| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Case Report
Volume 8, Number 7, July 2016, pages 544-547
Posterior Reversible Encephalopathy Syndrome in Henoch-Schonlein Purpura and Hemolytic Uremic Syndrome
Kibriya Fidana, d, Yasar Kandura, Murat Ucarb, Kivilcim Gucuyenerc, Oguz Soylemezoglua
aDepartment of Pediatric Nephrology, Faculty of Medicine, Gazi University, Ankara, Turkey
bDepartment of Radiology, Faculty of Medicine, Gazi University, Ankara, Turkey
cDepartment of Pediatric Neurology, Faculty of Medicine, Gazi University, Ankara, Turkey
dCorresponding Author: Kibriya Fidan, Department of Pediatric Nephrology, Faculty of Medicine, Gazi University Hospital, Besevler, Ankara 06500, Turkey
Manuscript accepted for publication April 07, 2016
Short title: Posterior Reversible Encephalopathy Syndrome
doi: http://dx.doi.org/10.14740/jocmr2157w
| Abstract | ▴Top |
Posterior reversible encephalopathy syndrome (PRES) is a clinico-radiological syndrome, composed of symptoms such as headache, seizures, visual disturbances, lethargy, confusion, stupor, focal neurologic findings and radiological findings of bilateral gray and white matter abnormalities suggestive of edema in the posterior regions of the cerebral hemispheres. PRES is associated with significant morbidity and mortality if it is not expeditiously recognized. Magnetic resonance image (MRI) represents the most sensitive imaging technique for recognizing PRES. PRES has been seen in various clinical settings including renal disorders such as acute glomerulonephritis, lupus nephritis, nephrotic syndrome, and drug usage such as calcineurin inhibitors. We aimed to present two study cases for such clinical setting. In this report, we present two patients with PRES in whom the primary diagnosis was hemolytic uremic syndrome (HUS) and Henoch-Schonlein purpura (HSP). Both of them were treated with anticonvulsant and proper antihypertensive drugs. A repeated MRI scan of the head, an ophthalmologic assessment, and a follow-up electroencephalogram produced normal results with no sequelae. Early recognition of PRES as a complication during different diseases and therapies in childhood may facilitate the appropriate treatment, so that intensive treatment should be performed as soon as possible to avoid neurological sequelae.
Keywords: Posterior reversible encephalopathy syndrome; Hemolytic uremic syndrome; Henoch-Schonlein purpura
| Introduction | ▴Top |
Posterior reversible encephalopathy syndrome (PRES) is a clinico-radiological syndrome, comprising symptoms such as headache, seizures, visual disturbances, lethargy, confusion, stupor, and focal neurologic findings [1]. In addition, radiological findings of bilateral gray and white matter abnormalities suggestive of edema in the posterior regions of the cerebral hemispheres are essential for the diagnosis. Since Hinchey et al first reported [2], PRES has been seen in various clinical settings, including renal disorders such as acute glomerulonephritis, lupus nephritis, and nephrotic syndrome [3, 4]. Cerebral hyperperfusion and consequent vasogenic edema have been considered as the major pathological events. The two theories regarding the pathophysiology of onset of vasogenic edema are hyperperfusion due to autoregulatory failure of the cerebral vasculature and hypoperfusion due to vasoconstriction of the cerebral artery. When blood pressure exceeds a certain set point in cases of significant systemic hypertension, autoregulation failure may occur. This results in cerebral hyperperfusion and breakdown of the blood-brain barrier, which in turn lead to vasogenic edema [2]. The major differential diagnosis is hypertensive encephalopathy. Furthermore, PRES can occur without significant hypertension. PRES is a relatively in the renal desorders but potentially a serious complication. In this report, we present two patients with PRES in whom the primary diagnosis was hemolytic uremic syndrome (HUS) and Henoch-Schonlein purpura (HSP).
