| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Original Article
Volume 8, Number 5, May 2016, pages 389-395
Diagnostic Value of Hook Wire Localization Technique for Non-Palpable Breast Lesions
Gokhan Demirala, Metin Senolb, g, Baris Bayraktarc, Hasan Ozturkd, Yahya Celike, Salih Bolukf
aRize State Hospital, General Surgery Clinic, Rize, Turkey
bPrivate Adatip Hospital, General Surgery Clinic, Sakarya, Turkey
cPrivate Uzmanlar Hospital, General Surgery Clinic, Yalova, Turkey
dRadiology Department, Faculty of Medicine, Ordu University, Ordu, Turkey
eGebze Fatih State Hospital, General Surgery Clinic, Kocaeli, Turkey
fCankiri State Hospital, General Surgery Clinic, Cankiri, Turkey
gCorresponding Author: Metin Senol, Private Adatip Hospital, General Surgery Clinic, Sakarya, Turkey
Manuscript accepted for publication February 23, 2016
Short title: Non-Palpable Breast Lesions
doi: http://dx.doi.org/10.14740/jocmr2498w
| Abstract | ▴Top |
Background: The aim of this study was to investigate the validity of hook wire localization biopsy for non-palpable breast lesions which were detected by ultrasonography (USG) or mammography (MMG).
Methods: In this retrospective study, USG or MMG-guided hook wire localization technique was performed on 83 patients who had non-palpable breast lesions. Then histopathological examination was performed on surgically removed specimens. All patients’ mammograms or ultrasonograms were categorized using Breast Imaging-Reporting and Data System (BI-RADS) classification.
Results: Radiologically, 27 (32.53%) patients were classified as BI-RADS 3, 49 (59.04%) BI-RADS 4, one (1.2%) BIRADS 5 and six (7.23%) BI-RADS 0. Histopathological results were benign in 68 (81.9%) and malignant in 15 (18.1%) patients. Twenty-seven patients were classified as BI-RADS 3 and definitive diagnoses for all were benign. Besides, 49 patients were classified as BI-RADS 4 and histopathologically 14 of them were reported as malignant, and 35 as benign. Sensitivity of MMG was 93% and specificity was 55%. For USG, the sensitivity was 100% and the specificity was 73%.
Conclusion: In early diagnosis of breast cancer, the validity of the imaging-guided hook wire localization biopsy of non-palpable breast lesions has been proved. The cooperation of surgeon, radiologist and pathologist increases the successfull results of hook wire localization technique.
Keywords: Breast cancer; Hook wire localization; Non-palpable breast lesion
| Introduction | ▴Top |
In recent years, extensive use of screening mammography (MMG) and women’s increased awareness of breast cancer have resulted in an increase in the number of non-palpable breast lesions [1]. The standard method used in the localization of non-palpable lesions is the hook wire localization technique [2-5]. The most common indication for a hook wire localization biopsy is the detection of a focus of suspicious microcalcifications or the diagnosis of a non-palpable lesion. Among the patients who have undergone a biopsy for a non-palpable lesion and diagnosed with malignancy, 98% of them had disease-free survival [6]. Thus, by facilitating the capture of malignant lesions at an early stage, the disease can be cured and the patient’s quality of life can be increased [4, 7].
The localization of a lesion is performed by MMG or ultrasonography (USG). All lesions visualized on USG must be localized by USG. The advantages of this technique over performance by MMG include lack of ionizing radiation, lack of requirement for a detailed equipment and patients’ horizontal positioning. Lesions with microcalcifications and structural parenchymal distortions that cannot be detected by USG must be localized by MMG (Fig. 1).
