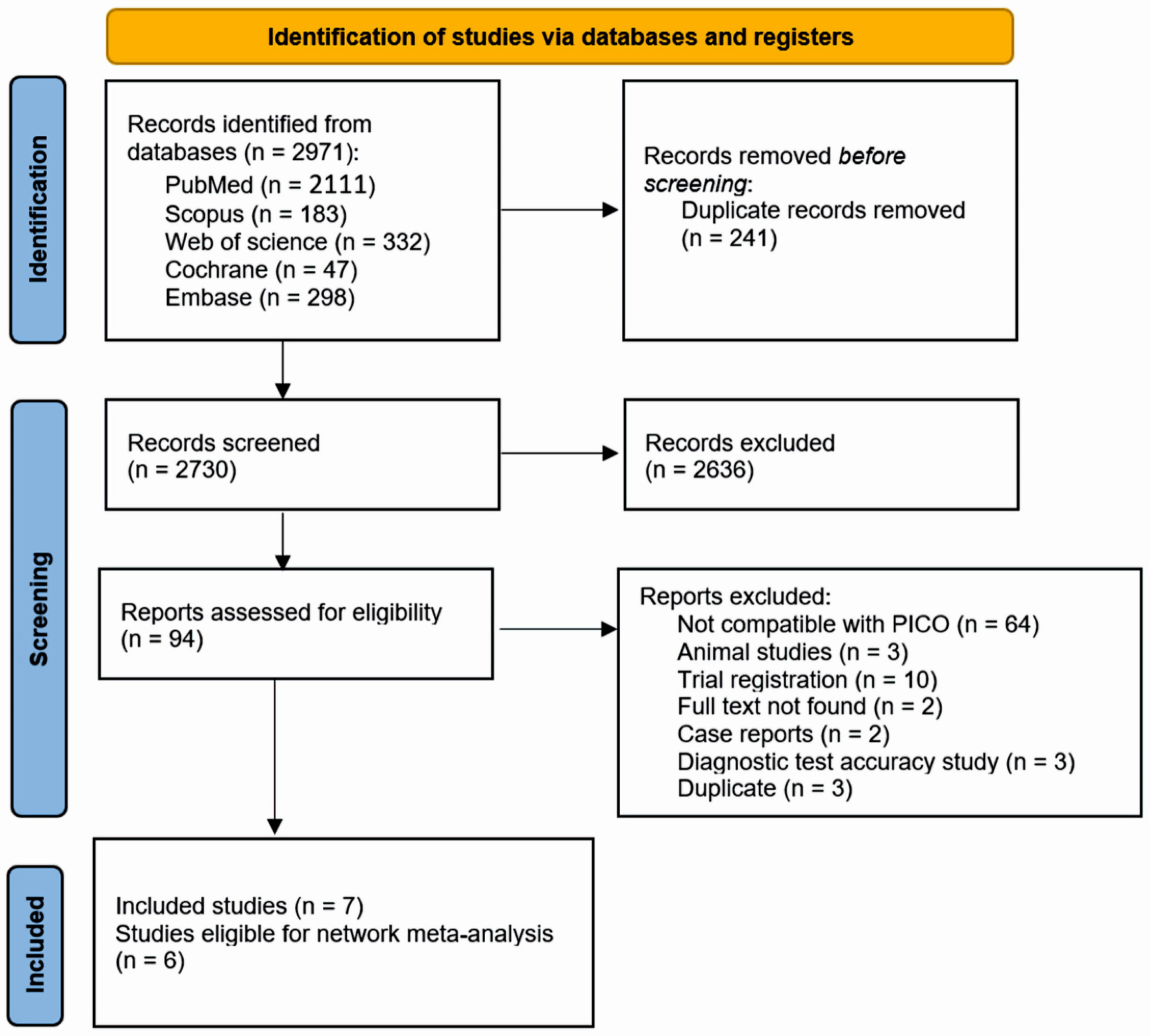
Figure 1. PRISMA flow diagram for literature search. PRISMA: the Preferred Reporting Items for Systematic Reviews and Meta-Analysis; PICO: patient, intervention, comparison, and outcome.
| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Original Article
Volume 16, Number 2-3, March 2024, pages 33-45
Evaluation of Alternative Treatment Strategies for Bile Acid Malabsorption in Inflammatory Bowel Disease Patients: A Network Meta-Analysis
Figures

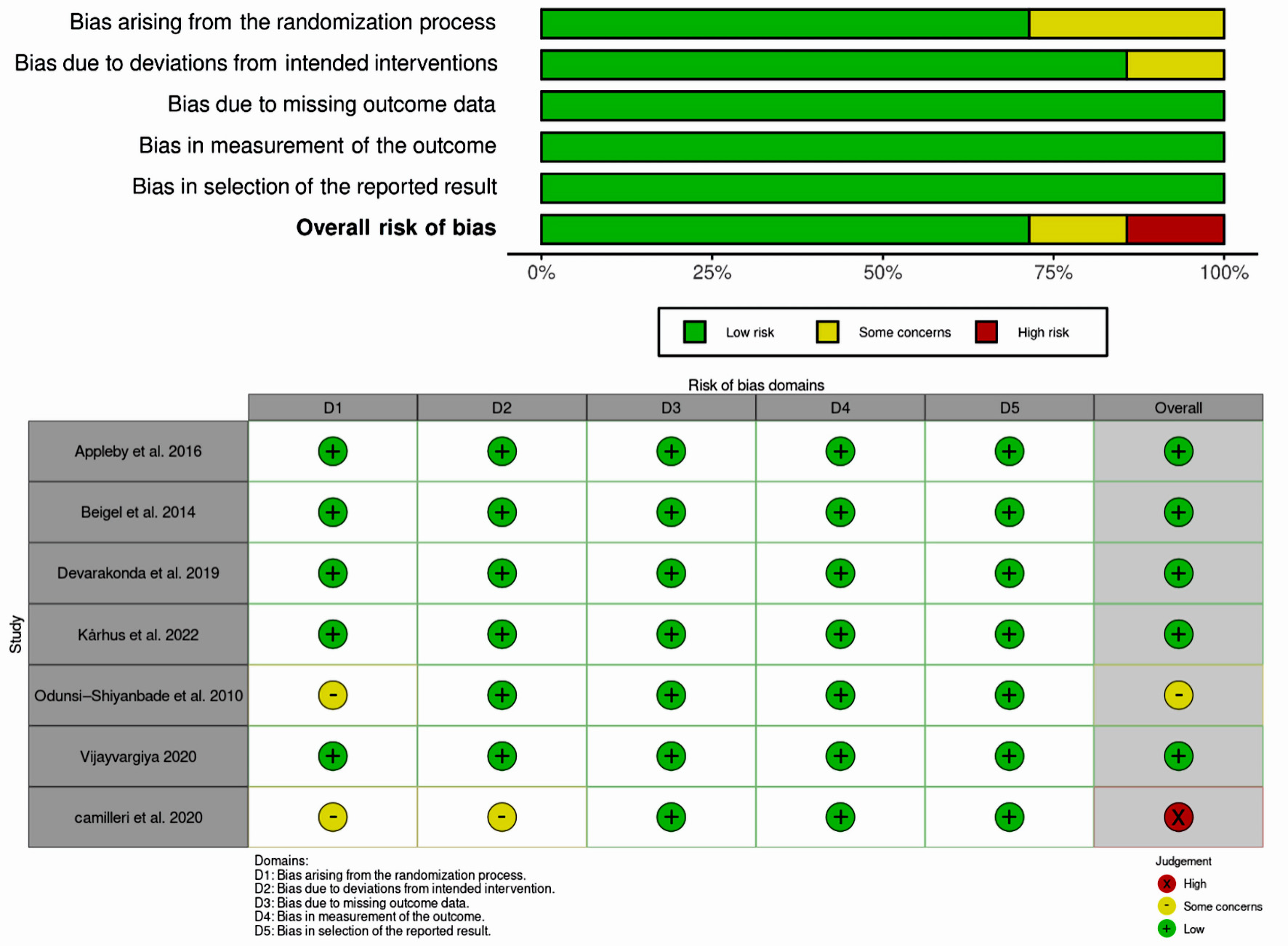
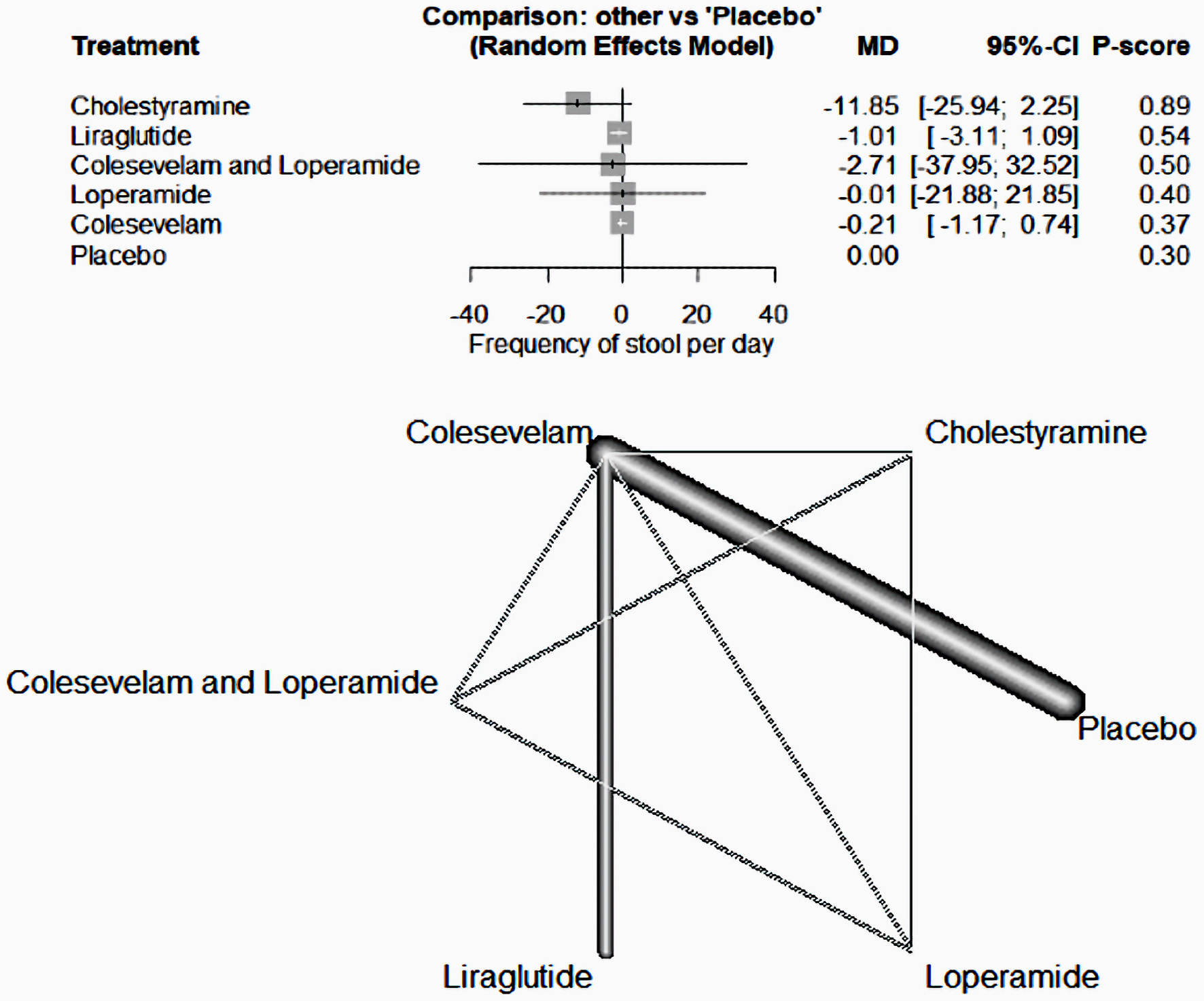
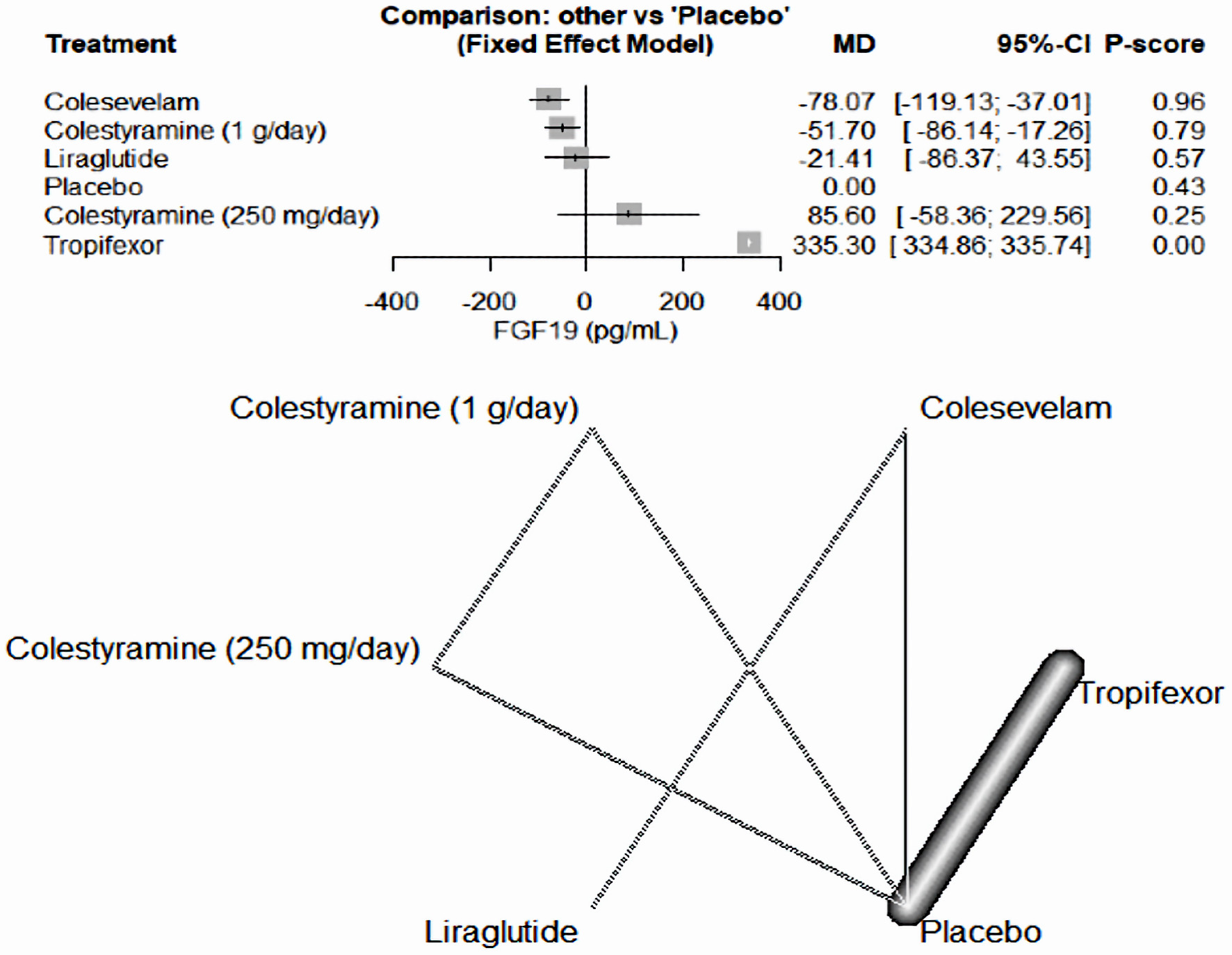
Tables
| References | Groups | Sample size | Design | Inclusion criteria | Regimen | Underlying GI problem | Findings | Country |
|---|---|---|---|---|---|---|---|---|
| RCT: randomized controlled study; BMI: body mass index; GI: gastrointestinal; CD: Crohn’s disease; BSFS: Bristol Stool Form Scale; 75SeHCAT: selenium-75-homocholic acid taurine; bid: twice daily; BAD: bile acid diarrhea; CDAI: Crohn’s disease activity index; BAM: bile acid malabsorption; IBS-D: irritable bowel syndrome with diarrhea; FGF19: fibroblast growth factor 19; BA: bile acid. | ||||||||
| Appleby et al, 2017 [16] | Cholestyramine (1 g/day) | 7 | RCT | Men and women aged 18 - 80 years with a BMI between 18.5 and 35: 1) three stools of BSFS 5 per day on average, calculated from 7 