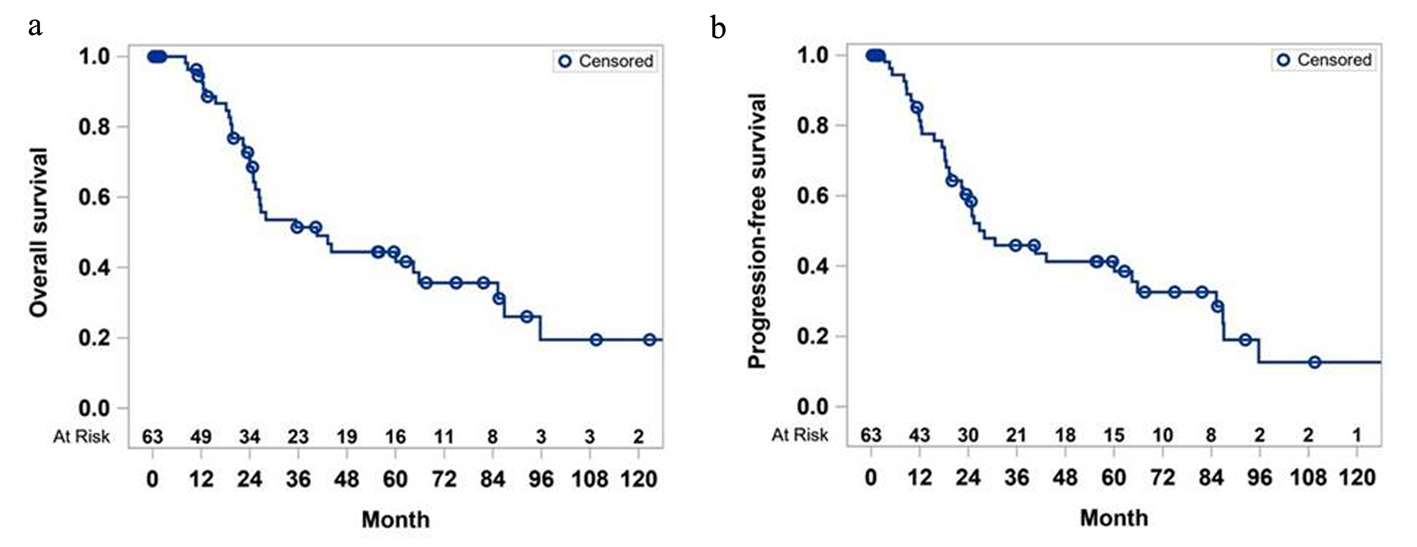
Figure 1. Overall survival (a) and progression-free survival (b) of study cohort.
| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Original Article
Volume 12, Number 9, September 2020, pages 560-567
The Clinical Benefit of Adjuvant Therapy in Long-Term Survival of Early-Stage Ampullary Carcinoma: A Single Institutional Experience
Figures

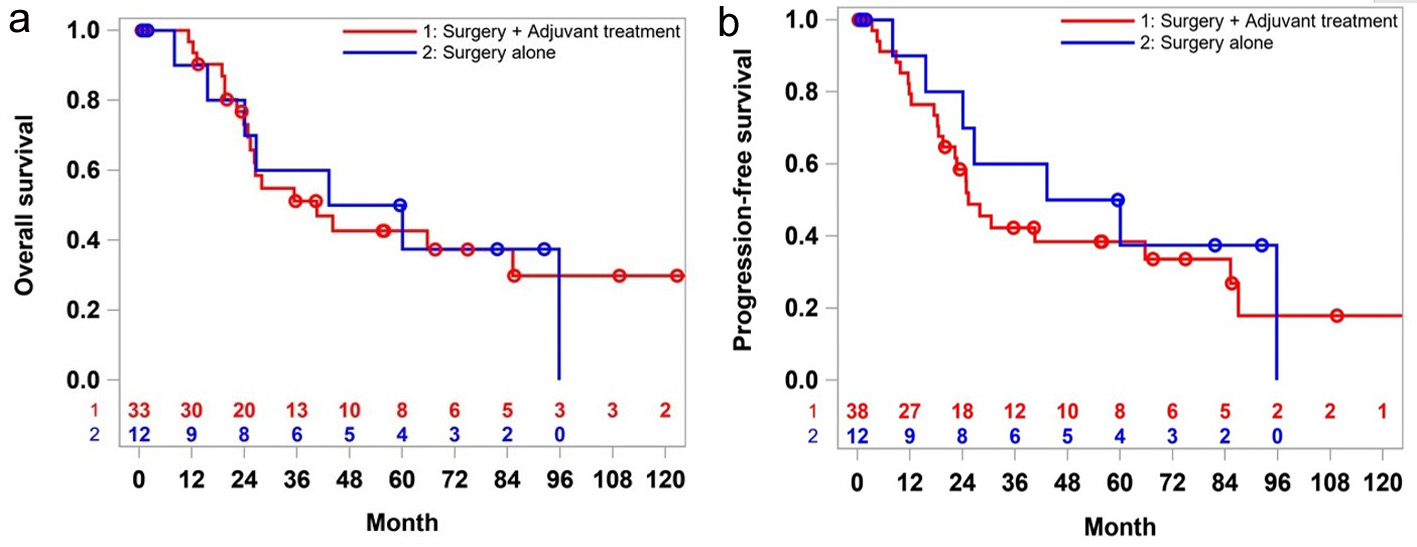
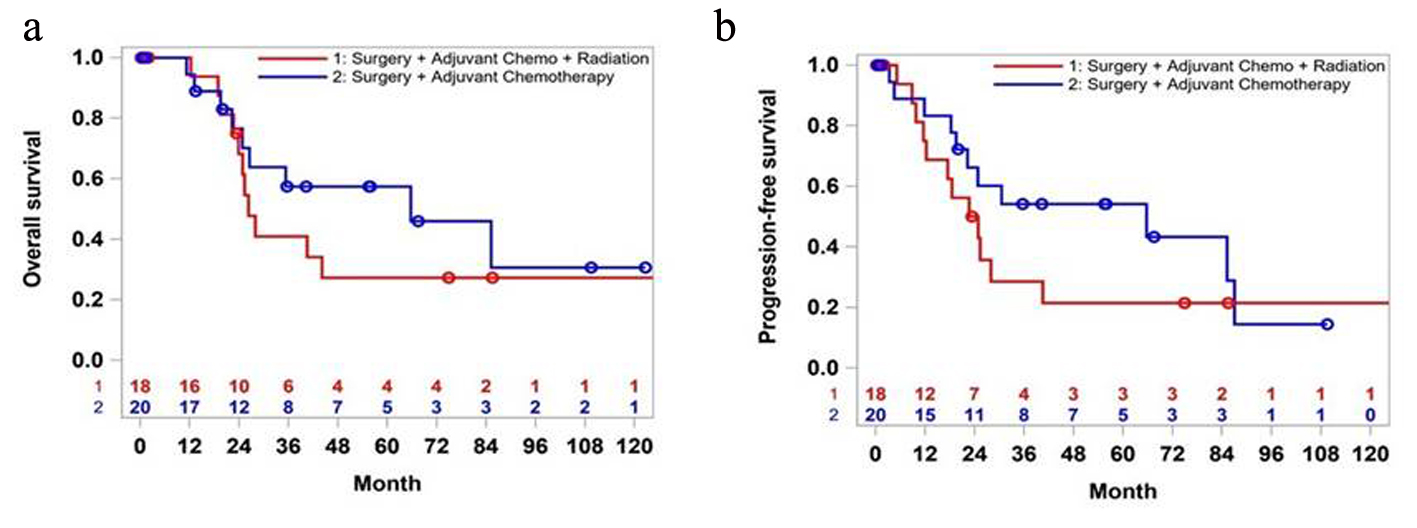
Tables
| Characteristics | Median (SD) or N (%) (N = 63) |
|---|---|
| SD: standard deviation; AA: Asian Americans. | |
| Age | 61.0 (10.8) |
| Gender (women) | 32 (50.8%) |
| Race | |
| White | 52 (82.5%) |
| AA | 8 (12.5%) |
| Asian | 3 (5%) |
| Smoking (yes) | 27 (43%) |
| Alcohol (yes) | 11 (17%) |
| History of pancreatitis (yes) | 7 (11.1%) |
| Diabetes | 15 (23.8%) |
| Albumin | |
| < 2 | 2 (3%) |
| 2 - 3.5 | 29 (46%) |
| > 3.5 | 28 (45%) |
| Not available | 4 (6%) |
| CA19 | 230.9 (438.8) |
| T stage | |
| Tis | 1 (1.6%) |
| T1 | 43 (68.3%) |
| T2 | 12 (19.1%) |
| T3 | 3 (4.8%) |
| Tx | 4 (6.4%) |
| N stage | |
| N0 | 42 (67%) |
| N1 | 20 (31.4%) |
| Unavailable | 1 (1.6%) |
| M stage | |
| M0 | 62 (98.4%) |
| M1 | 1 (1.6%) |