 Click for large image | Figure 1. Mammographic appearance of spiculated breast lesion with microcalcifications. |
The aim of this study was to investigate the validity of hook wire localization biopsy by imaging guidance for non-palpable breast lesions which were detected by USG or MMG.
| Materials and Methods | ▴Top |
The study protocol was approved by the Goztepe Research and Training Hospital Ethical Committee. Informed consent was obtained from all patients. At Goztepe Research and Training Hospital in Istanbul, 83 patients were analyzed retrospectively who had non-palpable breast lesions and were performed excisional biopsy via MMG or USG-guided hook wire localization technique in 2 years. These 83 patients were selected from breast cancer screening program that was done by general surgery outpatient clinic. The patients who had non-palpable breast lesions and malignancy suspicious criterion like microcalcifications, etc. at MMG or USG were included in this study. Age of the patients, Breast Imaging-Reporting and Data System (BI-RADS) values of the lesions and the radiomorphologic features assessed by MMG or USG were compared to histopathologic results to calculate the odds ratio (OR). For all cases, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), accuracy, positive and negative likelihood ratio (LR) values of MMG, USG and the frozen section were calculated. In addition, the incidence of histological results was calculated and in situ and invasive cancer numbers for each radiographic image were found.
Localization technique
In all patients, MMG and USG were performed in the Department of Radiology at the same hospital followed by hook wire localization. Lesions detectable with either MMG (Senographe Senix 600T; General Electric CGR, France) or USG (7.5 mHz, SSA-770A/80 Aplio; Toshiba, Japan, 5 - 11 mHz, Acuson 150; Siemens, Mountain View, USA and Logic 9; General Electric, USA) were localized with the used method whereas lesions detectable with both MMG and USG were localized via USG guidance (Figs. 2, 3). The localization was considered to be successful when the tip of the wire was within the lesion. For localization, a guide wire with a curved end (hook wire) (Hawkins III Hardwire BLN/Inter-V; Angiotech, Switzerland) was used and no local anesthesia was applied during the procedure.