days off therapy; 2) SeHCAT 7-day retention of < 10% (moderate or severe impairment), within the last 5 years with no change in symptoms since; 3) 21 bowel motions in the last 7 days before randomization, of which > 50% are BSFS ≥ 5; 4) macroscopically normal colonoscopy with no histological evidence of microscopic colitis within the last 5 years. | A3384 250 mg bid. or A3384 1 g bid., or placebo for 2 weeks | - | Serum FGF19 was suppressed, and bile acid production upregulated on conventional bile acid sequestrants, but not with A3384. This colonic-release formulation of colestyramine produced symptomatic benefit in patients with BAD. | Sweden |
| Cholestyramine (250 mg/day) | 6 | |||||||
| Placebo | 6 | |||||||
| Beigel et al, 2014 [17] | Colesevelam | 15 | RCT | CD patients with ≥ 3 and ≤ 15 liquid stools/day in clinical remission, defined as CD activity index (CDAI) ≤ 150 points and CRP value ≤ 1 mg/dL were eligible for the study, age ≥ 18 and ≤ 65 years, contraception in women, medication with either oral aminosalicylates, azathioprine, mercaptopurine, methotrexate, oral steroids (10 mg/day), or anti-TNF alpha antibody and cholestenone serum level of ≥ 50 ng/mL, which was centrally assessed at the screening visit. | Patients received either colesevelam (3 × 2 tablets a 625 mg/day) or identically shaped placebo (3 × 2 tablets/ day). | CD | We found significant differences in favor for colesevelam treatment compared to placebo treatment for CD patients with BAM regarding the reduction of the number of liquid stools/day and stool consistency. | Germany |
| Placebo | 11 | |||||||
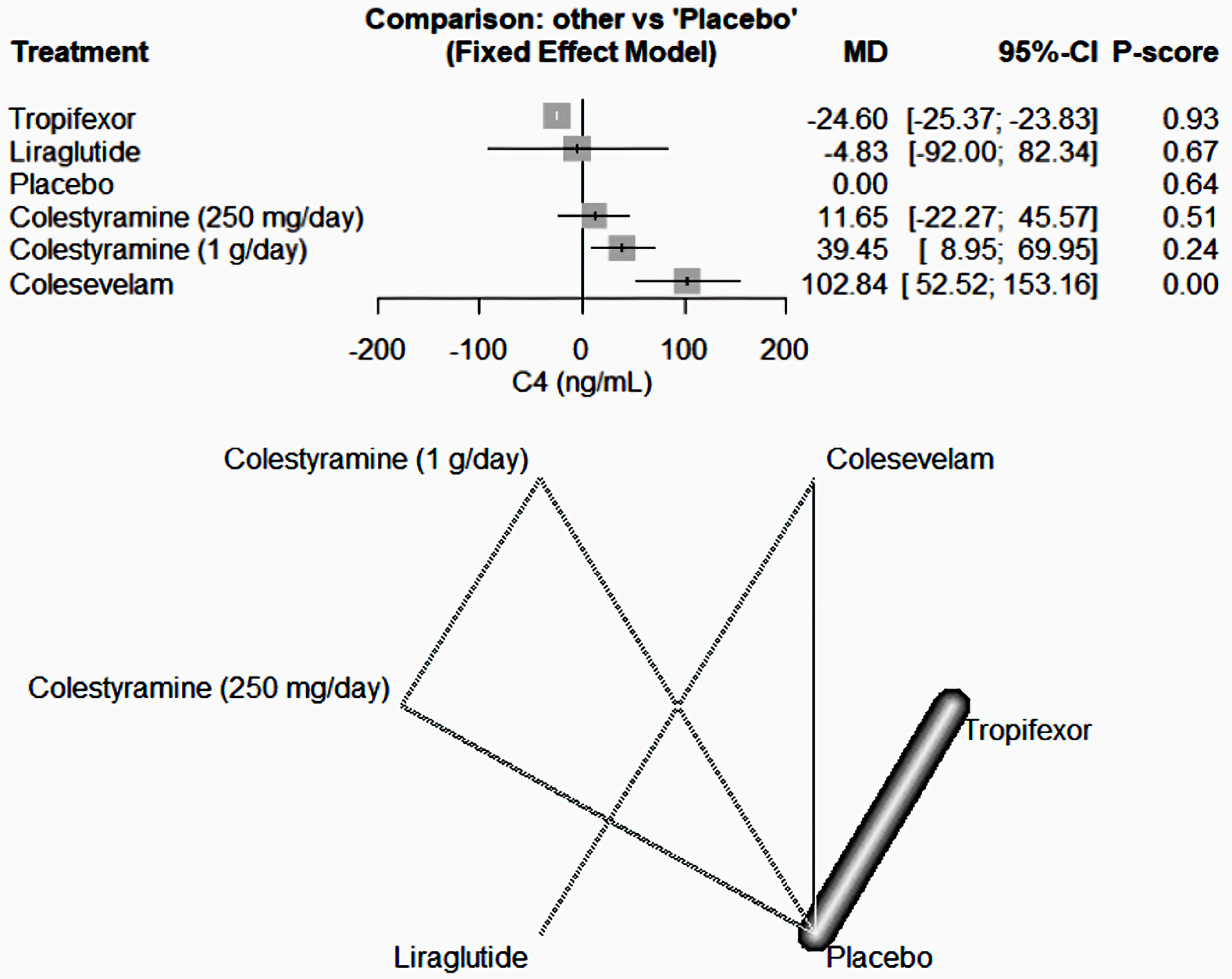
| Camilleri et al, 2020 [18] | Tropifexor (60 µg/day) - placebo | 10 | Cross over RCT | Patients with primary bile acid diarrhea, who had not used bile acid sequestrants, aged ≥ 18 years weighing ≥ 50 kg, with a history of diarrheal symptoms for at least 3 months prior to dosing, as defined by average stool frequency of ≥ 3 per day when off therapy and average stool form of > 5 on the Bristol Stool Chart; laboratory or radiological confirmation of bile acid malabsorption within the last 5 years as defined by evidence of fecal bile acid loss of ≥ 2,000 µmol/48 h with or 7-day 75SeHCAT retention of < 10%. | Tropifexor 60 µg/placebo or placebo/tropifexor 