| Case Reports | ▴Top |
Case 1
A previously healthy 6-year-old girl presented with abdominal pain, vomiting, and macroscopic hematuria. At the time of application, her vital signs except blood pressure were normal. Urinary microscopy revealed plenty of erytrocytes, and stick examination showed 3+ proteinuria. A full blood count revealed anemia (Hg 7.4 g/dL) and thrombocytopenia (42,000/mm3). Blood chemistry results were as follows: serum creatinine 4.6 mg/dL and lactic dehydrogenase (LDH) 1,200 IU/L. C3 and haptoglobulin levels were 7.18 and < 6 mg/dL, respectively. The clinical and laboratory findings led to the diagnosis of HUS. She was immediately treated with both plasmapheresis and hemodialysis. On the eighth day of her hospitalization, she had a generalized tonic-clonic seizure, became confused and complained of bilateral complete loss of vision. Neurologic examination showed normal findings, including her pupillary reflexes to light and normal accommodation. Her blood pressure was measured at 140/82 mm Hg. An electroencephalogram (EEG) showed diffuse slow waves at all leads. Her contrast-enhanced computed tomography showed no abnormality but cranial magnetic resonance image (MRI) revealed high signal abnormality. Brain MRI including diffusion-weighted images (DWI) showed faint signal intensities on bilateral parietal subcortical white matter without diffusion restriction (Fig. 1a, b). With the clinical and radiologic data, PRES was the diagnosis. She was put on both anticonvulsant and antihypertensive therapy. The next morning her vision was normal and she had no any other seizure.
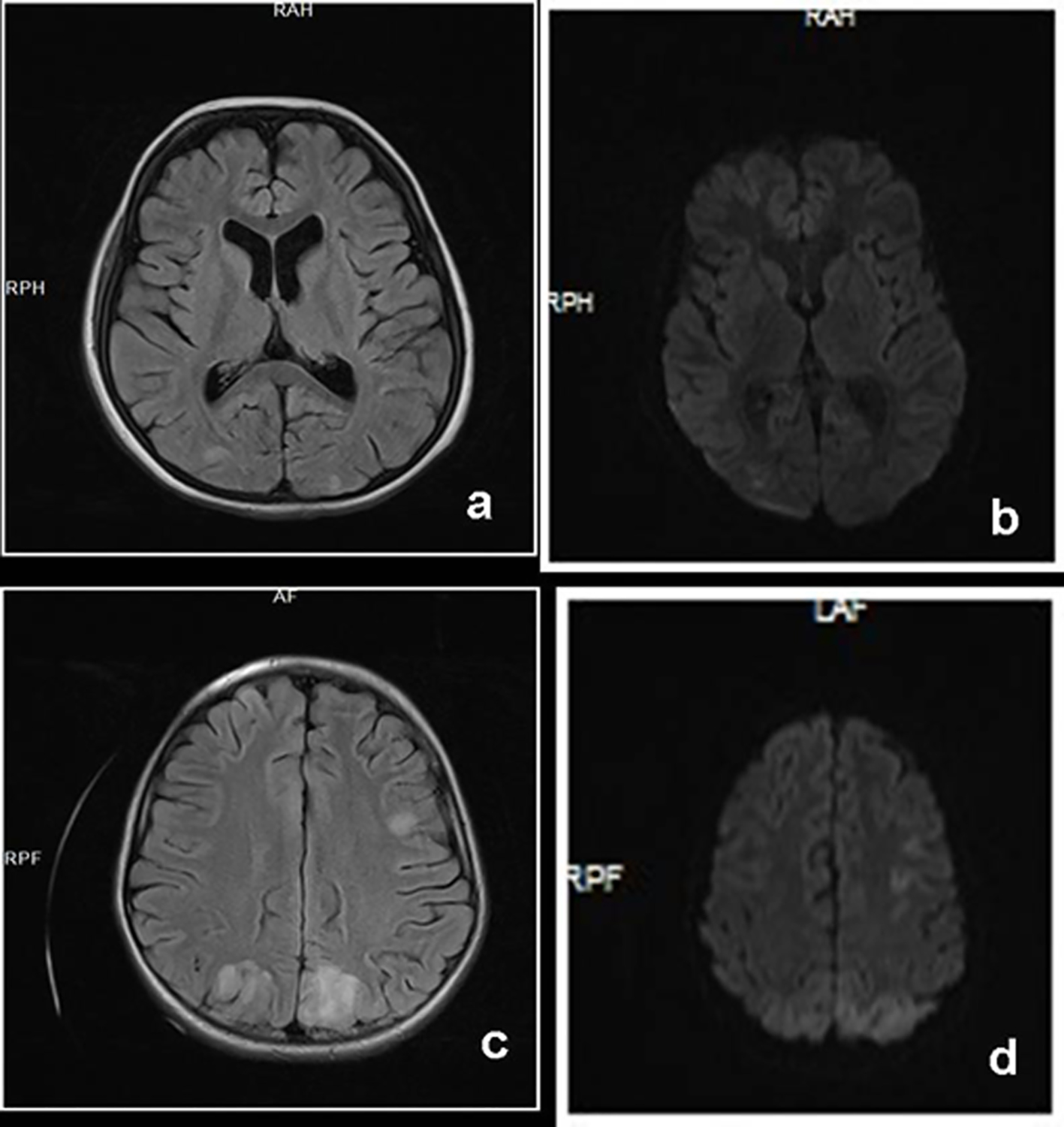 Click for large image | Figure 1. Axial FLAIR image (a) of case 1 shows faint signal intensities on both parietal white matter. Diffusion image (b) fails to show any restriction which is consistent with PRES. Axial FLAIR image (c) of case 2 and corresponding DWI image (d) show increased signal intensities at bilateral occipital and parietal lobes consistent with PRES. |
Case 2
A previously healthy 8-year-old girl presented with abdominal pain, hematochezia, rash on her legs, and macroscopic hematuria. With the diagnosis of HSP, she was put on oral prednisolone therapy. On the 10th day of her hospitalization, she had renal failure. Except a mild abdominal tenderness, the rest of her physical and neurological examinations and her vital sign were all within normal limits. Laboratory findings showed anemia (Hg 9.6 g/dL), proteinuria 3+, and hematuria. Serum Cr was higher than normal range (1.53 mg/dL). Renal ultrasonography revealed normal sized, moderately echogenic kidneys (grade 2-3). The renal biopsy due to the underlying HSP nephritic disease stage showed focal necrotizing