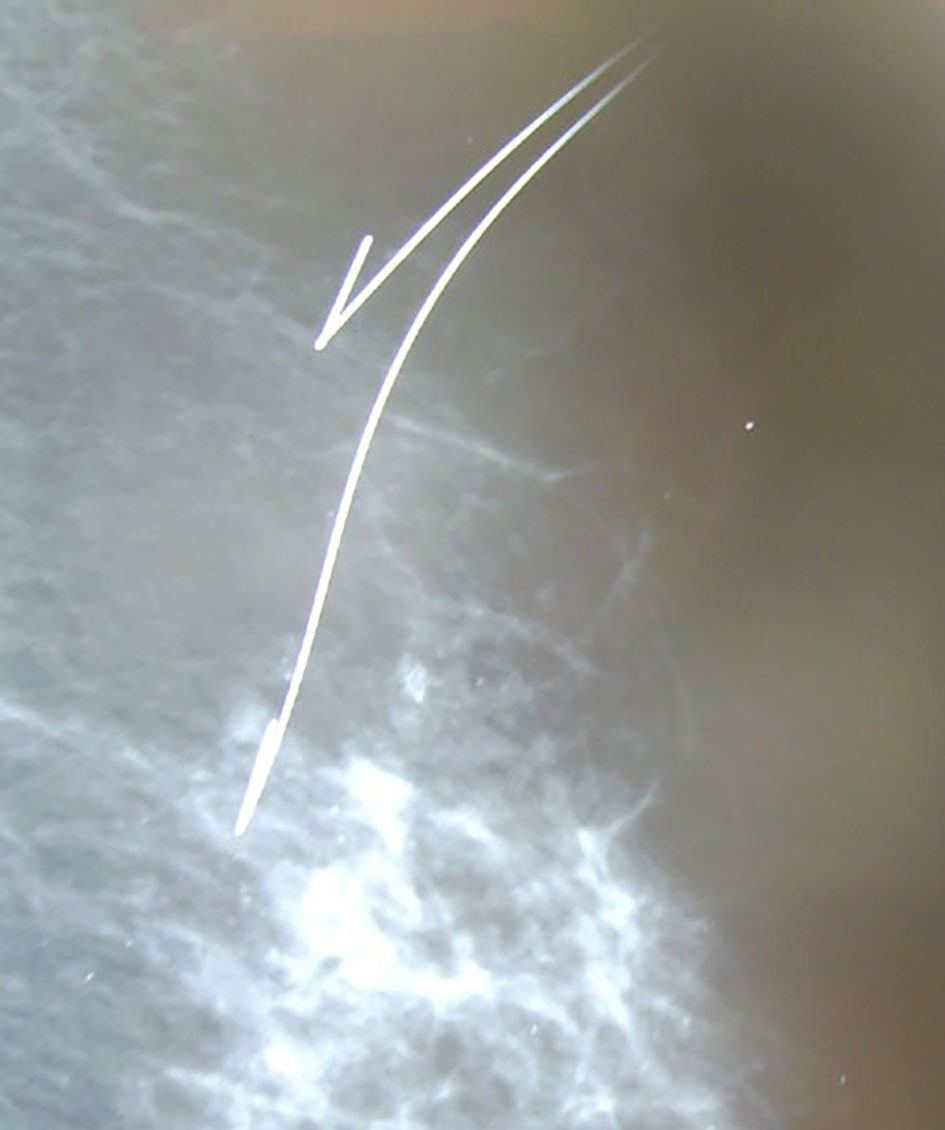 Click for large image | Figure 2. Hook wire localization of spiculated lesion by mammography. |
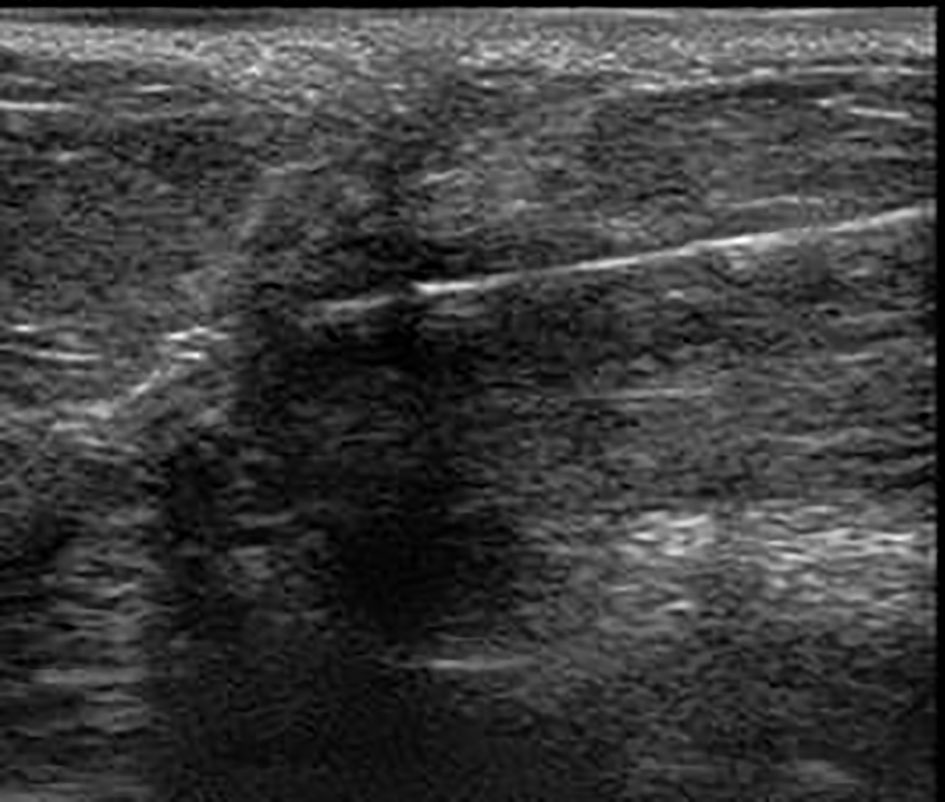 Click for large image | Figure 3. Hook wire localization by ultrasonography. |
After marking with the hook wire system in the Department of Radiology by the same radiologist, patients were sent to the Department of Surgery to be taken to operating room the same day, within a maximum interval of 4 h. The wire-marked area was excised to a margin of at least 1 cm of surrounding tissue under general anesthesia. The excised tissue was controlled via specimen graphy by X-ray before the pathological examination. The surgical team was informed after verification of the removal of the required piece in the specimen graphy. The borders of the specimen were marked with sutures in order to guide the pathologist. The intraoperatively excised piece of tissue in patients who underwent frozen section analysis was sent to pathology laboratory in a fresh state without being placed in an identification solution and examinations were finalized within 20 - 30 min after freezing the section and obtaining small slices. In benign assessments, the incision was closed without drain placement in the cavity. In a malignant frozen section, if the surgical margin was negative, the operation was completed with the performance of an axillary dissection. If the surgical margin was positive, a re-excision was performed and an axillary dissection was completed via separate incision. In patients with uncertain frozen sections, procedure was performed as if it was malignant. In others, the process was terminated and the paraffin result was awaited. Lesions recommended for excisional biopsy were classified according to BI-RADS classification.
Statistical analysis
Number Cruncher Statistical System (NCSS-2007) (Utah, USA) package program was used. In the evaluation of data and in comparison of qualitative data, the Fisher’s exact test, the relative ratio and a 95% confidence interval (CI) were used as well as descriptive statistical methods (average, standard deviation, and median). The sensitivity, specificity, PPV, NPV, accuracy and positive and negative LR of the variables were calculated. The results were evaluated to be significant at P < 0.05.
| Results | ▴Top |
