. 1990;76:257-264.
- Jenkins B, Lamar VL, Crumble JT. AIDS among African-Americans: a social epidemic. Journal of Black Psychology. 1993;19:108-122.
- Tsoukas CM, Bernard NF. Markers predicting progression of human immunodeficiency virus-related disease. Clin Microbiol Rev. 1994;7(1):14-28.
pubmed - Calabrese LH, Proffitt MR, Gupta MK, Easley KA, Walker JR, Rehm SJ, Valenzuela R,
et al . Serum beta 2-microglobulin and interferon in homosexual males: relationship to clinical findings and serologic status to the human T lymphotropic virus (HTLV-III). AIDS Res. 1984;1(6):423-438.
pubmed - Grieco MH, Reddy MM, Kothari HB, Lange M, Buimovici-Klein E, William D. Elevated beta 2-microglobulin and lysozyme levels in patients with acquired immune deficiency syndrome. Clin Immunol Immunopathol. 1984;32(2):174-184.
pubmed - Bethea M, Forman DT. Beta 2-microglobulin: its significance and clinical usefulness. Ann Clin Lab Sci. 1990;20(3):163-168.
pubmed - Bhalla RB, Safai B, Pahwa S, Schwartz MK. B2microglobulin as a prognostic marker for development of AIDS. Clin Chem. 1985;31:411-412.
- Fahey JL, Taylor JM, Detels R, Hofmann B, Melmed R, Nishanian P, Giorgi JV. The prognostic value of cellular and serologic markers in infection with human immunodeficiency virus type 1. N Engl J Med. 1990;322(3):166-172.
pubmed - Kofler H, Fuchs D, Hintner H, Wachter H, Fritsch P. Urinary neopterin: an early marker of HIV infection. Eur J Clin Microbiol. 1987;6(6):698-699.
pubmed - Lambin P, Desjobert H, Debbia M, Fine JM, Muller JY. Serum neopterin and beta 2-microglobulin in anti-HIV positive blood donors. Lancet. 1986;2(8517):1216.
pubmed - Lambin P, Lefrere JJ, Doinel C, Fine JM, Salmon D, Salmon C. Neopterin and beta 2-microglobulin in serum of HIV-seropositive subjects during a two-year follow-up. Clin Chem. 1988;34(6):1367-1368.
pubmed - Prince HE, Kleinman S, Williams AE. Soluble IL-2 receptor levels in serum from blood donors seropositive for HIV. J Immunol. 1988;140(4):1139-1141.
pubmed - Kloster BE, John PA, Miller LE, Rubin LA, Nelson DL, Blair DC, Tomar RH. Soluble interleukin 2 receptors are elevated in patients with AIDS or at risk of developing AIDS. Clin Immunol Immunopathol. 1987;45(3):440-446.
pubmed - Yagi MJ, Chu FN, Jiang JD, Wallace J, Mason P, Liu Y, Carafa J,
et al . Increases in soluble CD8 antigen in plasma, and CD8+ and CD8+CD38+ cells in human immunodeficiency virus type-1 infection. Clin Immunol Immunopathol. 1992;63(2):126-134.
pubmed - Griffin DE, McArthur JC, Cornblath DR. Soluble interleukin-2 receptor and soluble CD8 in serum and cerebrospinal fluid during human immunodeficiency virus-associated neurologic disease. J Neuroimmunol. 1990;28(2):97-109.
pubmed - Farzadegan H, Chmiel JS, Odaka N, Ward L, Poggensee L, Saah A, Phair JP. Association of antibody to human immunodeficiency virus type 1 core protein (p24), CD4+ lymphocyte number, and AIDS-free time. J Infect Dis. 1992;166(6):1217-1222.
pubmed - Sheppard HW, Ascher MS, McRae B, Anderson RE, Lang W, Allain JP. The initial immune response to HIV and immune system activation determine the outcome of HIV disease. J Acquir Immune Defic Syndr. 1991;4(7):704-712.
pubmed - Cianciolo GJ, Bogerd H, Snyderman R. Human retrovirus-related synthetic peptides inhibit T lymphocyte proliferation. Immunol Lett. 1988;19(1):7-13.
pubmed - Ugen KE, Goedert JJ, Boyer J, Refaeli Y, Frank I, Williams WV, Willoughby A,
et al . Vertical transmission of human immunodeficiency virus (HIV) infection. Reactivity of maternal sera with glycoprotein 120 and 41 peptides from HIV type 1. J Clin Invest. 1992;89(6):1923-1930.
pubmed - Lange JM, de Wolf F, Krone WJ, Danner SA, Coutinho RA, Goudsmit J. Decline of antibody reactivity to outer viral core protein p17 is an earlier serological marker of disease progression in human immunodeficiency virus infection than anti-p24 decline. AIDS. 1987;1(3):155-159.
pubmed - Chou MJ, Lee TH, Hatzakis A, Mandalaki T, McLane MF, Essex M. Antibody responses in early human immunodeficiency virus type 1 infection in hemophiliacs. J Infect Dis. 1988;157(4):805-811.
pubmed - Raska K
Jr. , Kim HC, Raska K3rd , Martin E, Raskova J, Saidi P. Human immunodeficiency virus (HIV) infection in haemophiliacs: long-term prognostic significance of the HIV serologic pattern. Clin Exp Immunol. 1989;77(1):1-6.
pubmed - Sekigawa I, Groopmen JE, Allan JD, Ikeuchi K, Biberfield G, Takatsuki K, Byrn RA. Characterization of autoantibodies to the CD4 molecule in human immunodeficiency virus infection. Clin Immunol Immunopathol. 1991;58(1):145-153.