| Final clinical stage | |
| 1 | 41 (65.1%) |
| 2 | 22 (34.9%) |
| Biliary stent (pre-operative) | 40 (63.5%) |
| Type of surgery | |
| Partial pancreatectomy | 60 (95.2%) |
| Total pancreatectomy | 1 (1.6%) |
| Ampullectomy | 2 (3.2%) |
| Gross morphology | |
| Tumor | 56 (88.9%) |
| Ulcer | 6 (9.5%) |
| Undefined | 1 (1.6%) |
| Tumor histology | |
| Ductal adenocarcinoma | 2 (3.2%) |
| Signet-ring cell carcinoma | 2 (3.2%) |
| Adenocarcinoma (not otherwise specified) | 57 (93.6%) |
| Margin status | |
| Involved | 3 (4.8%) |
| Uninvolved | 58 (92%) |
| N/A | 2 (3.2%) |
| Lymphovascular invasion (yes) | 15 (23.8%) |
| Peri-neural invasion (yes) | 10 (15.9%) |
| Peri-pancreatic soft tissue extension (yes) | 12 (19.1%) |
| Peri-nodal extension (yes) | 33 (52.4%) |
| Treatment modality | |
| Adjuvant chemotherapy | 25 (39%) |
| Adjuvant chemotherapy + radiation | 13 (22%) |
| No adjuvant therapy | 25 (39%) |
| Post-operative therapy for advanced stage | 5 (7%) |
| Unknown management | 13 (21%) |
| Characteristics | Overall survival | Progression-free survival | ||
|---|---|---|---|---|
| Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | |
| CI: confidence interval; PPE: peri-pancreatic extension; LN: lymph node; PNE: peri-nodal extension; PNI: peri-neural invasion; P stage: pathological stage; LVI: lymphovascular invasion; AA: Asian Americans. | ||||
| Age | 1.00 (0.96 - 1.03) | 0.8251 | 1.00 (0.97 - 1.04) | 0.796 |
| Gender (men vs. women) | 0.88 (0.43 - 1.80) | 0.7201 | 1.38 (0.70 - 2.73) | 0.3577 |
| Race (AA vs. non-AA) | 1.11 (0.39 - 3.18) | 0.8502 | 0.83 (0.29 - 2.36) | 0.7286 |
| Smoking (no vs. yes) | 0.90 (0.45 - 1.80) | 0.7549 | 1.26 (0.65 - 2.45) | 0.4591 |
| Diabetes | 0.65 (0.28 - 1.51) | 0.3173 | 0.79 (0.37 - 1.66) | 0.5325 |
| Albumin (> 3.5 vs. < 3.5) | 1.34 (0.65 - 2.79) | 0.4289 | 1.32 (0.66 - 2.61) | 0.4344 |
| LVI (yes vs. no) | 1.40 (0.57 - 3.44) | 0.5247 | 1.86 (0.83 - 4.16) | 0.1301 |
| LN metastasis (+ vs. -) | 2.23 (1.08 - 4.60) | 0.0305 | 2.94 (1.45 - 5.96) | 0.0027 |
| PNI (yes vs. no) | 0.69 (0.24 - 1.99) | 0.4965 | 0.71 (0.27 - 1.83) | 0.474 |
| Grade (vs. G1) | 1.30 (0.44 - 3.83) | 0.6368 | 1.24 (0.47 - 3.28) | 0.6703 |
| PPE (yes vs. no) | 3.89 (1.73 - 8.71) | 0.001 | 4.36 (1.93 - 9.87) | 0.0004 |
| PNE (yes vs. no) | 2.58 (1.25 - 5.31) | 0.0105 | 3.79 (1.84 - 7.79) | 0.0003 |
| P stage (> 2 vs. ≤ 2) | 2.91 (1.10 - 7.71) | 0.0314 | 3.71 (1.44 - 9.54) | 0.0065 |