| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Original Article
Volume 6, Number 5, October 2014, pages 345-353
Risk Factors for Subclavian Vein Thrombosis in Cancer Patients With Total Parenteral Nutrition
Ricardo Berea-Baltierraa, f, Rodolfo Rivas-Ruizb, Elpidia Vela-Martineza, Maria de la Luz Sevilla-Gonzalezc, Juan Osvaldo Talavera-Pinad, Elena Valencia-Jimeneze, Irene Perez-Francoe, Laura Escobedo-Hernandeze
aDepartment of Internal Medicine and Nutritional Support, Oncology Hospital, Centro Medico Nacional Siglo XXI, Mexico City, Mexico
bDepartment of Pediatrics, Pediatric Hospital, Centro Medico Nacional Siglo XXI, Instituto Mexicano del Seguro Social, Mexico City, Mexico
cPostgraduate Unit, Escuela Superior de Medicina, Instituto Politecnico Nacional, Mexico City, Mexico
dCentro de Adiestramiento en Investigacion Clinica, Coordinacionde Investigacion en Salud, Centro Medico Nacional Siglo XXI, Instituto Mexicano del Seguro Social, Mexico City, Mexico
eDepartment of Radiology, Oncology Hospital, Centro Medico Nacional Siglo XXI, Mexico City, Mexico
fCorresponding Author: Ricardo Berea-Baltierra, Hospital de Oncologia, CMN SXXI, Av Cuauhtemoc 330, Colonia Doctores, CP 06720, Mexico
Manuscript accepted for publication May 26, 2014
Short title: Risk Factors for Subclavian Thrombosis
doi: https://doi.org/10.14740/jocmr1862w
| Abstract | ▴Top |
Background: There are few reports on total parenteral nutrition (TPN) and its possible prothrombotic effect. The purpose of this study was to identify risk factors for subclavian vein thrombosis (SVT) in patients receiving TPN.
Method: Cancer patients with indwelling subclavian catheters and TPN were followed in a cohort study. Doppler ultrasound examination was performed 8 and 30 days after catheter placement.
Results: One hundred twenty-one patients were included, with a mean of 61 (± 11.8) years of age. We detected 36 SVT events at day 8 (29.8%) and 47 (38.8%) at day 30 after central catheter placement. Mean length of subclavian catheterization was 17.2 (± 8.2) days. Fifty-three point three percent of patients receiving ≥ 3,050 mOsm TPN in 24 hours developed SVT (relative risk (RR) = 2.01, 95% CI, 1.14 - 3.57; P = 0.016) at day 8 and 60% (RR = 1.67, 95% CI, 1.30 - 2.71; P = 0.038) at day 30 post-catheter placement. Protein administration of > 97.5 g/day was shown to be a risk factor for early thrombosis with a mean of 16.88 days for the development of SVT (95% CI, 10 - 23.7) versus 27.8 days (95% CI, 25.8 - 29.9) in the group with nutritional protein content < 97.5 g/day (P = 0.000).
Conclusion: High-osmolarity and high-protein nutrition formulas were shown to be risk factors for SVT in cancer patients receiving TPN.
Keywords: Subclavian vein thrombosis; Cancer; Parenteral nutrition; Risk factors
| Introduction | ▴Top |
Venous thrombosis is a frequent complication in hospitalized cancer patients, although subclavian vein thrombosis (SVT) is not commonly diagnosed, with trauma and venous catheterization being its main causes. Long-term venous catheterization is common in cancer patients in order for them to receive treatments such as chemotherapy or parenteral nutrition. Central veins are preferred in this setting due to a reduced risk of infections and thrombosis compared with peripheral veins [1]. There is great variability in the cumulative incidence of thrombosis reported in patients with central indwelling catheters, ranging from 12 to 66% [2, 3]; usually, these events are asymptomatic (71-90%). Trials with the highest cumulative incidences have been conducted using contrast media and invasive diagnostic procedures. Previous cohort studies had established the onset of SVT between day 8 (64-98%) and day 30 after catheter insertion (68-98%) [4-6]. The main risk factors proposed as SVT etiology include infections, older age, metastatic cancer, previous thrombosis events, etc. [7, 8]. Little is known about total parenteral nutrition (TPN) and high osmolarity venous infusions as risk factors for venous thrombosis, although in animal models, a potential prothrombotic effect of parenteral nutrition was previously shown [9].