A total of 83 cases with ages ranging from 17 to 80 years were examined. The average age of patients was 47.46 ± 10.254 years. Fifty-six patients underwent MMG-guided hook wire localization and 27 patients underwent USG-guided hook wire localization. Paraffin results of 68 patients (81.9%) were benign and of 15 patients (18.1%) were malignant. In some patients, there were also two or more histopathological results together (Table 1). The frozen section was evaluated in 47 patients.
 Click to view | Table 1. Distribution of Histopathological Results of Frozen and Permanent Incision |
In the 55 - 65 years age group, the presence of malignancy was found to be statistically significantly higher than in other groups (P = 0.01). In this group, the probability of malignancy was 5.81 times (1.59 - 21.2) higher than other age groups (Table 2). In addition, when the age group of ≥ 45 years was examined separately, the presence of malignancy was found to be statistically significantly higher than the age group of ≤ 45 years (P = 0.04). In the age group of ≥ 45 years, the possibility of malignancy was found to be 2.95 times (1.02 - 8.53) higher than the age group of ≤ 45 years.
 Click to view | Table 2. Distribution of Pathological Results According to Age Groups |
Twenty-seven cases were classified as BI-RADS 3, 49 cases as BI-RADS 4, one case as BI-RADS 5 and six cases as BI-RADS 0. According to BI-RADS classification, statistically significant differences at an advanced level were found between the results of pathological examinations of permanent sections. In the BI-RADS 3 group, the presence of malignancy was found to be statistically significantly lower than the other groups (P = 0.001). However, in the BI-RADS 4 group, the presence of malignancy was found to be statistically significantly higher than the other groups (P = 0.002). In the BI-RADS 4 group, the likelihood of malignancy was found to be 13.2 times (1.6 - 10.3) higher than the other groups (Table 3). No statistically significant difference was observed between the presence of malignancy in BI-RADS 5 and BI-RADS 0 groups.
 Click to view | Table 3. Distribution of Pathological Results According to BI-RADS |
While examining BI-RADS 4 sub-classes, a statistically significant presence of malignancy was found in BI-RADS 4b and BI-RADS 4c (P = 0.035 and P = 0.023). In addition, the likelihood of malignancy was found to be 3.86 times higher in BI-RADS 4b and 4.83 times higher in BI-RADS 4c (Table 4).
