
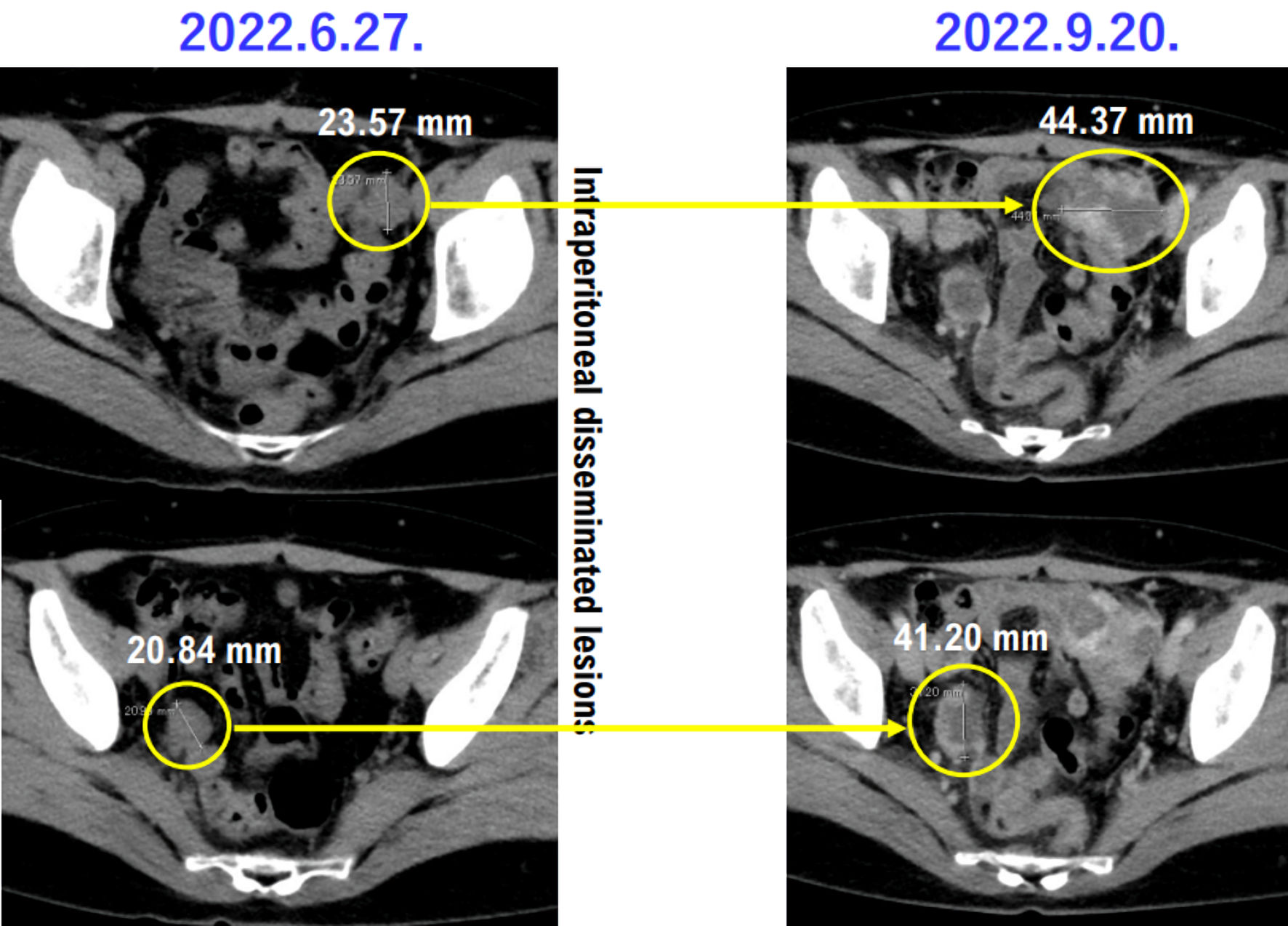
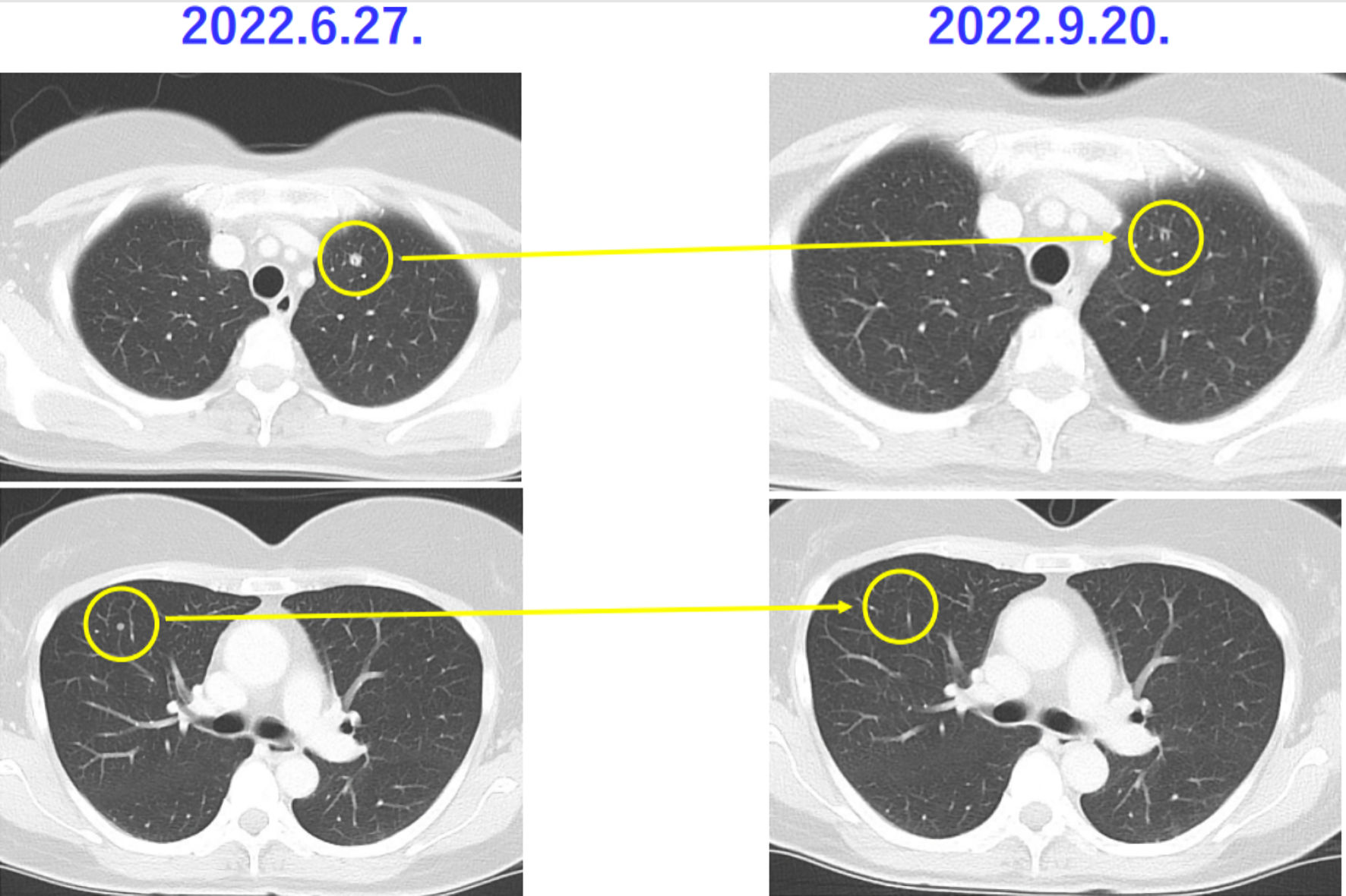
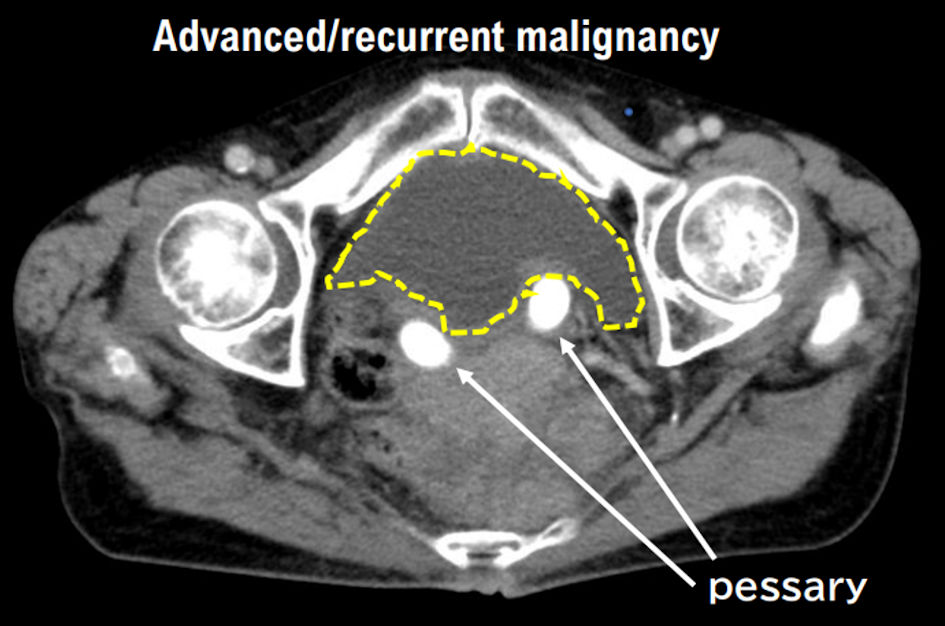
Figure 1. Histopathological findings of case 1. The patient in case 1 was diagnosed with uterine leiomyosarcoma by histopathological examination. Histopathological examination revealed no malignant tumor cells in the ovaries and fallopian tubes.
| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Case Report
Volume 15, Number 10-11, December 2023, pages 461-468
Treatment With Antitumor Agents Recommended by Cancer Genome Panel for Uterine Leiomyosarcoma
Figures

Tables
| Biomarker findings | Therapies with clinical relevance (in patient’s tumor type) | Therapies with clinical relevance (in other tumor types) |
