Figures
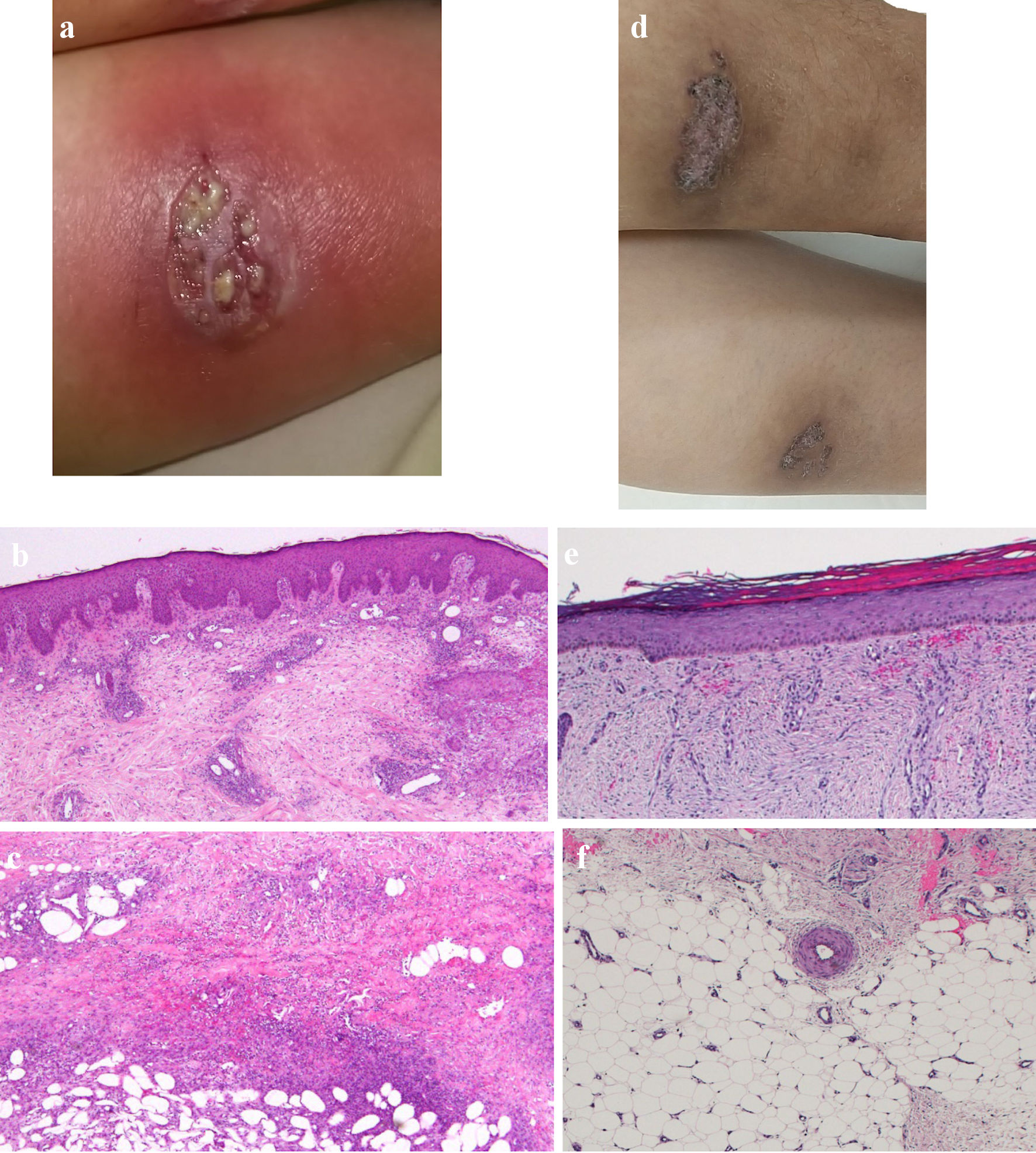
Figure 1. Skin findings of pyoderma gangrenosum on the lower legs. (a) On admission, gross inspection shows deep ulcers with erythema on the flexion side of the left lower leg as the representative lesion. (b, c) On admission, histopathological findings show diffusely necrotizing suppurative inflammation and perivascular infiltrates with lack of vasculitis due to dominant neutrophils rather than lymphocytes, and focal fibrosis in the dermis (b, × 40), and necrotizing suppurative inflammation in the subcutaneous adipose tissue due to dominant neutrophils (c, × 40). (d) At 10 weeks after starting combination therapy, gross inspection shows healing of the ulcer with re-epithelization. (e, f) At 10 weeks after starting combination therapy, ulcers show re-epithelization with mild edema and regeneration of venules in the dermis (e, × 40), and mild fibrosis in the subcutaneous tissue (f, × 40).