cresentic glomerulonephritis. Due to both renal and gastrointestinal involvement of HSP, prednisolone was started. Fourteen days later, she suddenly complained of loss of vision; thereafter, she had generalized tonic seizure. Blood pressure was found to be 130/90 mm Hg at the time and amlodipine was started and followed by enalapril and propanolol for persistant hypertension. The contrast-enhanced cranial computed tomography was normal but the cranial MRI revealed bilateral hyperintense white matter lesions in the occipital and posterior parietal regions on T2-weighted fluid-attenuated inversion recovery (FLAIR) and DWI (Fig. 1c, d). An EEG showed diffuse slow waves at all leads and there were focal isolated temporooccipital sharp wave discharges. Loss of vision resolved after 2 days, and her consciousness normalized. An anticonvulsant therapy was started. For the primary disease of HSP, she was given oral steroid and cyclophosphamide for the last 4 months. So she had no problem at all.
| Discussion | ▴Top |
We described PRES in two children with HSP and HUS. Although they are completely different diseases with different etiologies, the vascular systems are affected in both diseases and hypertension was the common finding in two children.
PRES was originally defined in 1996, with the clinical signs of renal insufficiency, hypertension, or immunosuppressive status, exhibiting headaches, altered mental functioning, seizures, and visual disturbance. The neuroimaging findings were characteristic of posterior brain edema without infarction, suggesting capillary leakage and breakdown of blood-brain barrier. The clinical symptoms and neuroradiological findings of PRES are typically indistinguishable, regardless of etiology and underlying factors [2]. Beside its specific clinical setting which comprises mostly nephrologic patients to date, there is no evidence supporting genetic susceptibility to PRES.
Although there are some limitations, cranial MRI is the gold standard of imaging studies for detecting the lesions of PRES [5]. Typical cranial images show focal regions of relatively symmetric hemispheric edema and the parietal and occipital lobes are most commonly affected. T2-weighted images and FLAIR images have been frequently employed. The differential diagnosis of PRES includes cerebral vasculitis, cerebral venous trombosis, encephalitis, hypertensive encephalopathy, cerebral findings secondary to hypoglycemia and hyponatremia [6].
The mainstays of the management of PRES are control of blood pressure, treatment of seizures, and removal or reduction of other causative factors. After ruling out cerebral infarction, blood pressure should be lowered to the mean arterial pressure by 25% within the first hour.