 Click to view | Table 4. Distribution of Pathological Results According to BI-RADS 4 Sub-Classes |
For permanent incision/MMG, the sensitivity was calculated as 0.93, the specificity as 0.55, PPV as 0.41, NPV as 0.96, the accuracy of the test as 0.64 and LR(+) as 2.05. In other words, for a patient identified as malignant in MMG, the probability of being malignant in paraffin also was 2.05 times more than benign (Table 5).
 Click to view | Table 5. Analysis of Pathological Results of Mammography, Ultrasound, Frozen and Permanent Incision |
For permanent incision/USG, the sensitivity was calculated as 1.00, the specificity as 0.73, the PPV as 0.13, the NPV as 1.00, the accuracy of the test as 0.74 and LR(+)as 3.71. In other words, for a patient identified as malignant in USG, the probability of being malignant in paraffin also was 3.71 times more than benign (Table 5).
For permanent incision/frozen, the sensitivity was calculated as 0.60, the specificity as 0.97, the PPV as 0.86, the NPV as 0.90, the accuracy of the test as 0.89 and the LR(+) as 21.6. In other words, for a patient identified as malignant in frozen, the probability of being malignant in paraffin also was 21.6 times more than benign. A significant consistency was observed between routine permanent section tests and frozen section examinations (Table 5).
| Discussion | ▴Top |
The localization of a lesion is performed by MMG or USG. Specimen radiography should be performed in surgically excised lesions with microcalcifications and with masses or distortions where removal cannot be ensured. In specimen radiography, lesion’s being excised or not and distance to the surgical margin have to be evaluated [2, 3, 8]. In this study, specimen radiography was performed for all lesions that were mammographically marked.
Breast localization is generally performed using hook wire pins developed by Kopans. MMG and USG enable the bidirectional localization of the hook wire and the surgeon to reach the lesion by localizing the projection of the hook wire on the skin’s surface. More than one wire can be used if the boundary of the lesion is not clear [5, 7].
Although the hook wire localization technique is highly effective, it still has some disadvantages. Surgeon’s entry point of the wire being far from the ideal location of incision may result in the excision of a large area by the surgeon. The most common complication during localization is a vasovagal reaction (10%). Additional complications and disadvantages include difficulty in localizing the hook wire into dense breasts, shifting of the hook wire, patient’s discomfort, bleeding, infection, pneumothorax, hook wire’s being cut and residual tumors in the biopsy cavity [8, 9]. It is considered unsuccessful if the lesion cannot be removed after localization. A failure rate of 0-18% (average 2.6%) has been reported in the literature. These situations are encountered mostly in lesions with unclear boundaries like microcalcifications and in MMG-guided localizations. Localizations and excisions by experienced surgeons have lower complication rates as well as being more successful [10]. In this study, only five patients experienced a vasovagal reaction (5.9%).
The frequency of detecting breast cancer in patients who underwent localization biopsy in non-palpable lesions varies between 10% and 36% [2-4]. Therefore, this necessitates being more selective in determining biopsy indications. Meanwhile, in situ cancer detection rate has increased with this new method and reported to be between 18% and 34% in various studies [3, 4, 11]. In Ozdemir’s series, the rate of in situ/invasive cancer has been reported as 36% and in this study it was emphasized that the rate of correct diagnosis has increased in time especially for early stage cancers and the frequency of unnecessary biopsies has decreased gradually [4]. Ductal carcinoma in situ (DCIS) accounts for 15-20% of all breast cancers and is detected via MMG rather than by physical examination [12]. In our study, consistent with the literature, the paraffin results of 68 (81.9%) patients were benign and of 15 (18.1%) patients were malignant. The pathology results of five patients out of 15 who were detected with cancer were reported as DCIS. The rate of in situ carcinomas among all malignancies was figured as 33.3% and the rate of in situ/invasive cancer was 50%. In this study, the reason for the low malignancy rate can be explained by the lower number of BI-RADS 5 cases that consist of uncertain microcalcifications and by the low rate of opacities compatible with microcalcifications. Two out of five patients diagnosed with DCIS were performed breast-conserving surgery whereas the other three patients underwent modified radical mastectomy.
