| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Letter to the Editor
Volume 6, Number 5, October 2014, pages 392-394
Potential Biomarker for Human Uterine Leiomyosarcoma
Takuma Hayashia, b, c, n, Akiko Horiuchid, Hiroyuki Aburatanie, Osamu Ishikof, Nobuo Yaegashig, Yae Kanaih, i, Dorit Zharharyc, j, Tanri Shiozawak, Susumu Tonegawal, Ikuo Konishim
aDepartment of Immunology and Infectious Disease, Shinshu University School of Medicine, Matsumoto, Nagano 390-8621, Japan
bPromoting Business Using Advanced Technology, Japan Science and Technology Agency (JST), Chiyoda, Tokyo 102-8666, Japan
cSIGMA-Aldrich Collaboration Laboratory
dHoriuchi Ladies Clinic, Matsumoto, Nagano 390-0821, Japan
eThe Cancer System Laboratory, Research Center for Advanced Science and Technology, The University of Tokyo, Meguro, Tokyo 153-9804, Japan
fDepartment of Obstetrics and Gynecology, Osaka City University Graduate School of Medicine, Osaka, Osaka 545-8585, Japan
gDepartment of Obstetrics and Gynecology, Tohoku University Graduate School of Medicine, Sendai, Miyagi 980-8574, Japan
hPathology Division, National Cancer Center Research Institute, Chuoku, Tokyo 104-0045, Japan
iThe International Human Epigenome Consortium (IHEC) and CREST, Japan Science and Technology Agency (JST), Chiyoda, Tokyo 102-8666, Japan
jSigma-Aldrich Israel Ltd, Rehovot 76100, Israel
kDepartment of Obstetrics and Gynecology, Shinshu University School of Medicine, Matsumoto, Nagano 390-8621, Japan
lPicower Institution and Department of Biology, Massachusetts Institute of Technology, Cambridge, MA 02139-4307, USA
mDepartment of Obstetrics and Gynecology, Kyoto University Graduate School of Medicine, Kyoto, Kyoto 606-8507, Japan
nCorresponding Author: Takuma Hayashi, Department of Immunology and Infectious Disease, Shinshu University Graduate School of Medicine, 3-1-1, Asahi, Matsumoto, Nagano 390-8621, Japan
Manuscript accepted for publication May 15, 2014
Short title: Human Uterine Leiomyosarcoma
doi: https://doi.org/10.14740/jocmr1867e
| To the Editor | ▴Top |
Sarcomas are a rare form of malignant tumor, with less than 15,000 new cases being diagnosed each year in the United States. In spite of their rarity, sarcomas are highly debilitating malignancies that are often associated with significant morbidity and mortality. They are also biologically very heterogeneous because they originate from many different tissues and cell types. Sarcomas have classically been defined by their tissue of origin and are additionally stratified according to their histopathology or the age of the patient at diagnosis. Uterine mesenchymal tumors that develop in the myometrium have traditionally been divided into benign uterine usual LMA, cellular LMA and malignant Ut-LMS based on cytological atypia, mitotic activity and other criteria. Ut-LMS is relatively rare, having an estimated annual incidence of 0.64 per 100,000 women [1], and is resistant to chemotherapy and radiotherapy; therefore, surgical interventions are virtually the only means of treatment [2, 3]. The prognosis of patients with Ut-LMS is poor, and the 5-year survival rate is approximately 35%. Uterine LMA may occur in 70-80% of women by the age of 50 years [4]. Difficulties have been reported in distinguishing Ut-LMS from other uterine mesenchymal tumors including uterine LMA, and a diagnosis generally requires surgery and cytoscopy. Diagnostic categories for uterine mesenchymal tumors and morphological criteria are used to assign cases. The non-standard subtypes of uterine mesenchymal tumors such as the epithelioid and myxoid types are classified in a different manner using these features; therefore, a diagnostic method needs to be established that can identify non-standard smooth muscle differentiation [5, 6].