Long-term prognosis is an important issue in children with PRES, but evidence is scarce. Another important neurological problem is the development of epilepsy at a later period [7]. These may suggest the necessity of long-term follow-up even including those who show complete clinical and radiological recovery in the short term period. Subtle neurological disabilities, including subclinical developmental delay and learning problems, are of particular significance in some children. In the literature, there are also some cases diagnosed as HSP and HUS [8, 9] who developed PRES during the natural course of their diseases. Our patients demonstrated the characteristic radiologic signs and clinical features of PRES, including headache, altered sensorium, seizures and visual disturbances. So that both of them were treated with anticonvulsant and proper antihypertensive drugs. A repeated MRI scan of the head, an ophthalmologic assessment, and a follow-up EEG produced normal results with no sequelae.
Hemodynamic changes due to severe hypertension and renal insufficiency may be involved in the pathogenesis of PRES associated with HSP. The vertebrobasilar and posterior cerebral arteries have a tender sympathetic innervation [2]. In HUS, platelet-fibrin thrombus by occluding predominantly renal but rarely brain vessels subsequently may induce endothelial cell damages [10]. Stimulated endothelial cells can produce cytokines and chemokines, i.e., interleukin-1, 6, 8, TNF-alpha, and von-Willebrand factor in situ [11]. These substrates can induce vasospasms in cerebral vessels, which may lead to additional microemboli and breakdown of blood-brain barrier, which resemble to the underlying pathogenesis of PRES [2]. Although PRES was initially supposed to be a reversible and treatable disease, unusual PRES cases associated with irreversible brain lesions have been accumulated [12]. However, that was not the case in our patients.
Clinical symptoms leading to consideration of PRES should be first evaluated for the primary disease especially in nephrologic patients and patients with hypertension. Afterwards if there is no suspicion of the complication of the primary disease, cerebral infarction and hypertensive encephalopathy, PRES should be considered for the diagnosis. In that cases MRI becomes important and should be performed without hesitation before the resolution of the edema. Morever, the EEG findings also should be assessed, in order to exclude the later development of epilepsy.
Conflicts of Interest
The authors report no conflicts of interest.
| References | ▴Top |
- Kinoshita T, Moritani T, Shrier DA, Hiwatashi A, Wang HZ, Numaguchi Y, Westesson PL. Diffusion-weighted MR imaging of posterior reversible leukoencephalopathy syndrome: a pictorial essay. Clin Imaging. 2003;27(5):307-315.
doi - Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, Pessin MS, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334(8):494-500.
doi pubmed - Ishikura K, Ikeda M, Hamasaki Y, Hataya H, Shishido S, Asanuma H, Nishimura G, et al. Posterior reversible encephalopathy syndrome in children: its high prevalence and more extensive imaging findings. Am J Kidney Dis. 2006;48(2):231-238.
doi pubmed - Ishimori ML, Pressman BD, Wallace DJ, Weisman MH. Posterior reversible encephalopathy syndrome: another manifestation of CNS SLE? Lupus. 2007;16(6):436-443.
doi pubmed - Lamy C, Oppenheim C, Meder JF, Mas JL. Neuroimaging in posterior reversible encephalopathy syndrome. J Neuroimaging. 2004;14(2):89-96.
doi pubmed - Ringelstein EB, Knecht S. Cerebral small vessel diseases: manifestations in young women. Curr Opin Neurol. 2006;19(1):55-62.
doi - de Laat P, Te Winkel ML, Devos AS, Catsman-Berrevoets CE, Pieters R, van den Heuvel-Eibrink MM. Posterior reversible encephalopathy syndrome in childhood cancer. Ann Oncol. 2011;22(2):472-478.
doi pubmed - Woolfenden AR, Hukin J, Poskitt KJ, Connolly MB. Encephalopathy complicating Henoch-Schonlein purpura: reversible MRI changes. Pediatr Neurol. 1998;19(1):74-77.
doi - Fujii K, Matsuo K, Takatani T, Uchikawa H, Kohno Y. Multiple cavitations in posterior reversible leukoencephalopathy syndrome associated with hemolytic-uremic syndrome. Brain Dev. 2012;34(4):318-321.
doi pubmed - Tarr PI, Gordon CA, Chandler WL. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet. 2005;365(9464):1073-1086.
doi - Moake JL. Thrombotic microangiopathies. N Engl J Med. 2002;347(8):589-600.
doi pubmed - Antunes NL, Small TN, George D, Boulad F, Lis E. Posterior leukoencephalopathy syndrome may not be reversible. Pediatr Neurol. 1999;20(3):241-243.
doi
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