60 µg. In treatment period 1, patients received either tropifexor 60 µg or placebo orally once daily for 14 days. | - | Tropifexor 60 µg once daily had acceptable safety and tolerability. Changes in FGF19 and C4 showed effective target engagement; however, higher doses may be required to observe stool frequency changes. Slowing of ascending colon emptying suggests therapeutic potential of tropifexor in patients with primary bile acid diarrhea. | USA |
| Placebo - tropifexor (60 µg/day) | 10 | |||||||
| Devarakonda et al, 2019 [19] | Colesevelam | 17 | RCT | - | CD | We have demonstrated that colesevelam is an effective treatment for postoperative BAM in CD. Both colesevelam and cholestyramine were associated with a reduction in stool frequency but only colesevelam was associated with a reduction in CDAI and an improvement in quality of life. | UK | |
| Cholestyramine | 10 | |||||||
| Loperamide | 12 | |||||||
| Colesevelam and loperamide | 5 | |||||||
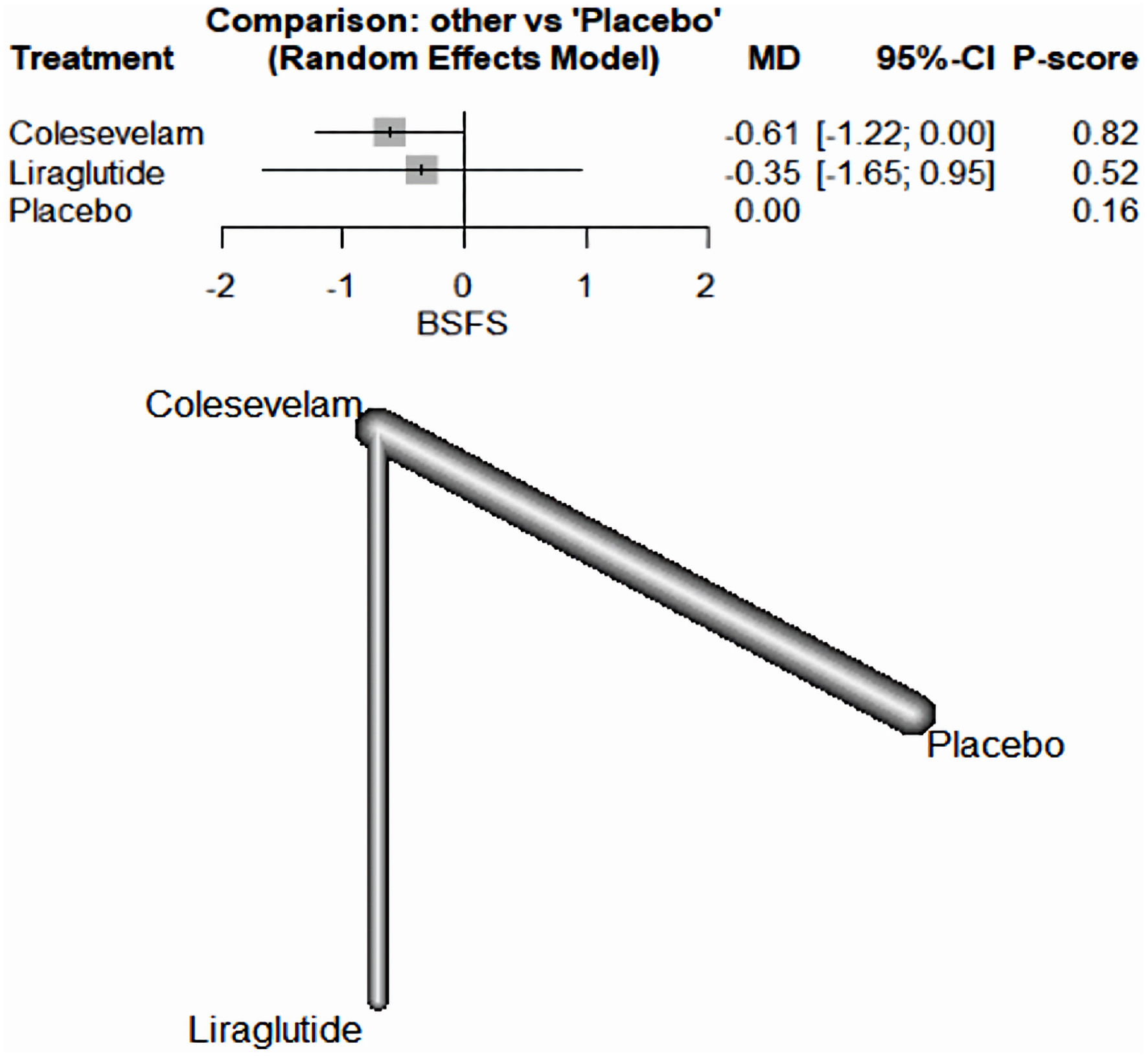
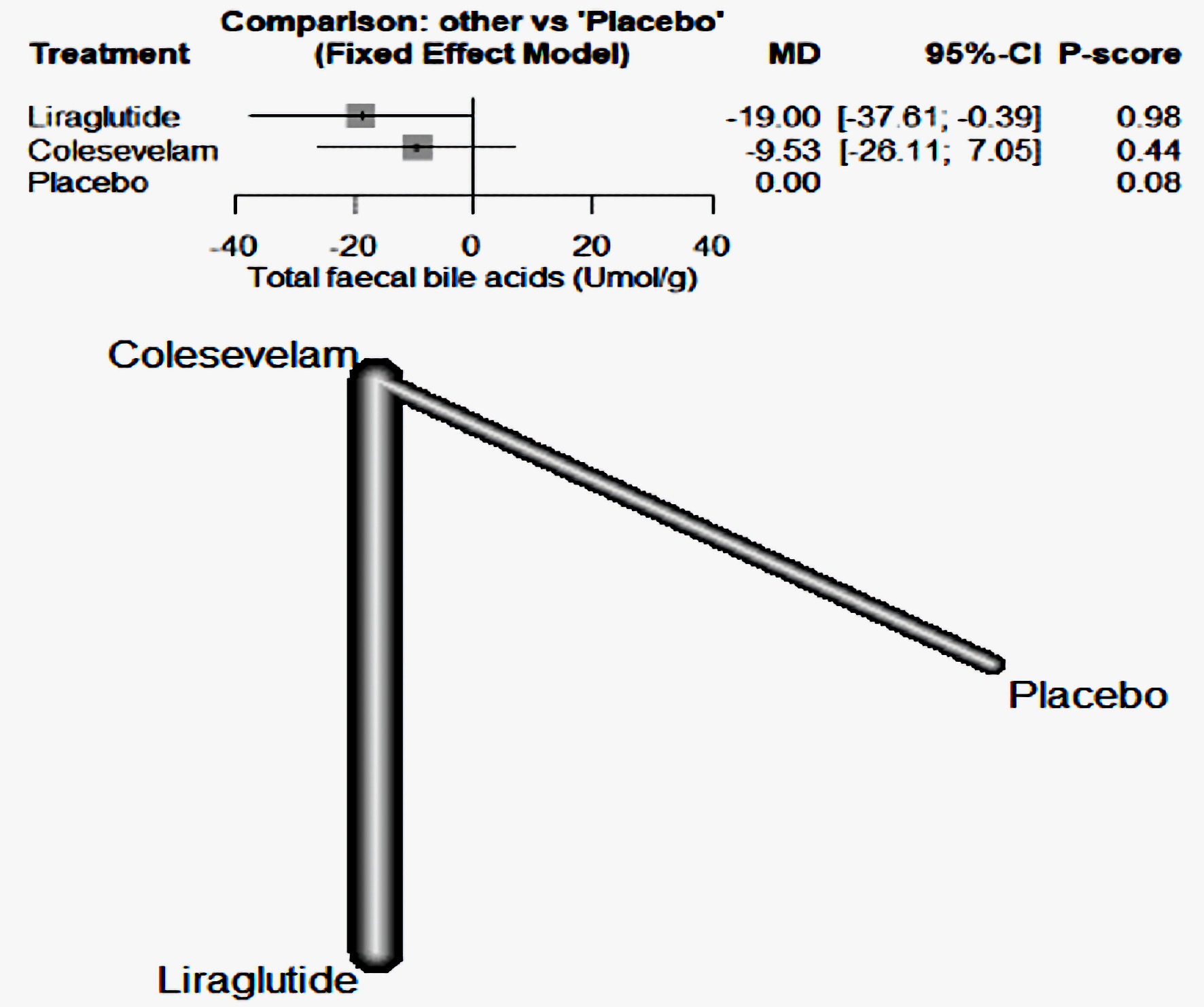