pubmed - Kiprov DD, Anderson RE, Morand PR, Simpson DM, Chermann JC, Levy JA, Ross AR. Antilymphocyte antibodies and seropositivity for retroviruses in groups at high risk for AIDS. N Engl J Med. 1985;312(23):1517.
pubmed - Eyster ME, Ballard JO, Gail MH, Drummond JE, Goedert JJ. Predictive markers for the acquired immunodeficiency syndrome (AIDS) in hemophiliacs: persistence of p24 antigen and low T4 cell count. Ann Intern Medx. 1989;110(12):963-969.
pubmed - Moss AR, Bacchetti P, Osmond D, Krampf W, Chaisson RE, Stites D, Wilber J,
et al . Seropositivity for HIV and the development of AIDS or AIDS related condition: three year follow up of the San Francisco General Hospital cohort. Br Med J (Clin Res Ed). 1988;296(6624):745-750.
pubmed - Lahdevirta J, Maury CP, Teppo AM, Repo H. Elevated levels of circulating cachectin/tumor necrosis factor in patients with acquired immunodeficiency syndrome. Am J Med. 1988;85(3):289-291.
pubmed - Mintz M, Rapaport R, Oleske JM, Connor EM, Koenigsberger MR, Denny T, Epstein LG. Elevated serum levels of tumor necrosis factor are associated with progressive encephalopathy in children with acquired immunodeficiency syndrome. Am J Dis Child. 1989;143(7):771-774.
pubmed - Gendelman HE. Biomarkers, laboratory, and animal models for the design and development of adjunctive therapies for HIV-1 dementia and other neuroinflammatory disorders. J Neuroimmune Pharmacol. 2007;2(1):8-13.
pubmed - Carbone J, Sarmiento E, Rodriguez-Molina JJ, Gil J, Fernandez-Cruz E. Immunoglobulin levels and prediction of progression to AIDS in HIV-infected injection drug users. AIDS Patient Care STDS. 2004;18(12):685-686.
pubmed - Heggelund L, Flo T, Berg K, Lien E, Mollnes TE, Ueland T, Aukrust P,
et al . Soluble toll-like receptor 2 in HIV infection: association with disease progression. Aids. 2004;18(18):2437-2439.
pubmed - Lau B, Sharrett AR, Kingsley LA, Post W, Palella FJ, Visscher B, Gange SJ. C-reactive protein is a marker for human immunodeficiency virus disease progression. Arch Intern Med. 2006;166(1):64-70.
pubmed - Fernandes AP, Goncalves MA, Zavanella RB, Figueiredo JF, Donadi EA, Rodrigues ML. HLA markers associated with progression to AIDS are also associated with susceptibility to cytomegalovirus retinitis. Aids. 2003;17(14):2133-2136.
pubmed - Sacktor N, Haughey N, Cutler R, Tamara A, Turchan J, Pardo C, Vargas D,
et al . Novel markers of oxidative stress in actively progressive HIV dementia. J Neuroimmunol. 2004;157(1-2):176-184.
pubmed - Lau B, Gange SJ, Phair JP, Riddler SA, Detels R, Margolick JB. Rapid declines in total lymphocyte counts and hemoglobin concentration prior to AIDS among HIV-1-infected men. Aids. 2003;17(14):2035-2044.
pubmed - Jacobson LP, Li R, Phair J, Margolick JB, Rinaldo CR, Detels R, Munoz A. Evaluation of the effectiveness of highly active antiretroviral therapy in persons with human immunodeficiency virus using biomarker-based equivalence of disease progression. Am J Epidemiol. 2002;155(8):760-770.
pubmed - Ioannidis JP, Contopoulos-Ioannidis DG, Rosenberg PS, Goedert JJ, De Rossi A, Espanol T, Frenkel L,
et al . Effects of CCR5-delta32 and CCR2-64I alleles on disease progression of perinatally HIV-1-infected children: an international meta-analysis. Aids. 2003;17(11):1631-1638.
pubmed - Ioannidis JP, Rosenberg PS, Goedert JJ, Ashton LJ, Benfield TL, Buchbinder SP, Coutinho RA,
et al . Effects of CCR5-Delta32, CCR2-64I, and SDF-1 3'A alleles on HIV-1 disease progression: An international meta-analysis of individual-patient data. Ann Intern Med. 2001;135(9):782-795.
pubmed - McClure JB. Are biomarkers useful treatment aids for promoting health behavior change? An empirical review. Am J Prev Med. 2002;22(3):200-207.
pubmed - Price RW, Epstein LG, Becker JT, Cinque P, Gisslen M, Pulliam L, McArthur JC. Biomarkers of HIV-1 CNS infection and injury. Neurology. 2007;69(18):1781-1788.
pubmed - Hulgan T, Shepherd BE, Raffanti SP, Fusco JS, Beckerman R, Barkanic G, Sterling TR. Absolute count and percentage of CD4+ lymphocytes are independent predictors of disease progression in HIV-infected persons initiating highly active antiretroviral therapy. J Infect Dis. 2007;195(3):425-431.
pubmed - Nilsson J, Boasso A, Velilla PA, Zhang R, Vaccari M, Franchini G, Shearer GM,
et al . HIV-1-driven regulatory T-cell accumulation in lymphoid tissues is associated with disease progression in HIV/AIDS. Blood. 2006;108(12):3808-3817.
pubmed - Kiepiela P, Smith AN, Rosenberg E. Laboratory markers associated with progression of HIV infection. Best Pract Res Clin Obstet Gynaecol. 2005;19(2):243-254.
pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


