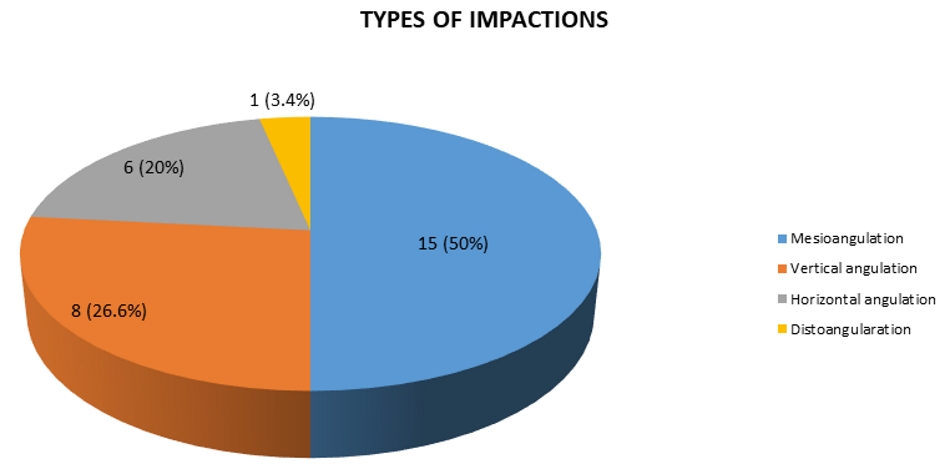
Figure 1. Types of impactions in the patients of this research study.
| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Original Article
Volume 11, Number 7, July 2019, pages 501-508
Dexamethasone Injection Into Pterygomandibular Space Versus Sublingual Space on Post-Operative Sequalae of Lower Third Molar Intervention
Figures

Tables
| Inclusion criteria selection of the patients |
|---|
| The patient has bilateral impacted lower third molars symmetrically positioned on both sides of the mandible of which surgical removal consists of flap operation, bone removal, and tooth section |
| Aged between 18 - 45 years |
| No history of allergy to dexamethasone, amoxicillin, or acetaminophen |
| No use of other medicine 1 month before and during the study period |
| The patient is able to understand and carry out the instructions given by the investigators |
| The patient has provided their consent for the study |
| Exclusion criteria selection of the patients |
| Pregnancy or current lactation |
| Patients with cardiovascular problems, renal and/or liver failure, or other serious medical conditions |
| Allergic to local anesthetics and other drugs that were used in this study |
| Patient with facial deformities that may interfere with the injections, surgery or evaluation |
| The existence of acute infection and/or swelling and pain at the time of surgery |
| Patients taking any medication during the previous 1 month prior to the surgery |
| Inability to follow the instructions or cooperate during the study |
| Duration of treatment more than 1 h |
| Data consideration | 8 mg dexamethasone | Total | Percentage | |
|---|---|---|---|---|
| Pterygomandibular space injection | Sublingual space injection | |||
| Position A: the part of the lower third molar is above the occlusal plane of the lower second molar; Position B: the highest portion of the lower third molar is between the occlusal plane and the cervical line of the second molar; Class I: there is sufficient space of accommodation of the mesio-distal diameter of the lower third molar; Class II: the space of accommodation of the mesio-distal diameter of the lower third molar is less than the mesio-distal diameter of the lower third molar. | ||||
| Number | 30 | 30 | 60 | 100 |
| Age | ||||
| 16 - 25 years | 28 | 28 | 56 | 93.3 |
| 26 - 32 years | 2 | 2 | 4 | 6.6 |
| Sex | ||||
| Male | 13 | 13 | 26 | 43.3 |
| Female | 17 | 17 | 34 | 56.7 |
| Position | ||||
| A | 9 | 9 | 18 | 30 |
| B | 21 | 21 | 42 | 70 |
| Class | ||||
| I | 12 | 12 | 24 | 40 |
| II | 18 | 18 | 36 | 60 |
| Data consideration | 8 mg dexamethasone | P value | |
|---|---|---|---|
| Pterygomandibular space injection (SD) | Sublingual space injection (SD) | ||
| Duration of operation (min) | 19.93 (2.71) | 20.50 (2.77) | 0.225 |
| Data evaluation | 8 mg dexamethasone | P value | |
|---|---|---|---|
| Pterygomandibular space injection (SD) | Sublingual space injection (SD) | ||
| Day 0: immediate after operation; Day 1: first day after operation; Day 2: second day after operation; Day 3: third day after operation; Day 7: seventh day after operation. | |||
| VAS | |||
| