| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Original Article
Volume 5, Number 2, April 2013, pages 97-100
Polymyxins and Doripenem Combination Against KPC-Producing Klebsiella pneumoniae
Grace C. Leea, b, d, David S. Burgessc
aUniversity of Texas Health Science Center at San Antonio, Pharmacotherapy Education and Research Center, 7703 Floyd Curl Drive - MC 6220, San Antonio, TX, U.S
bCollege of Pharmacy, University of Texas at Austin, 1 University Station, Austin, TX U.S
cDepartment of Pharmacy Practice and Science, University of Kentucky College of Pharmacy, 789 S. Limestone, 292K, Lexington, KY 40536, U.S
dCorresponding author: Grace C. Lee, University of Texas Health Science Center, Pharmacotherapy Education and Research Center, School of Medicine, 7703 Floyd Curl Drive, MSC-6220, San Antonio, TX 78229-3900, U.S
Manuscript accepted for publication January 15, 2013
Short title: Polymyxins and Doripenem Combination
doi: https://doi.org/10.4021/jocmr1220w
| Abstract | ▴Top |
Background: Most KPC-producing organisms have maintained susceptibility to polymyxins; however, development of resistance to polymyxins has been increasingly reported. One potential treatment modality is to optimize the use of combination therapy. Therefore, we evaluated the in vitro activity of doripenem, colistin sulfate, polymyxin B alone and in combination against KPC- producing K. pneumoniae.
Methods: In-vitro time-kill assays were performed for four non-duplicate KPC-3 producing K. pneumoniae isolates with the following antibiotics: doripenem, polymyxin B and colistin sulfate alone and in combination. Bacterial densities were determined at 0, 4, 8, 12, 24 and 48 hours. Bactericidal activity was defined as ≥ 3-log10 CFU/mL reduction from the starting inoculum. Synergism was defined as ≥ 2-log10 reduction with the combination when compared to the most active single agent at 24 hours.
Results: Minimum inhibitory concentrations (MICs) for polymyxin B and colistin sulfate ranged from 0.0625 to 0.25 µg/mL, and all isolates were resistant to doripenem (MICs ranged 16 - 32 µg/mL). Monotherapy with colistin sulfate and polymyxin B displayed bacterialcidal activity within 12 hours; however, significant re-growth occurred by 24 hours in all isolates. Monotherapy with doripenem did not show bactericidal activity in any isolate. Synergy occurred with combinations of both colistin sulfate and polymyxin B with doripenem against all isolates and was sustained at 48 hours. Combinations of colistin sulfate or polymyxin B with doripenem demonstrated rapid bactericidal activity by 4 hours in all isolates and was sustained for 24 hours.
Conclusion: Polymyxin B and colistin sulfate in combination with doripenem may be an important treatment modality in treating KPC-producing organisms.
Keywords: Klebsiella pneumoniae; KPC; Carbapenemase; Time-kill; Doripenem; Colistin; Polymyxin B; Carbapenem; Synergy
| Introduction | ▴Top |
Over the last decade, the detection of Klebsiella pneumoniae carbapenemase (KPC)-producing organisms have been increasing at an alarming rate in the United States and throughout the world [1]. Since its first report in 2001, 10 additional KPC variants have been subsequently discovered, with KPC-2 and KPC-3 as the most commonly reported [2, 3]. KPC enzymes have the capacity to confer resistance to all β-lactams including carbapenems. In addition, organisms harboring the blakpc gene are frequently resistant to other antibiotics commonly used against Enterobacteriaceae infections, further limiting therapeutic options [4]. As a result of their multi-drug resistant profiles, infections caused by KPC-producing organisms have been associated with increased treatment failures, longer hospital stays and high mortality rates [5-8].
Despite the rapid rise of Klebsiella pneumoniae carbapenemase (KPC) producing organisms, the optimal treatment for these infections has not been well established. KPC-producing organisms have historically maintained susceptibility to polymyxins (for example, colistin sulfate and polymyxin B). Unfortunately, polymyxin resistance can develop rapidly when used as monotherapy and incidences of polymyxin resistance are being reported with increasing frequency [9-11]. Several in vitro studies have demonstrated that while polymyxins can exhibit rapid killing against KPC-producing organisms, re-growth has been observed by 24 hours [12, 13]. In the era of increasing resistance coupled with a decreasing antibiotic armamentarium, attention must be brought to the role of combination therapy. We evaluated the in vitro activity of colistin sulfate, polymyxin B, doripenem alone and in combination against KPC- producing K. pneumoniae.
| Methods | ▴Top |
Four non-duplicate KPC-3-producing K. pneumoniae isolates were collected from hospitalized patients previously described in an observational clinical case series [14]. Minimum inhibitory concentrations (MICs) were determined for doripenem, colistin sulfate, and polymyxin B by broth microdilution according to the Clinical and Laboratory Standards Institute (CLSI).