Doppler ultrasound has high sensitivity (78-94%) and specificity (82-96%) for the diagnosis of SVT [10, 11]; being a non-invasive test, and safer than venography, it is known as the diagnostic gold standard. Our objective in this study was to find out the cumulative incidence of SVT in patients on parenteral nutrition and to identify risk factors associated with this complication.
| Materials and Methods | ▴Top |
Consecutive cancer patients requiring subclavian vein catheterization in order to receive TPN were included. All patients were receiving thromboprophylaxis with subcutaneous enoxaparin (1 mg/kg/day). Patients with previous subclavian catheter placement or previous thrombotic events were excluded.
Study design
This was a prospective cohort study. A subclavian vein Doppler ultrasound (HDI 5000 Philips ATL broadband linear transducer, 12 to 5 MHz) was performed 8 (± 1) and 30 days (± 1) after catheter placement looking for venous thrombosis. By direct interviews, we collected data about age, comorbidities (diabetes, hypertension, autoimmune disorders, etc.) and smoking index. From medical records, we obtained data on type of cancer, body mass index (BMI), TPN formula received (osmolarity, calories, amounts of protein, carbohydrates and lipids), side and complications during catheter insertion, coagulation tests and catheter tip cultures. Our dependent variable, SVT, was defined as total or partial obstruction by a thrombus diagnosed by Doppler ultrasound examination. The study was carried out at the Oncology Hospital, Centro Medico Siglo XXI, IMSS, one of the main cancer reference centers in Mexico.
Statistical analysis
Chi-square or Mann-Whitney U tests were performed depending on the type of variable. Relative risks (RRs) were established for variables with statistical significance. A Kaplan-Meier test was employed to analyze significant variables eliminating confounding factors, and a Cox proportional hazards model was used to establish the relationship between the cumulative incidence of thrombosis events and the number of days elapsed after catheter placement. Multiple logistic regression analyses helped us to establish a possible relationship between significant variables and SVT. A P < 0.05 was considered statistically significant. IBM SPSS Statistics 20 software was employed for statistical analysis.
Ethical aspects
The study was considered to be a minimal risk research; it was reviewed and accepted by the Investigation Committee at the Oncology Hospital, Centro Medico Nacional Siglo XXI, in compliance with internationally accepted ethical codes and guidelines. All study subjects gave their written informed consent.
| Results | ▴Top |
From January 2012 to February 2013, 121 patients were included, 63 male (52.1%) and 58 female (47.9%), with a mean of 61 (± 11.8) years of age. The most common cancer locations were colorectal (37.2%), stomach (29.8%) and pancreas (19%). Thirty-six thrombosis events were detected at day 8 from catheter insertion for TPN (29.8%) and 47 at day 30 (38.8%) (Fig. 1).