Although the rate of benign lesions in series has been reported as 69-82%, these rates are not considered determinative for success. If the rate is too high, it may lead to unnecessary biopsies and when the rate is too low, it may lead to the omission of potentially malignant lesions that are too small to detect [13, 14]. Therefore, decision-making as a team and experience are very important. It should be emphasized that each biopsy procedure should aim to achieve cosmetic results while preserving the normal tissue as well as removing the lesion correctly. It is a fact that as the number of procedures increases in time, experience increases too.
Age appears to be the most significant independent risk factor [3]. There are articles stating that in non-palpable breast lesions, the age of the patient is reported as criterion for malignancy as well as the mammographic features of the lesion [3]. In this study, significantly higher rate of malignancy has been detected in patients over the age of 55 in respect to patients under 55. The presence of malignancy in the 55 - 65 age group was found to be statistically significantly higher than other age groups (P = 0.001). The probability of malignancy in this group has been detected as 5.81 times higher than other age groups (1.59 - 21.2).
BI-RADS, despite being a system developed for MMG scanning, has been in use in examinations via USG too [7]. Studies show that malignancy rate in BI-RADS 3 lesions is less than 8%, whereas in BI-RADS 4 lesions rate varies between 4% and 34%, and in BI-RADS 5 lesions between 54% and 97% [15, 16]. In this study, numbers of patients in BI-RADS 3, 4 and 5 groups were detected as 32.5%, 59% and 1.2%, respectively. Statistically significant differences as per BI-RADS degrees have been detected between permanent section pathology examination results. The presence of malignancy in BI-RADS 3 group (0%) has been detected as statistically significantly lower than other groups (P = 0.001). On the other hand, the presence of malignancy in BI-RADS 4 group (28.5%) has been detected as statistically significantly higher than other groups (P = 0.002). The probability of malignancy in BI-RADS 4 group has been detected as 13.2 times (1.6 - 10.3) higher than other groups. In this study, in BI-RADS 4 sub-classes, statistically significant malignancy presence has been detected in BI-RADS 4b and BI-RADS 4c (P = 0.035 and P = 0.023). The probability of malignancy has been detected as 3.86 times higher in BI-RADS 4b and as 4.83 times higher in BI-RADS 4c.
In this study, all 27 patients in the BI-RADS 3 group were identified with benign diseases. In the BI-RADS 4 group, 14 patients out of 49 (28.5%) were diagnosed with malignancy. In the BI-RADS 0 group, five cases were benign whereas one was malignant. In the BI-RADS 5 group, there was only patient and he/she was detected with benign pathology. No malignancy being detected in BI-RADS 3 group indicates more careful selection of patients. In addition, with only one patient in BI-RADS 5 group, it is not possible to compare this group with the current literature.
After determining PPVs according to the BI-RADS categories, approaches were also determined for radiologically detected lesions. A biopsy might be recommended in highly benign BI-RADS 3 lesions while considering patient’s anxiety, preferences and other risk factors. It is stated that 3- to 6-month follow-up is appropriate for patients without any biopsy. Biopsies are recommended for BI-RADS 4 and 5 groups too [15, 17].
MMG holds a very special place in the diagnosis of non-palpable breast lesions and its sensitivity for detecting breast cancer ranges between 85% and 95% in various publications [2, 18, 19]. In another study conducted by Burhenne et al, the sensitivity of MMG was detected as 85% and the specificity as 30% [20]. In this study, sensitivity of MMG was 93% and specificity was 55%. For USG, the sensitivity was 100% and the specificity was 73%.