We evaluated the activity of the following regimens by conducting time-kill assays: doripenem, colistin sulfate, polymyxin B, colistin sulfate plus doripenem, and polymyxin B plus doripenem. Concentrations representative of achievable serum levels were used: doripenem concentration of 6 μg/mL and 2× MIC for colistin sulfate and polymyxin B. The methodology used for time-kill assays were previously described [15]. Bacterial densities were determined at 0, 4, 8, 12, 24 and 48 hours post inoculation. Fifty μLs were plated on trypticase soy agar plates using a spiral plater (Spiral Biotech, Bethesda, MD) then incubated for 24 hours before colony counts were enumerated using a laser colony counter (Q-Counter, Spiral Biotech, Bethesda, MD). Bactericidal activity was defined as ≥ 3-log10 CFU/mL reduction from the starting inoculum. Synergism and antagonism were defined as ≥ 2-log10 reduction or increase, respectively, in combination when compared to the most active single agent at 24 hours. The limit of quantification was 102 CFU/mL. All tests were performed in duplicate. Isolates exposed to colistin sulfate or polymyxin B with viable colonies after 24 hours were subjected to susceptibility testing to evaluate the development of resistance. Development of resistance was defined by an MIC increase of > 4 times the initial MIC.
| Results | ▴Top |
All strains were resistant to doripenem (MICs: 16 - 32 μg/mL) and susceptible to colistin sulfate and polymyxin B (Table 1).
 Click to view | Table 1. Minimum Inhibitory Concentrations (μg/mL) for KPC-3 Producing Klebsiella pneumoniae |
The average bacterial density of the starting inoculum was 5.52 ± 0.12 log10. For doripenem monotherapy, an initial reduction in log CFU/mL was observed at 4 hours followed by regrowth at 8 hours for all isolates (Fig. 1). Doripenem alone did not achieve bactericidal activity in any isolate at any time point. Colistin sulfate alone exhibited rapid bactericidal activity in 3 of 4 (75%) isolates by 4 hours and in all (4/4) isolates by 8 hours. Significant regrowth occurred in all 4 isolates exposed to colistin sulfate monotherapy with an average colony count of 6.72 ± 1.11 log10 at 24 hours and to growth-control quantities by 48 hours. Likewise, bactericidal activity was not sustained after 8 hours for polymyxin B alone. At 24 hours, there were significant regrowth among all of the strains exposed to polymyxin B monotherapy with an average colony count of 6.97 ± 1.25 log10.
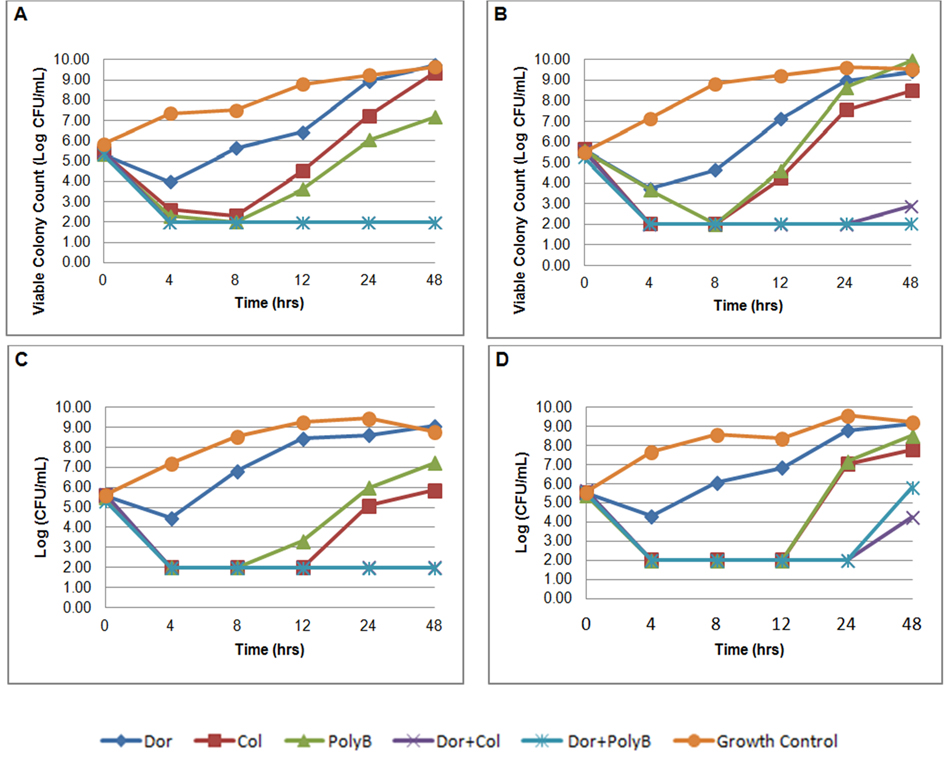 Click for large image | Figure 1. In-vitro time-kill assays of doripenem, colistin sulfate, polymyxin B alone and in combination against four KPC-3-producing Klebsiella pneumoniae. A) isolate C16; B) isolate C17; C) isolate C33; D) isolate C50. |
