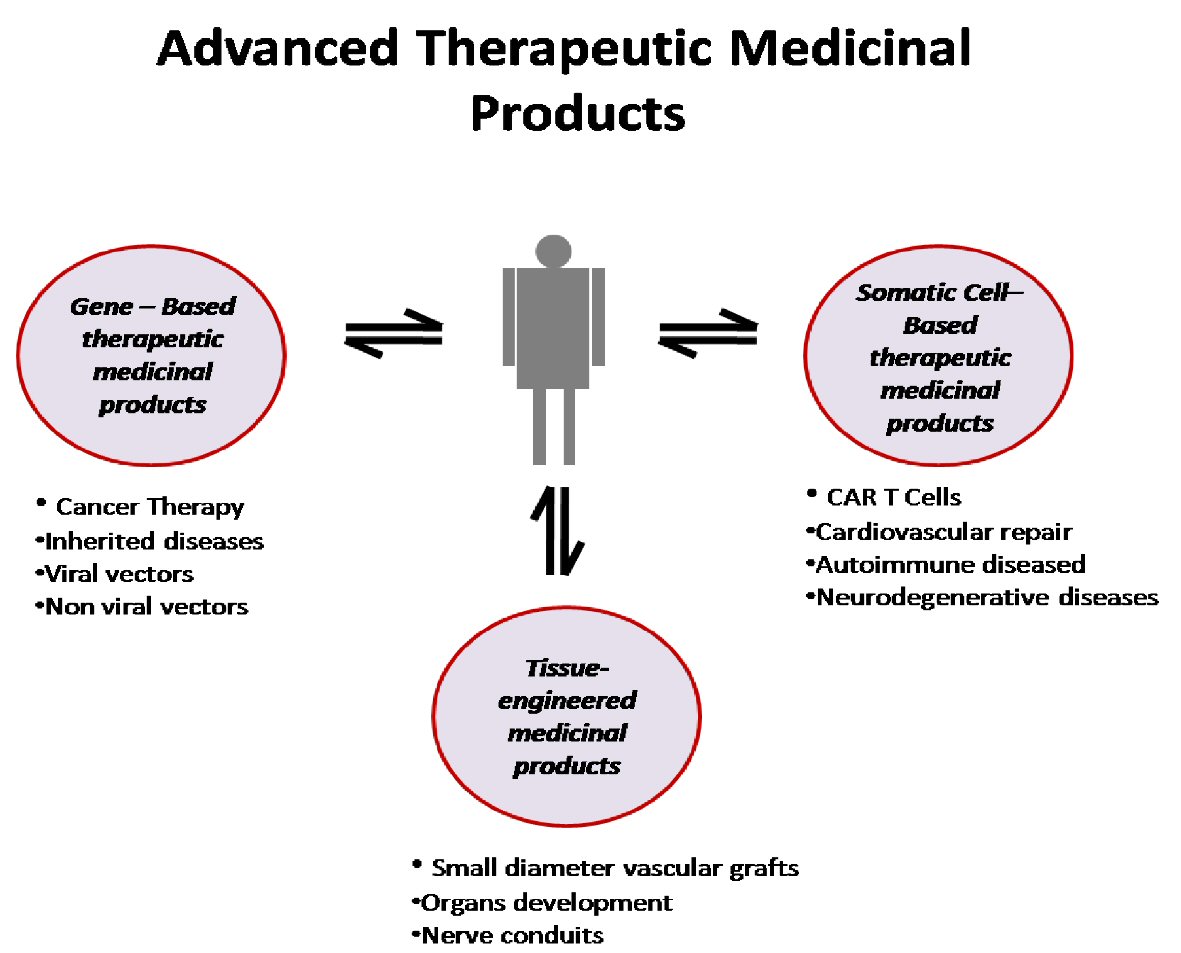
Figure 1. Schematic representation of advanced medicinal therapeutic products. CAR: chimeric antigen receptor.
| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Review
Volume 12, Number 12, December 2020, pages 780-786
Advanced Therapy Medicinal Products Challenges and Perspectives in Regenerative Medicine
Figure

Tables
| Classification | Definition | Applications |
|---|---|---|
| ATMPs: advanced therapy medicinal products; ALS: amyotrophic lateral sclerosis. | ||
| Gene-based therapeutic medicinal products | Gene transfer-based approaches which are leading to therapeutic, prophylactic or diagnostic effect. | Inherited disease |
| Cancer therapy | ||
| Tissue regeneration (e.g., lost of sight) | ||
| Somatic cell therapeutic medicinal products | Cell-based approaches which are including in vitro manipulating cells or tissues with therapeutic, prophylactic or diagnostic effect. | Products against immune disease |
| Parkinson’s disease, ALS, Alzheimer’s disease | ||
| Cartilage defect | ||
| Product for cardiac repair | ||
| Skin replacement | ||
| Cancer immunotherapy | ||
| Tissue-engineered medicinal products | Tissue engineered-based approaches that can be applied to repair, regenerate or replace human tissues or organs. | Small diameter vascular grafts |
| Trachea replacement | ||
| Tissue-engineered esophagus | ||
| Liver and kidney implantation | ||
| Nerve conduits | ||
| Advance therapy medicinal products borderlines | |
|---|---|
| Advantages | Disadvantages |
| ATMPs: advanced therapy medicinal products. | |
| Highly personalized therapy | Complex manufacturing |
| Direct application through infusion | High up-front cost |
| Longer lasting effect | One-time treatment |
| Address complex diseases | Specific regulatory and pharmacovigilance demands |
| Improving health-related quality of life | Highly specific storage requirements: short shelf life |
| Fewer hospitalizations, comorbidities and associate treatment | |