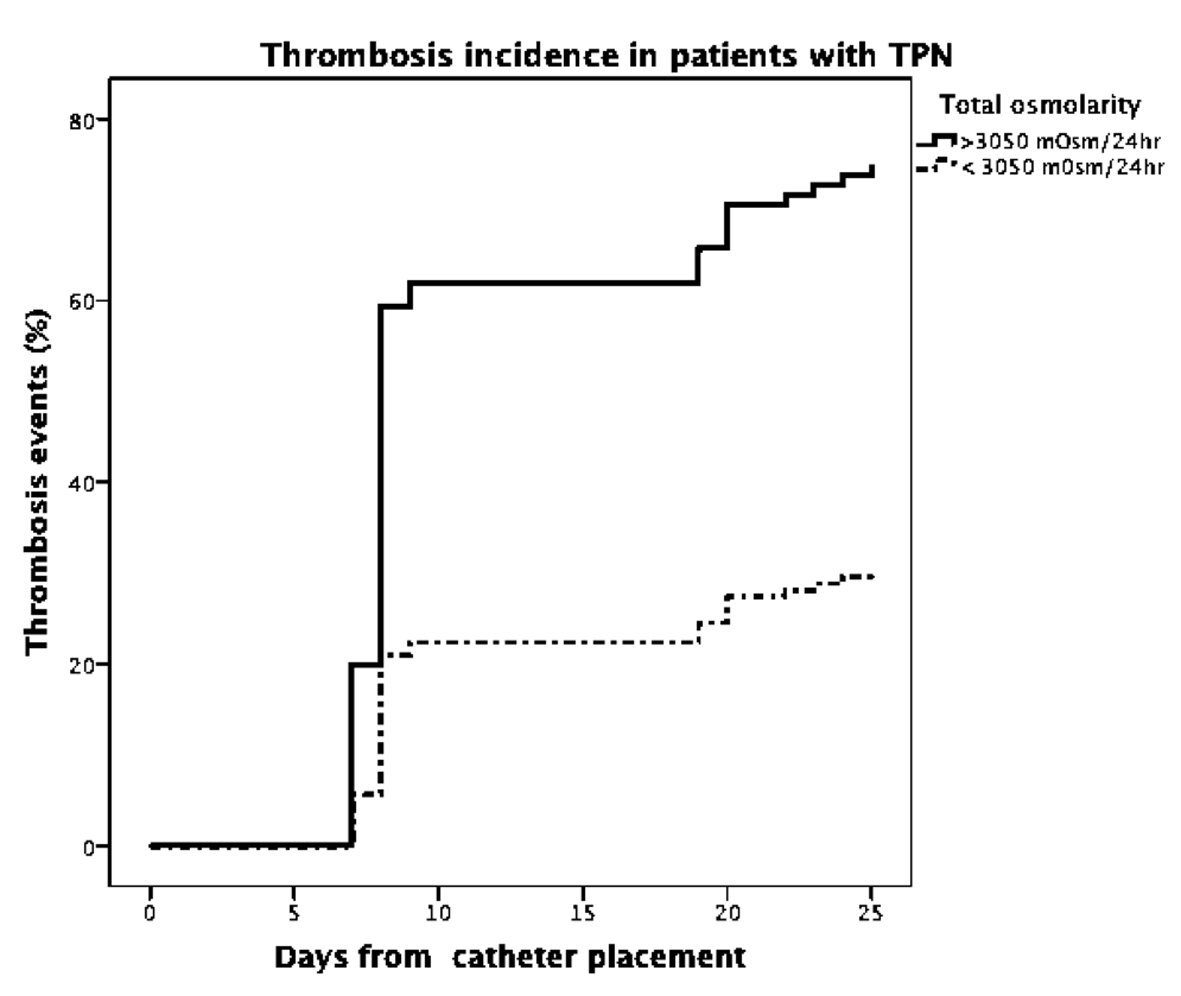 Click for large image | Figure 1. Subclavian vein thrombosis incidence in patients with central catheter for TPN. |
| Discussion | ▴Top |
High cumulative incidence of SVT was found in high-risk patients with cancer, recent surgery and parenteral nutrition, in spite of antithrombotic prophylaxis.
This incidence is comparable to that in previous reports in non-oncologic patients receiving TPN without thrombotic prophylaxis [12]. Symptomatic events showed similar incidence as in previously reported studies with non-radiologic diagnosis of thrombosis (3.3%). Most thrombosis events were observed at day 7 from catheter placement (76.5%), similar as the 64% reported by De Cicco and colleagues using flebography in cancer patients [13].