Studies have reported that the sensitivity of USG in detection of malignancy in non-palpable breast lesions is 89% and its PPV is 86% [21]. In this study, for USG, sensitivity was calculated as 100% and specificity as 73%.
The conduct of an intraoperative frozen section examination in non-palpable lesions is controversial. Although it is stated that this process may miss a small invasive carcinoma or microinvasive disease, it may still be used at centers with experienced pathology units. Frozen examination is recommended when there is enough tissue and if there is a significant expectation that it may change the course of surgery [22, 23]. If there is consistency between frozen examination and pathologic result, the operation can continue in the same session rather than patient’s waiting for a second surgery. In this study, frozen section was conducted in 47 cases with the appropriate mass diameter and in cases where the course of the operation is likely to change as mastectomy re-excisions. In diagnosis of malignancy in frozen, the presence of malignancy in permanent section too was detected statistically significantly higher than other findings (P = 0.0001). The possibility of malignancy in diagnosis of malignancy in frozen was found to be 10.4 times (5.04 - 22.8) higher than other findings.
In this study, for permanent section/frozen examination, sensitivity was calculated as 60%, specificity as 97%, PPV as 86%, NPV as 90%, the accuracy of the test as 89% and the LR(+) as 21.6. In a study conducted by Dorel-LeTheo et al, sensitivity and specificity between frozen and permanent section tests were detected as 87.6% and 100%, respectively [24]. Breast-conserving surgery (BCS) was performed in six suspected, five malignant and two benign patients diagnosed as a result of frozen section examinations. In cases where BCS was performed due to frozen being uncertain, the result of paraffin test was malignant in two patients whereas benign in four. In cases where BCS was performed due to frozen being malignant, the result of paraffin test was malignant in all five patients. In cases where BCS was performed due to frozen being benign, the result of paraffin test was malignant in one patient whereas benign in one. There were no false positives. In this study, low sensitivity was detected (10/47) due to large number of suspected results at the frozen test.
Conclusion
In early diagnosis of breast cancer, the validity of the imaging-guided hook wire localization biopsy of non-palpable breast lesions has been proved. In our study, the rate of detecting breast cancer with this method was determined as 18.1%. No malignancy has been detected in BI-RADS 3 group patients but close follow-up is recommended. Since significantly high rate of malignancy has been detected in BI-RADS 4 group, hook wire localization biopsy is definitely recommended. The success rate in hook wire localization technique increases when departments of surgery, radiology and pathology work in harmony.
Conflict of Interest
No conflict of interest was declared by the authors.
Financial Disclosure
The authors declared that this study has received no financial support.
| References | ▴Top |
- Altomare V, Guerriero G, Giacomelli L, Battista C, Carino R, Montesano M, Vaccaro D, et al. Management of nonpalpable breast lesions in a modern functional breast unit. Breast Cancer Res Treat. 2005;93(1):85-89.
doi pubmed - Balci P, Gunes N, Kocdor MA, et al. The results of preoperative localization at non-palpable breast lesions: mamographic analysis of the lesions. Meme Hastaliklari Dergisi. 1997;4:123-127.
- Bilgen IG, Memis A, Ustun EE. The retrospective analysis of 550 nonpalpable breast lesions with localisation biyopsy. Turk J Diagn Intervent Radiol. 2002;8:487-495.
- Ozdemir A. The analysis of 381 preoperatively localized nonpalpable breast lesions. Tan?sal ve Giri?imsel Radyoloji. 2000;6:314-322.
- Liberman L, Kaplan J, Van Zee KJ, Morris EA, LaTrenta LR, Abramson AF, Dershaw DD. Bracketing wires for preoperative breast needle localization. AJR Am J Roentgenol. 2001;177(3):565-572.
doi pubmed - Reintgen D, Cox C, Greenberg H, Baekey P, Nicosia S, Berman C, Clark R, et al. The medical legal implications of following mammographic breast masses. Am Surg. 1993;59(2):99-105.
pubmed - Kopans DB. Ultrasound and breast evaluation, 2nd ed. Philedelphia: Lippincott-Raven Publishers. 1998. p. 409-443.