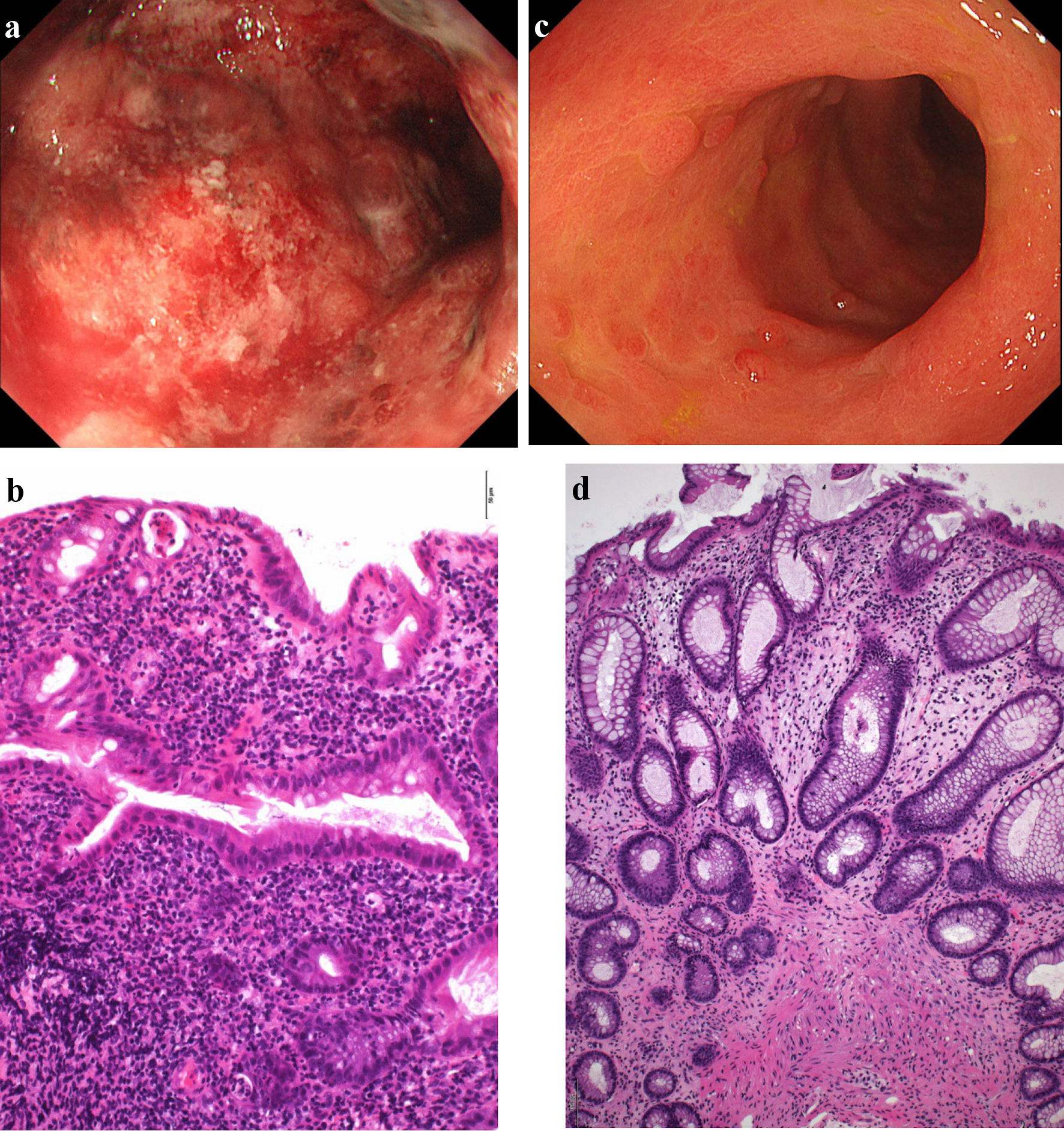
Figure 2. Findings from colonoscopy. (a) On admission, colonoscopy of the descending colon shows edematous mucosa and ulcerations with marked erythema and spontaneous bleeding. (b) On admission, histopathological findings based on Geboes score (grade 5.4) show severe diffuse or multifocal abnormalities of crypts and a marked increase in chronic inflammatory infiltrates, with no increase in eosinophils and a marked increase in neutrophils in the lamina propria. Over 50% of crypts in the epithelium showed infiltration of neutrophils, unequivocal crypt destruction and ulcer or granulation tissue (× 200). (c) At 10 weeks after starting combination therapy, colonoscopy of the descending colon shows marked erythema, no clear vascular pattern with no ulcerated surfaces. (d) At 10 weeks after starting combination therapy, histopathological findings based on Geboes score (grade 2A.1) show no crypt abnormality, a mild increase in chronic inflammatory infiltrates, a mild increase of eosinophils and no increase of neutrophils in the lamina propria, and no neutrophil infiltration in the epithelial crypts, no crypt destruction and no erosion, ulceration, or granulation tissue (× 100).