We included clinical variables previously shown to be risk factors for thrombotic events [8], adding the nutritional formula elements and characteristics. No significant differences were found in age group, presence of bacterial colonization, puncture side or complications during catheter placement. A higher incidence of subclavian thrombosis was found in gastric cancer patients; there are previous reports of C-protein-acquired resistance leading to a hypercoagulable state [14, 15]. None of these patients had known metastases at the time of the study.
Analyzing the nutritional formula characteristics, we found that high amino acid concentrations (> 97.5 g) and high osmolarity (> 3,050 mOsm) administered in 24 h were related to SVT with statistical significance.
We included laboratory parameters not previously considered as being related to SVT. In this study, fibrinogen levels < 550 mg/dL were found to be a risk factor for subclavian thrombosis; this is an element of the coagulation cascade that at high levels is associated with atherosclerosis and prothrombotic states [16]; whereas low levels are related mainly with disseminated intravascular coagulation. In subjects with systemic inflammatory responses and in cancer patients, a 200 to 400% increase in fibrinogen levels is expected [17]; in our study, we found a significant relationship between subclavian thrombosis events and normal to 50%-higher levels of fibrinogen. Some studies had found coagulation tests anomalies in patients with cervix [18], lung [19, 20], ovarian [21] or breast cancer [22]. We think that this finding is likely to be related to a pro-inflammatory effect rather than a risk factor.
This study had some limitations. First, our patients were not assessed with an initial ultrasound, they were asymptomatic prior to the catheterization, and we considered that the only way to install a venous catheter was using a permeable vein. Another limitation was that D-dimer levels were not measured, as this is not a routine laboratory test in our hospital because levels are considered to be usually high in cancer patients. The present study gave us some insights on the incidence of this complication and possible risk factors. We propose close monitoring of venous patency in patients receiving high-osmolarity TPN formulas, and we also hope that this report will provide grounds for new studies on this topic.
Conclusion
High-osmolarity and high-protein nutrition formulas were shown to be risk factors for SVT in cancer patients receiving TPN. We propose close monitoring in high-risk patients with Doppler ultrasound testing 30 days after catheter insertion for prompt treatment.
Financial Support
No financial support received.
Conflict of Interest
None of the authors have a conflict of interest to report.
| References | ▴Top |
- Hamilton HC, Foxcroft DR. Central venous access sites for the prevention of venous thrombosis, stenosis and infection in patients requiring long-term intravenous therapy. Cochrane Database Syst Rev. 2007;(3):CD004084.
- Yilmaz KB, Akinci M, Dogan L, Yologlu Z, Atalay C, Kulacoglu H. Central venous catheter-associated thrombosis in the perioperative period: a frequent complication in cancer patients that can be detected early with doppler examination. Tumori. 2010;96(5):690-694.
pubmed - Timsit JF, Farkas JC, Boyer JM, Martin JB, Misset B, Renaud B, Carlet J. Central vein catheter-related thrombosis in intensive care patients: incidence, risks factors, and relationship with catheter-related sepsis. Chest. 1998;114(1):207-213.
doi pubmed - Kuter DJ. Thrombotic complications of central venous catheters in cancer patients. Oncologist. 2004;9(2):207-216.
doi pubmed - Balestreri L, De Cicco M, Matovic M, Coran F, Morassut S. Central venous catheter-related thrombosis in clinically asymptomatic oncologic patients: a phlebographic study. Eur J Radiol. 1995;20(2):108-111.
doi - Lokich JJ, Becker B. Subclavian vein thrombosis in patients treated with infusion chemotherapy for advanced malignancy. Cancer. 1983;52(9):1586-1589.
doi - Alikhan R, Cohen AT, Combe S, Samama MM, Desjardins L, Eldor A, Janbon C,
et al . Risk factors for venous thromboembolism in hospitalized patients with acute medical illness: analysis of the MEDENOX Study. Arch Intern Med. 2004;164(9):963-968.
doi pubmed - Verso M, Agnelli G, Kamphuisen PW, Ageno W, Bazzan M, Lazzaro A, Paoletti F,
et al . Risk factors for upper limb deep vein thrombosis associated with the use of central vein catheter in cancer patients. Intern Emerg Med. 2008;3(2):117-122.
doi pubmed - van der Poll T, Levi M, Braxton CC, Coyle SM, Roth M, ten Cate JW, Lowry SF. Parenteral nutrition facilitates activation of coagulation but not of fibrinolysis during human endotoxemia. J Infect Dis. 1998;177(3):793-795.
doi pubmed - Koksoy C, Kuzu A, Kutlay J, Erden I, Ozcan H, Ergin K. The diagnostic value of colour Doppler ultrasound in central venous catheter related thrombosis. Clin Radiol. 1995;50(10):687-689.
doi - Chin EE, Zimmerman PT, Grant EG. Sonographic evaluation of upper extremity deep venous thrombosis. J Ultrasound Med. 2005;24(6):829-838, quiz 839-840.
pubmed - Valerio D, Hussey JK, Smith FW. Central vein thrombosis associated with intravenous feeding--a prospective study. JPEN J Parenter Enteral Nutr. 1981;5(3):240-242.
doi pubmed - De Cicco M, Matovic M, Balestreri L, Panarello G, Fantin D, Morassut S, Testa V. Central venous thrombosis: an early and frequent complication in cancer patients bearing long-term silastic catheter. Central venous thrombosis: an early and frequent complication in cancer patients bearing long-term silastic catheter. Thromb Res. 1997;86(2):101-113.
doi - Lip GY, Chin BS, Blann AD. Cancer and the prothrombotic state. Lancet Oncol. 2002;3(1):27-34.
doi - Green D, Maliekel K, Sushko E, Akhtar R, Soff GA. Activated-protein-C resistance in cancer patients. Haemostasis. 1997;27(3):112-118.
pubmed - Kamath S, Lip GY. Fibrinogen: biochemistry, epidemiology and determinants. QJM. 2003;96(10):711-729.
doi - Wallach J. Interpretation of diagnostic tests. 6th ed. Boston: Little, Brown and Company; 1996.
- Gadducci A, Baicchi U, Marrai R, Facchini V, del Bravo B, Fosella PV, Fioretti P. Pretreatment plasma levels of fibrinopeptide-A (FPA), D-dimer (DD), and von Willebrand factor (vWF) in patients with operable cervical cancer: influence of surgical-pathological stage, tumor size, histologic type, and lymph node status. Gynecol Oncol. 1993;49(3):354-358.
doi pubmed - Seitz R, Rappe N, Kraus M, Immel A, Wolf M, Maasberg M, Egbring R,
et al . Activation of coagulation and fibrinolysis in patients with lung cancer: relation to tumour stage and prognosis. Blood Coagul Fibrinolysis. 1993;4(2):249-254.
doi pubmed - Nakanishi M, Yagawa K, Hayashi S, Ogino H, Ogata K, Yatsunami J, Miyagawa Y,
et al . Plasma thrombosis-inducing activity in 120 patients with primary lung cancer. Oncology. 1991;48(4):297-300.
doi pubmed - Mirshahi SS, Pujade-Lauraine E, Soria C, Mirshahi M, Fretault J, Bernadou A, Soria J. D-dimer and CA 125 levels in patients with ovarian cancer during antineoplastic therapy. D-dimer and CA 125 levels in patients with ovarian cancer during antineoplastic therapy. Cancer. 1992;69(9):2289-2292.
doi - McCulloch P, Lowe GD, Douglas JT, Murray GD, George WD. Haemostatic abnormalities and outcome in patients with operable breast cancer. Eur J Cancer. 1990;26(9):950-953.
doi
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