Both combinations of colistin sulfate plus doripenem and polymyxin B plus doripenem rapidly achieved bactericidal activity by 4 hours and were maintained through 24 hours in all strains. Synergy was observed for colistin sulfate plus doripenem in all isolates. Similarly, polymyxin B in combination with doripenem was synergistic and bactericidal at 24 hours. At 48 hours, the combination of colistin sulfate and doripenem demonstrated synergy against all 4 isolates and was bactericidal against 2 (50%) of the 4 isolates (C16 and C33). The combination of polymyxin B and doripenem demonstrated synergy against all 4 isolates at 48 hours and remained bactericidal in 3 (75%) of 4 isolates (C16, C17, and C33). Antagonism was not identified in any combination. Repeat MICs were performed for all 4 isolates with viable colonies after 24 hours of exposure to colistin sulfate and polymyxin B. All isolates exposed to colistin sulfate and polymyxin B alone developed resistance (MICs: 8 - 128 μg/mL) after 24 hours. Exposure to polymyxins had no change on doripenem MICs. Cross resistance between colistin sulfate and polymyxin B was also observed. After 24 hours of colistin sulfate exposure, the MIC for polymyxin B increased greater than about 500 fold. Colistin sulfate MICs increased about 32 - 2,000 fold post polymyxin B exposure.
| Discussion | ▴Top |
The incidence of infections caused by KPC-producing organisms is increasing at an alarming rate. These pathogens are highly resistant to multiple antimicrobial classes. While KPC-producing bacteria have historically maintained susceptibility to polymyxins, resistance to these last line agents is now well-documented. Studies evaluating the role of combination therapies against these pathogens are urgently needed. Herein, we sought to evaluate the effectiveness of colistin sulfate and polymyxin B in combination with doripenem. To our knowledge, this is the first study to compare activities of both colistin sulfate and polymyxin B in combination with doripenem against KPC isolates.
Overall, our study demonstrated that while polymyxin B and colistin sulfate exhibited rapid bactericidal activity within 8 hours, regrowth occurred by 24 hours. None of the monotherapy regimens sustained bactericidal killing at 24 hours. In comparison, with the addition of doripenem to colistin sulfate and polymyxin B, bactericidal killing was achieved and sustained through 24 hours in all isolates. Moreover, an important clinical observation was the bactericidal and synergistic activity of these combinations using concentrations of polymyxins (0.12 - 0.5 μg/mL) lower than traditionally studied. Implications of these preliminary findings suggest that when used in combination with doripenem, aggressive dosing of polymyxins to achieve higher serum concentrations may not be necessary. Toward this end, the risk of polymyxin dose-limiting toxicities including nephrotoxicity and neurotoxicity may be minimized. It is unknown, however, if higher concentrations of polymyxins may have maintained bactericidal killing in combination in all 4 isolates at 48 hours.
Conclusion
Polymyxins in combination with doripenem provided synergistic effects and achieved bactericidal activity minimizing the development of polymyxin resistance. Clinical studies and further in vitro studies evaluating a larger number of isolates and wider range of MICs are necessary to corroborate our findings.