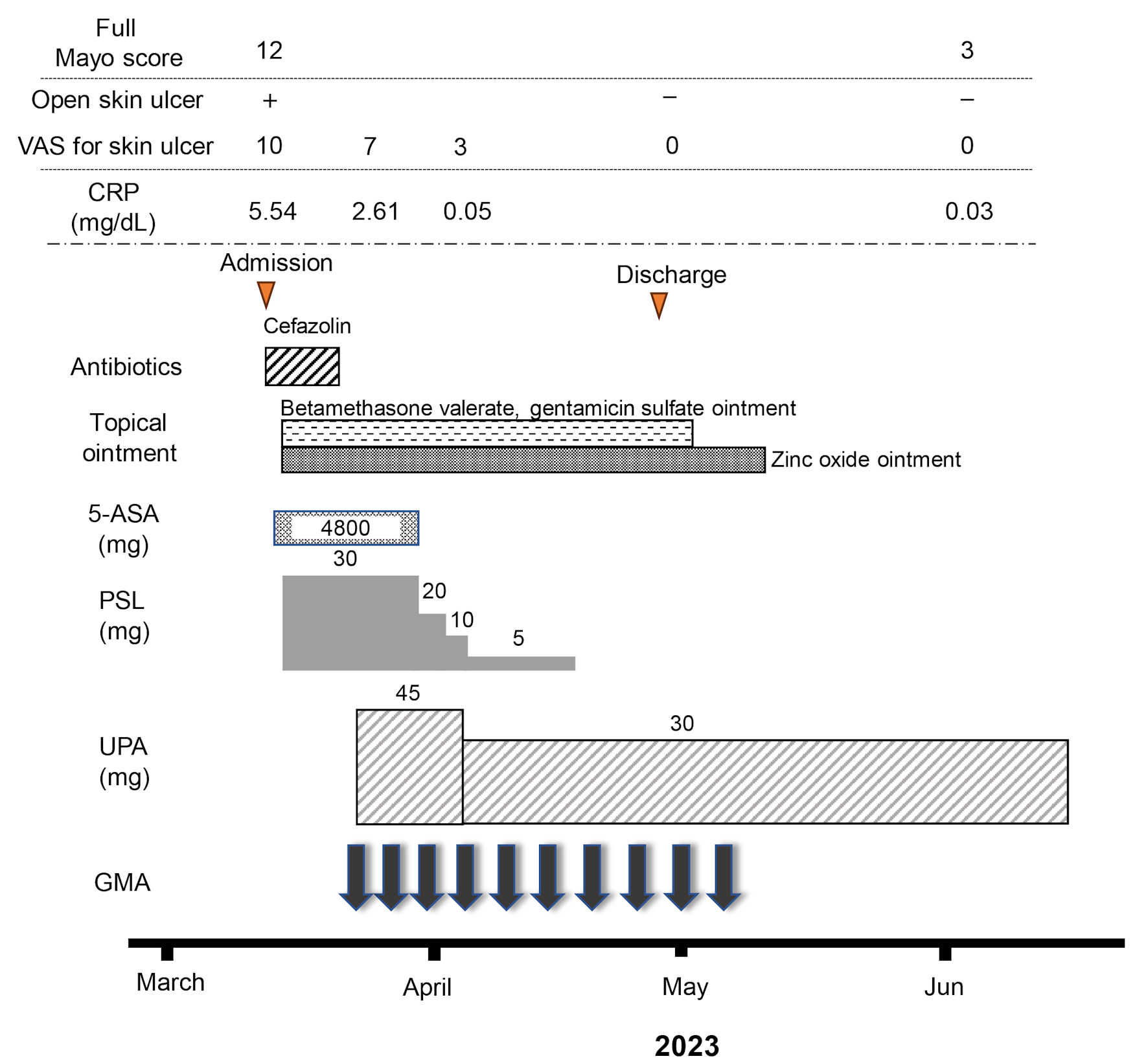
Figure 3. Clinical course for 10 weeks. The patient was admitted for treatment of active pyoderma gangrenosum (PG) associated with severe ulcerative colitis (UC) in March, 2023. Full Mayo score on admission as an activity index for UC was 12, including a Mayo endoscopic subscore of 3. Intravenous administration of prednisolone (PSL) was started at 30 mg/day, accompanied by mesalazine at 4,800 mg/day. However, combination therapy with upadacitinib (UPA) at 45 mg/day plus intensive granulocyte and monocyte adsorptive apheresis (GMA) was started as corticosteroid-refractory disease was seen. Mesalazine was then discontinued because the patient proved allergic to the drug. In addition, the dose of upadacitinib was decreased from 45 to 30 mg/day because anemia was identified 10 days after starting upadacitinib. An ointment combining betamethasone valerate and gentamicin sulfate and zinc oxide ointment had been continued until the PG ulcer healed. The dose of PSL was tapered off at the end of April. At the beginning of June, after 10 weeks of combination therapy using upadacitinib plus intensive GMA, clinical improvement (full Mayo score, 3), histologic mucosal improvement of UC, ulcer healing and marked histologic improvement of PG were achieved. 5-ASA: 5-aminosalicylic acid; CRP: C-reactive protein.
