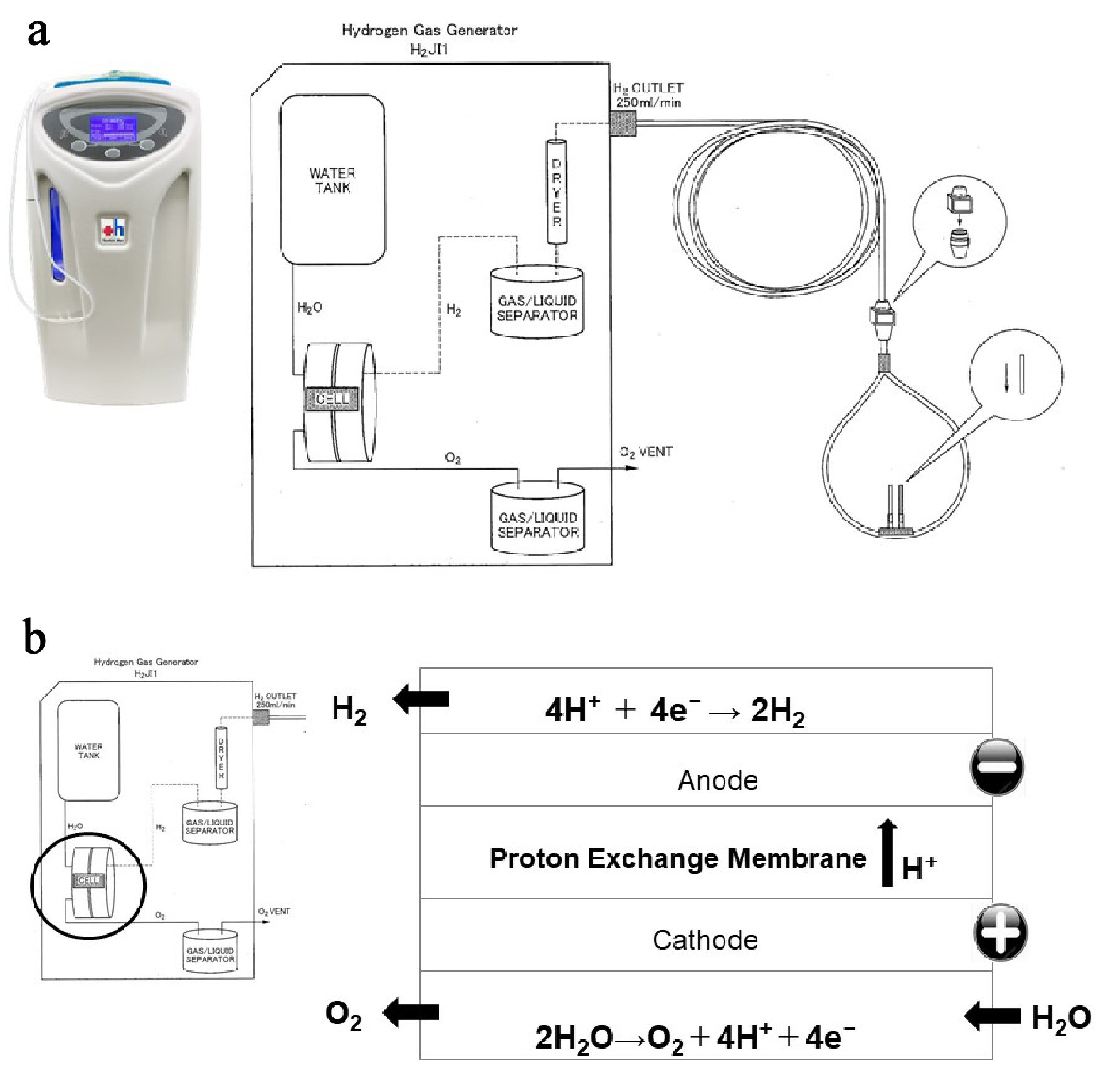
Figure 1. Hydrogen gas (H2) supply system from an H2 gas generator. (a) Specifications of H2 gas generator. The H2 gas generator is capable of continuously administering 100% H2 at a flow rate of 250 mL/min, 24 h a day, 365 days a year. (b) Structure of the electrolyzer. The electrolysis reaction occurs in an electrolyzer, which consists of two electrodes separated by an ion exchange membrane. When voltage is continuously applied to the electrodes in the electrolyzer, two electrons are removed from a water molecule at the anode (negative electrode) to form one oxygen molecule (O2) and four hydrogen ions. The O2 is safely released into the atmosphere, and the four hydrogen ions pass through the ion exchange membrane and are attracted to the cathode. At the cathode (positive electrode), electrons are combined with hydrogen ions to produce hydrogen gas (H2).