- Derici H, Tansug T, Nazli O, et al. The stereotactic localisation and surgical excision of nonpalpable breast lesions. Meme Sagligi Dergisi. 2007;3:10-13.
- Mitnick JS, Vazquez MF, Harris MN, Buchbinder SS. Localization of transected wire. AJR Am J Roentgenol. 1991;156(4):866.
doi pubmed - Abrahamson PE, Dunlap LA, Amamoo MA, Schell MJ, Braeuning MP, Pisano ED. Factors predicting successful needle-localized breast biopsy. Acad Radiol. 2003;10(6):601-606.
doi - Tinnemans JG, Wobbes T, Holland R, Hendriks JH, Van der Sluis RF, De Boer HH. Treatment and survival of female patients with nonpalpable breast carcinoma. Ann Surg. 1989;209(2):249-253.
doi pubmed - Evans A. The diagnosis and management of pre-invasive breast disease: radiological diagnosis. Breast Cancer Res. 2003;5(5):250-253.
doi pubmed - Meyer JE, Amin E, Lindfors KK, Lipman JC, Stomper PC, Genest D. Medullary carcinoma of the breast: mammographic and US appearance. Radiology. 1989;170(1 Pt 1):79-82.
doi pubmed - Tukel S. Hook wire localisation before biyopsy at nonpalpable breast lesions. Tanisal ve Girisimsel Radyoloji. 1995;1:425-430.
- Sickles EA. Nonpalpable, circumscribed, noncalcified solid breast masses: likelihood of malignancy based on lesion size and age of patient. Radiology. 1994;192(2):439-442.
doi pubmed - Orel SG, Kay N, Reynolds C, Sullivan DC. BI-RADS categorization as a predictor of malignancy. Radiology. 1999;211(3):845-850.
doi pubmed - Nguyen M, McCombs MM, Ghandehari S, Kim A, Wang H, Barsky SH, Love S, et al. An update on core needle biopsy for radiologically detected breast lesions. Cancer. 1996;78(11):2340-2345.
doi - Bassett LW, Manjikian V, 3rd, Gold RH. Mammography and breast cancer screening. Surg Clin North Am. 1990;70(4):775-800.
pubmed - Liberman L, Morris EA, Dershaw DD, Abramson AF, Tan LK. Ductal enhancement on MR imaging of the breast. AJR Am J Roentgenol. 2003;181(2):519-525.
doi pubmed - Warren Burhenne LJ, Wood SA, D'Orsi CJ, Feig SA, Kopans DB, O'Shaughnessy KF, Sickles EA, et al. Potential contribution of computer-aided detection to the sensitivity of screening mammography. Radiology. 2000;215(2):554-562.
doi pubmed - van Dam PA, Van Goethem ML, Kersschot E, Vervliet J, Van den Veyver IB, De Schepper A, Buytaert P. Palpable solid breast masses: retrospective single- and multimodality evaluation of 201 lesions. Radiology. 1988;166(2):435-439.
doi pubmed - McGreevy JM, Loftus TJ. Outcomes evaluation for operative and nonoperative management of the abnormal mammogram. Am J Surg. 1998;175(1):69-72.
doi - Jackson VP. The current role of ultrasonography in breast imaging. Radiol Clin North Am. 1995;33(6):1161-1170.
pubmed - Dorel-LeTheo M, Dales JP, Garcia S, Ramuz O, Andrac-Meyer L, Bonnier P, Piana L, et al. [Accuracy of intraoperative frozen section diagnosis in non palpable breast lesions: a series of 791 cases]. Bull Cancer. 2003;90(4):357-362.
pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