Acknowledgments
We thank the following PharmD students at the University of Texas at Austin College of Pharmacy and UT Health Science Center San Antonio, Pharmacotherapy Education and Research Center in the School of Medicine: Emily Gordon, Isaac Pan, and Wilbur Rutter for their laboratory assistance.
Conflict of Interest Statement
All authors have no disclosures.
| References | ▴Top |
- Nordmann P, Cuzon G, Naas T. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect Dis. 2009;9(4):228-236.
pubmed - Yigit H, Queenan AM, Anderson GJ, Domenech-Sanchez A, Biddle JW, Steward CD, Alberti S,
et al . Novel carbapenem-hydrolyzing beta-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob Agents Chemother. 2001;45(4):1151-1161.
pubmed - Gupta N, Limbago BM, Patel JB, Kallen AJ. Carbapenem-resistant Enterobacteriaceae: epidemiology and prevention. Clin Infect Dis. 2011;53(1):60-67.
pubmed - Yigit H, Queenan AM, Rasheed JK, Biddle JW, Domenech-Sanchez A, Alberti S, Bush K,
et al . Carbapenem-resistant strain of Klebsiella oxytoca harboring carbapenem-hydrolyzing beta-lactamase KPC-2. Antimicrob Agents Chemother. 2003;47(12):3881-3889.
pubmed - Guidance for control of infections with carbapenem-resistant or carbapenemase-producing Enterobacteriaceae in acute care facilities. MMWR Morb Mortal Wkly Rep. 2009;58(10):256-260.
pubmed - Neuner EA, Yeh JY, Hall GS, Sekeres J, Endimiani A, Bonomo RA, Shrestha NK,
et al . Treatment and outcomes in carbapenem-resistant Klebsiella pneumoniae bloodstream infections. Diagn Microbiol Infect Dis. 2011;69(4):357-362.
pubmed - Gasink LB, Edelstein PH, Lautenbach E, Synnestvedt M, Fishman NO. Risk factors and clinical impact of Klebsiella pneumoniae carbapenemase-producing K. pneumoniae. Infect Control Hosp Epidemiol. 2009;30(12):1180-1185.
pubmed - Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect Control Hosp Epidemiol. 2008;29(12):1099-1106.
pubmed - Neonakis IK, Samonis G, Messaritakis H, Baritaki S, Georgiladakis A, Maraki S, Spandidos DA. Resistance status and evolution trends of Klebsiella pneumoniae isolates in a university hospital in Greece: ineffectiveness of carbapenems and increasing resistance to colistin. Chemotherapy. 2010;56(6):448-452.
pubmed - Zarkotou O, Pournaras S, Voulgari E, Chrysos G, Prekates A, Voutsinas D, Themeli-Digalaki K,
et al . Risk factors and outcomes associated with acquisition of colistin-resistant KPC-producing Klebsiella pneumoniae: a matched case-control study. J Clin Microbiol. 2010;48(6):2271-2274.
pubmed - Elemam A, Rahimian J, Mandell W. Infection with panresistant Klebsiella pneumoniae: a report of 2 cases and a brief review of the literature. Clin Infect Dis. 2009;49(2):271-274.
pubmed - Deshpande LM, Rhomberg PR, Sader HS, Jones RN. Emergence of serine carbapenemases (KPC and SME) among clinical strains of Enterobacteriaceae isolated in the United States Medical Centers: report from the MYSTIC Program (1999-2005). Diagn Microbiol Infect Dis. 2006;56(4):367-372.
pubmed - Pournaras S, Vrioni G, Neou E, Dendrinos J, Dimitroulia E, Poulou A, Tsakris A. Activity of tigecycline alone and in combination with colistin and meropenem against Klebsiella pneumoniae carbapenemase (KPC)-producing Enterobacteriaceae strains by time-kill assay. Int J Antimicrob Agents. 2011;37(3):244-247.
pubmed - Le J, Castanheira M, Burgess DS, McKee B, Iqbal R, Jones RN. Clonal dissemination of Klebsiella pneumoniae carbapenemase KPC-3 in Long Beach, California. J Clin Microbiol. 2010;48(2):623-625.
pubmed - Le J, McKee B, Srisupha-Olarn W, Burgess DS. In vitro activity of carbapenems alone and in combination with amikacin against KPC-producing Klebsiella pneumoniae. J Clin Med Res. 2011;3(3):106-110.
pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