| Karhus et al, 2022 [20] | Liraglutide | 25 | RCT | White individuals aged 18 - 75 years with SeHCAT verified moderate-to-severe primary bile acid diarrhea (≤ 10% 7-day bile acid retention), with no diabetes, other GI diseases including CD, ileal resection, previous history of radiation to the abdomen or the pelvis, or recent or active malignancy. | Liraglutide (one daily subcutaneous injection up-titrated from 0.6 mg to 1.8 mg over 3 weeks) or colesevelam (three capsules of 625 mg twice daily) and the opposite dummy for 6 weeks. | IBS-D | The superiority of liraglutide compared with colesevelam in reducing stool frequency suggests consideration of liraglutide as a potential new treatment modality for bile acid diarrhea, although larger confirmatory trials powered for superiority are warranted | Denmark |
| Colesevelam | 25 | |||||||
| Odunsi-Shiyanbade et al, 2010 [10] | Colesevelam | 12 | RCT | All participants had fasting plasma 7alpha-C4 (C4) levels measured to assess for underlying BAM and had serum FGF19 levels measured. Participants also completed the following questionnaires: Hospital Anxiety and Depression Scale,16 Symptom Checklist 90 (SCL-90) (somatization), and bowel function by validated daily diaries including BSFS scores. | Colesevelam HCl (625-mg tablets) and the placebo (312.5-mg capsules) | IBS-D | Sodium chenodeoxycholate in health and colesevelam in IBS-D patients have opposite effects on colonic transit and fecal parameters. | USA |
| Placebo | 12 | |||||||
| Vijayvargiya et al, 2020 [21] | Colesevelam | 15 | RCT | Females and males aged 18 - 75. An IBS diagnosis based on the Rome III criteria for at least 3 months, with onset at least 6 months previously, of recurrent abdominal pain or discomfort. Biomarkers serum alpha C4 ≥ 40 ng/mL or FGF19 ≤ 80 pg/mL or fecal bile acid > 2,000 µmol/48 h | Colesevelam, 1,875mg (three tablets, 625 mg each), or matching placebo (ratio 1:1), taken orally, twice daily. | IBS-D | In a randomized trial, we found that colesevelam increases delivery of total and secondary BAs to stool, hepatic BA synthesis, and colonic mucosal expression of genes that regulate BA, farnesoid X, and GPBAR1 receptors. Larger studies are needed to determine the effects on clinical responses. | USA |
| Placebo | 15 | |||||||
| Drug | Mechanism of action |
|---|---|
| Colestyramine | Cholestyramine resin adsorbs and combines with the bile acids in the intestine to form an insoluble complex which is excreted in the feces. This results in a partial removal of bile acids from the enterohepatic circulation by preventing their absorption. |
| Colesevelam | Colesevelam hydrochloride is a non-absorbed, lipid-lowering polymer that binds bile acids in the intestine, impeding their reabsorption. |
| Tropifexor | Activation of tropifexor inhibits bile acid synthesis and increases bile acid conjugation, transport, and excretion, thereby protecting the liver from the harmful effects of bile accumulation, leading to considerable interest in tropifexor as a therapeutic target for the treatment of cholestasis and nonalcoholic steatohepatitis. |
| Loperamide | Loperamide, a synthetic opiate agonist, decreases peristaltic activity and inhibits secretion, resulting in the reduction of fluid and electrolyte loss and an increase in stool consistency. |
| Liraglutide | Through liraglutide-induced prolongation of small intestinal transit time allowing a greater degree of passive and active bile acid reabsorption through the small intestine and thereby reducing spillover of bile acids. |