High estrogen levels have been shown to significantly influence the development of tumors in the uterine body [7]. However, the molecular mechanisms underlying the transformation of uterine LMA and development of Ut-LMS remain unknown. Tumors that have developed and grown in the myometrium increase in size due to the influence of the female hormone, estrogen, which leads to the generation of more tumors. However, a relationship has not yet been reported between the development of Ut-LMS and hormonal conditions, and no obvious risk factors have been identified. The identification of risk factors associated with the development of human Ut-LMS will contribute significantly to the development of preventive and therapeutic treatments. Cytoplasmic proteins are mostly degraded by a protease complex referred to as the 20S proteasome, which has many substrates that consist of twenty-eight 20 to 30 kDa subunits [8, 9]. Proteasomal degradation is essential for many cellular processes, including the cell cycle, regulation of gene expression and immunological function [10]. A previous study reported that an interferon (IFN)-γ treatment induced the expression of large numbers of responsive genes, the β-ring subunits of proteasomes, i.e., low-molecular mass polypeptide (LMP)2/β1i, LMP7/β5i and LMP10/multicatalytic endopeptidase complex-like (MECL)-1/β2i [11]. Ut-LMS was detected in female LMP2/β1i-deficient mice at 6 months or older, and its incidence at 14 months was approximately 40% [12]. Histopathological studies of LMP2/β1i-lacking uterine tumors have revealed the characteristic abnormalities of Ut-LMS [12].
The non-standard subtypes of uterine mesenchymal tumors such as the epithelioid and myxoid types have been classified in a different manner using these features; therefore, a diagnostic method needs to be established that can identify non-standard smooth muscle differentiation [5, 6]. Pathological studies have been performed to demonstrate the validity and reliability of LMP2/β1i as a diagnostic biomarker when combined with other candidate molecules, such as cyclin E and calponin h1, which reportedly function as anti-oncogenic factors in human Ut-LMS. Pathological examinations revealed that the ability to induce the expression of LMP2/β1i and calponin h1 was markedly lower in human Ut-LMS tissues than in uterine LMA or a normal myometrium located in the same section, and the expression of cyclin E was markedly high in human Ut-LMS tissues only [13-16]. The histological findings of skeletal muscle and rectum lesions were consistent with metastatic Ut-LMS [13, 14]. Western blotting and RT-PCR experiments revealed that LMP2/β1i was expressed in a normal myometrium, but not in human Ut-LMS, and both findings strongly supported the pathological results [13, 14, 16, 17]. Although we previously demonstrated that the abnormal expression of the ovarian steroid receptors, Tp53, ki-67 and mutations in Tp53 were frequently associated with Ut-LMS, the defective expression of LMP2/β1i and calponin h1 appeared to be more characteristic of human Ut-LMS than these factors [14, 15, 18-20] (Fig. 1).