|---|---|---|
| VAF: variant allele fraction. | ||
| Tumor mutational burden (14 Muts/Mb) | Pembrolizumab | Atezolizumab |
| Dostarlimab | Avelumab | |
| Cemiplimab | ||
| Durvalumab | ||
| Nivolumab | ||
| Nivolumab + ipilimumab | ||
| Microsatellite status - MS-equivocal | No therapies or clinical trials. See biomarker findings section | |
| Genomic findings | Therapies with clinical relevance (in patient’s tumor type) | Therapies with clinical relevance (in other tumor types) |
| ARAF - E89K VAF 32.47% | None | None |
| Variants to consider for follow-up germline testing in selected cancer susceptibility genes | ||
| MSH6 - F1088fs*5 VAF 1.51% | MUTYH - splice site 892-2A>G VAF 50.32% | |
| Biomarker findings | Therapy and clinical trial implications | |
|---|---|---|
| VAF: variant allele fraction. | ||
| Microsatellite status - MS-stable | No therapies or clinical trials. See biomarker findings section | |
| Tumor mutational burden (2 Muts/Mb) | No therapies or clinical trials. See biomarker findings section | |
| Genomic findings | Therapies with clinical relevance (in patient’s tumor type) | Therapies with clinical relevance (in other tumor types) |
| AKT1 - E17K VAF 11.56% | None | Pazopanib |
| Variants to consider for follow-up germline testing in selected cancer susceptibility genes | ||
| ATRX - L1250* VAF 39.98% | RB1 - loss exons 18-27 CN0 | |
| MED12 - G44S VAF 44.88% | TP53 - G187V, K132N VAF 44.89% | |