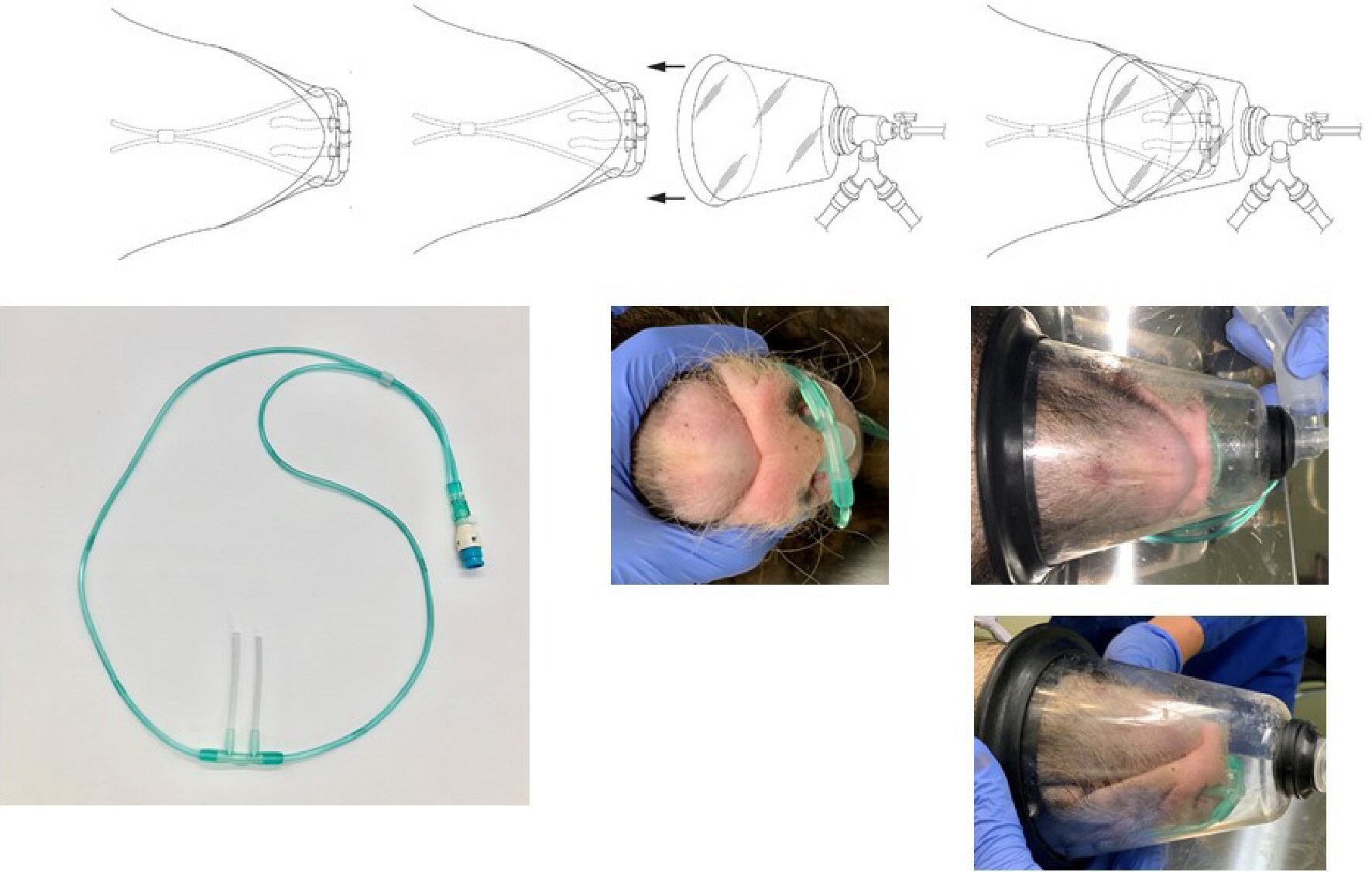
Figure 2. Nasal cannula and oxygen mask for micro miniature pigs. The length of the part of the nasal cannula that is inserted into the nostrils was modified from the original 12 mm to 62 mm by inserting a 10-mm silicon tube. The nasal cannula was then inserted deep into the nasal cavity of a spontaneously breathing pig, and 100% H2 produced by the H2 gas generator was administered at a flow rate of 250 mL/min. A veterinary anesthesia mask was placed over the nasal cannula, and a veterinary anesthesia delivery system was used to supply oxygen and anesthesia to the animals.