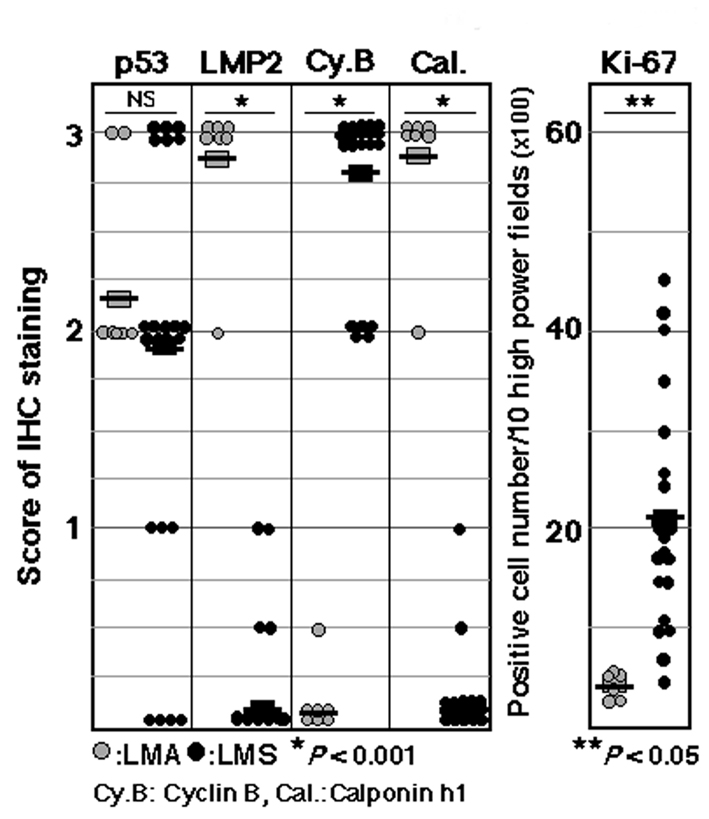 Click for large image | Figure 1. Summary of IHC experiments for p53, LMP2, cyclin B, calponin h1 and ki-67 expression levels in leiomyoma (LMA) and leiomyosarcoma (LMS). IHC experiments were performed using seven LMA tissue samples and 23 LMS tissue samples obtained from patients with LMA and/or LMS. Data were quantified using WinROOF Ver.6.3.0 software (Mitani Co., Ltd, Fukui, Japan). P vales were generated using a t-test. Data are representative of three experiments. +++: diffuse-positive (homogeneous distribution with more than 90% of cells stained), -: negative (no stained cells). |
We are currently collaborating with several clinical research facilities to investigate the reliability and characteristics of LMP2/β1i as a diagnostic indicator. The histopathological characteristics of human uterine mesenchymal tumors including mitotically active leiomyoma, bizarre leiomyoma, lipoleiomyoma, undifferentiated endometrial sarcoma, epithelioid variant leiomyosarcoma, myxoid variant leiomyosarcoma, smooth muscle tumors of uncertain malignant potential (STUMP) and leiomyomatoid angiomatous neuroendocrine tumor (LANT) have already been summarized [21-24]. Therefore, clarifying the relationships between these factors and the development of human Ut-LMS, and identifying specific risk factors may lead to the development of new clinical treatments for this disease.
Acknowledgments
This study was supported in part by grants from the Ministry of Education, Culture, Science and Technology, and The Foundation of Osaka Cancer Research, The Foundation for the Promotion of Cancer Research, The Kanzawa Medical Research Foundation, and The Takeda Foundation for Medical Science.
Competing Interests
The authors declare that they have no conflicts of interest. The authors alone are responsible for the content and writing of this manuscript.
| References | ▴Top |
- Zaloudek C, Hendrickson MR. Mesenchymal tumors of the uterus. In: Kurman RJ, ed. Blaustein’s Pathology of the Female Genital Tract. 5th edition. New York: Springer-Verlag. 2002:561-578.
- Wu TI, Chang TC, Hsueh S, Hsu KH, Chou HH, Huang HJ, Lai CH. Prognostic factors and impact of adjuvant chemotherapy for uterine leiomyosarcoma. Gynecol Oncol. 2006;100(1):166-172.
doi pubmed - Leitao MM, Soslow RA, Nonaka D, Olshen AB, Aghajanian C, Sabbatini P, Dupont J,
et al . Tissue microarray immunohistochemical expression of estrogen, progesterone, and androgen receptors in uterine leiomyomata and leiomyosarcoma. Cancer. 2004;101(6):1455-1462.
doi pubmed - http://cancer.gov/cancertopics/pdq/treatment/uterinesarcoma/HealthProfessional.
- Kurma RJ. Pathology of the Female Genital Tract. 4th edition. New York: Springer-Verlag, 2001:499.
- Diagnostic Criteria for LMS, Adapted from 2003 WHO Guidelines. World Health Organization Classification of Tumours: Pathology and Genetics, Pathology and Genetics of Tumours of the Breast and Female Genital Organs. France: IARC Press; 2003.
- Lin JF, Slomovitz BM. Uterine sarcoma 2008. Curr Oncol Rep. 2008;10(6):512-518.
doi - Peters JM, Franke WW, Kleinschmidt JA. Distinct 19 S and 20 S subcomplexes of the 26 S proteasome and their distribution in the nucleus and the cytoplasm. J Biol Chem. 1994;269(10):7709-7718.
pubmed - Lodish H, Berk A, Matsudaira P, Kaiser CA, Krieger M, Scott MP, Zipursky SL, Darnell J. “3”. Mol Cell Biol. 5th edition. New York: W.H. Freeman and CO. 2004:66- 72.