Table
Table 1. Laboratory Findings on Admission
| Results | Normal range |
|---|
| WBC: white blood cell; RBC: red blood cell; Hb: hemoglobin; Ht: hematocrit; MCV: mean corpuscular volume; MCH: mean corpuscular hemoglobin; PLT: platelet; TP: total protein; T-Bil: total bilirubin; Alb: albumin; AST: aspartate aminotransferase; ALT: alanine aminotransferase; LDH: lactate dehydrogenase; γ-GTP: γ-glutamyl transferase; BUN: blood urea nitrogen; Cr: creatinine; CRP: C-reactive protein; PR: proteinase; ANCA: antineutrophil cytoplasmic antibody; MPO: myeloperoxidase; HBs: hepatitis B surface; HBc: hepatitis B core; HBV; hepatitis B virus; HCV: hepatitis C; EB: Epstein-Barr; Ig: immunoglobulin; EBNA: Epstein Barr virus nuclear antigen. |
| Hematology | | |
| WBC, /µL | 11,300 | 3,300 - 8,600 |
| Neutrophil, % | 77.3 | 40.4 - 70.0 |
| Lymphocyte, % | 11.1 | 25.0 - 45.0 |
| RBC, × 104/µL | 372 | 386 - 492 |
| Hb, g/dL | 10.6 | 11.6 - 14.8 |
| Ht, % | 33.0 | 35.1 - 44.4 |
| MCV, fL | 88.7 | 83.6 - 98.2 |
| MCH, pg | 28.5 | 27.5 - 33.2 |
| PLT, × 103/µL | 346 | 158 - 348 |
| Serum biochemistry | | |
| TP, g/dL | 6.6 | 6.6 - 8.1 |
| Alb, g/dL | 3.0 | 4.1 - 5.1 |
| T-Bil, mg/dL | 0.3 | 0.4 - 1.5 |
| AST, U/L | 14 | 13 - 30 |
| ALT, U/L | 14 | 7 - 23 |
| LDH, U/L | 112 | 124 - 222 |
| γ-GTP, U/L | 20 | 9 - 32 |
| BUN, mg/dL | 7.4 | 8 - 20 |
| Cre, mg/dL | 0.81 | 0.46 - 0.79 |
| Glu, mg/dL | 107 | 73 - 109 |
| Na, mmol/L | 138 | 138 - 145 |
| K, mmol/L | 3.3 | 3.6 - 4.8 |
| Cl, mmol/L | 102 | 101 - 108 |
| Fe, µg/dL | 13 | 40 - 188 |
| CRP, mg/dL | 5.54 | ≤ 0.30 |
| Serology | | |
| Antigenemia (C-7HRP) | Negative | |
| QuantiFERON | Negative | |
| β-D-glucan, pg/mL | 8.3 | ≤ 20.0 |
| EB VCA IgG, times | 80 | |
| EB VCA IgM, times | < 10 | |
| EB EBNA, times | 40 | |
| Anti-nuclear antibodies, times | < 40 | |
| PR3-ANCA, U/mL | 2.3 | < 4 |
| MPO-ANCA, U/mL | < 1.0 | < 4 |
| HBs antigen, IU/mL | 0.01 | 0.00 - 0.04 |
| HBs antibody, mIU/L | 0.8 | < 10.0 |
| HBc antibody, sample/cut-off | 0.11 | < 1.00 |
| HBV DNA | Not detected | |
| HCV antibody, sample/cut-off | 0.08 | 0.00 - 0.99 |
| Stool culture | No pathogenic microbe | |
| Clostridium difficile (-), CD toxin A/B (-) | |