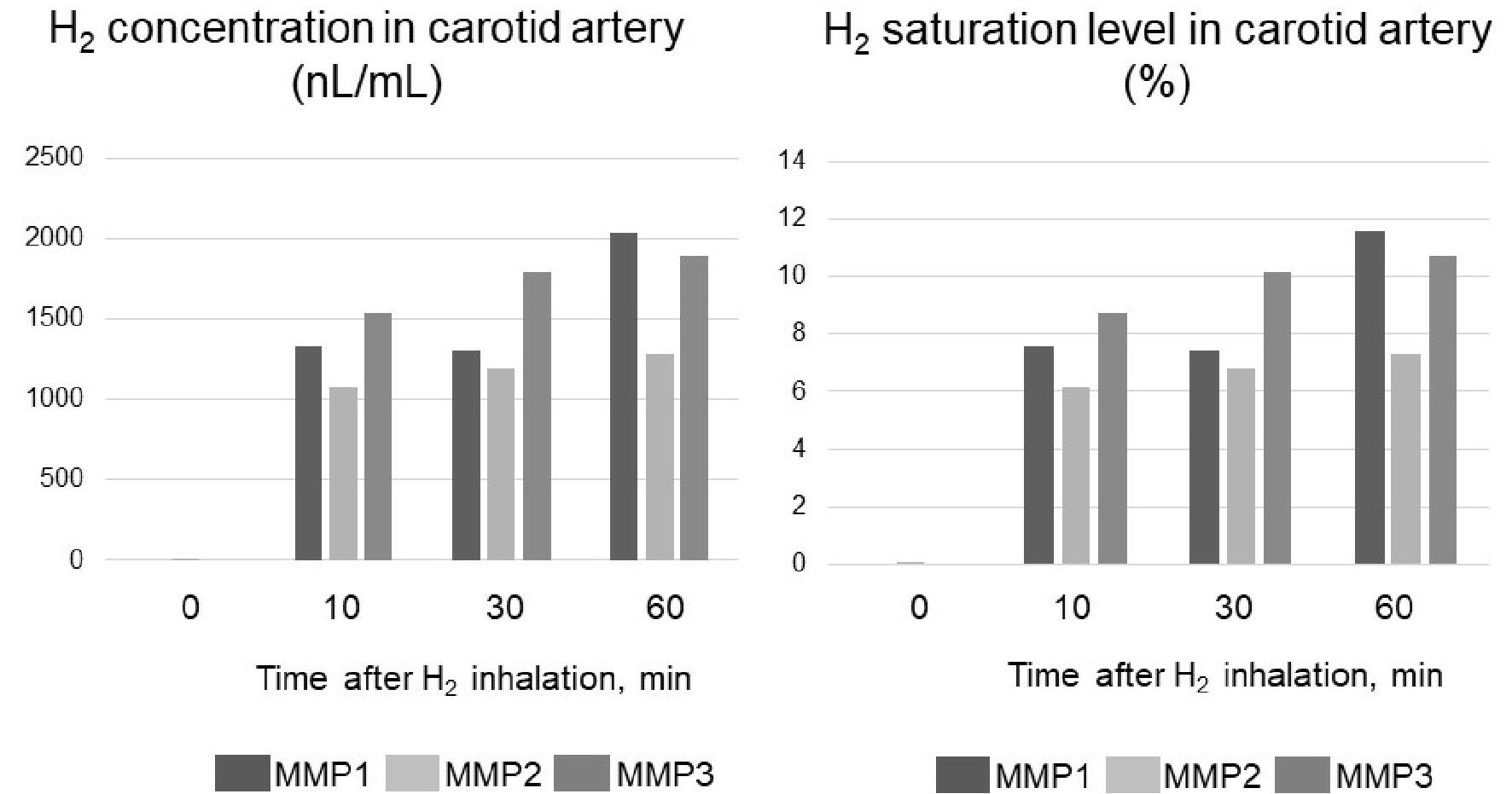
Figure 3. Carotid artery hydrogen (H2) concentration in three micro miniature pigs (MMP1 - 3) during H2 inhalation. The concentration of H2 when it is completely dissolved in water is 17,600 nL/mL. Therefore, H2 saturation was determined by dividing the measured value (concentration) by 17,600 nL/mL.