- Konstantinova IM, Tsimokha AS, Mittenberg AG. Role of proteasomes in cellular regulation. Int Rev Cell Mol Biol. 2008;267:59-124.
doi - Wang J, Maldonado MA. The ubiquitin-proteasome system and its role in inflammatory and autoimmune diseases. Cell Mol Immunol. 2006;3(4):255-261.
pubmed - Hayashi T, Faustman DL. Development of spontaneous uterine tumors in low molecular mass polypeptide-2 knockout mice. Cancer Res. 2002;62(1):24-27.
pubmed - Hayashi T, Kobayashi Y, Kohsaka S, Sano K. The mutation in the ATP-binding region of JAK1, identified in human uterine leiomyosarcomas, results in defective interferon-gamma inducibility of TAP1 and LMP2. Oncogene. 2006;25(29):4016-4026.
doi pubmed - Hayashi T, Horiuchi A, Sano K, Hiraoka N, Kasai M, Ichimura T, Sudo T,
et al . Potential role of LMP2 as tumor-suppressor defines new targets for uterine leiomyosarcoma therapy. Sci Rep. 2011;1:180.
doi pubmed - Zhai YL, Kobayashi Y, Mori A, Orii A, Nikaido T, Konishi I, Fujii S. Expression of steroid receptors, Ki-67, and p53 in uterine leiomyosarcomas. Int J Gynecol Pathol. 1999;18(1):20-28.
doi pubmed - Hayashi T, Horiuchi A, Sano K, Hiraoka N, Kasai M, Ichimura T, Sudo T,
et al . Potential role of LMP2 as an anti-oncogenic factor in human uterine leiomyosarcoma: morphological significance of calponin h1. FEBS Lett. 2012;586(13):1824-1831.
doi pubmed - Hayashi T, Horiuchi A, Konishi I. Tumor growth arrest: involvement of the mutation in the catalytic region of JAK1. Eur J Res Med Sci. 2013;1:8-21.
- Horiuchi A, Nikaido T, Ito K, Zhai Y, Orii A, Taniguchi S, Toki T,
et al . Reduced expression of calponin h1 in leiomyosarcoma of the uterus. Lab Invest. 1998;78(7):839-846.
pubmed - Horiuchi A, Nikaido T, Taniguchi S, Fujii S. Possible role of calponin h1 as a tumor suppressor in human uterine leiomyosarcoma. J Natl Cancer Inst. 1999;91(9):790-796.
doi pubmed - Zhai YL, Nikaido T, Shiozawa T, Orii A, Fujii S. Expression of cyclins and cyclin-dependent kinases in smooth muscle tumors of the uterus. Intl J Cancer. 1999;84:224-250.
doi - Sakashita N, Yamada M, Nakagawa T, Yamasaki H, Takeya M. A leiomyomatoid angiomatous neuroendocrine tumor of the myometrium: case study with ultrastructural analysis. Hum Pathol. 2008;39(5):788-792.
doi pubmed - Vajtai I, Sahli R, Kappeler A, Christ ER, Seiler RW. Leiomyomatoid angiomatous neuroendocrine tumor (LANT) of the pituitary: a distinctive biphasic neoplasm with primitive secretory phenotype and smooth muscle-rich stroma. Acta Neuropathol. 2006;111(3):278-283.
doi pubmed - Avritscher R, Iyer RB, Ro J, Whitman G. Lipoleiomyoma of the uterus. AJR Am J Roentgenol. 2001;177(4):856.
doi pubmed - Takeuchi M, Matsuzaki K, Harada M. Preliminary observations and clinical value of lipid peak in high-grade uterine sarcomas using in vivo proton MR spectroscopy. Eur Radiol. 2013;23(9):2358-2363.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


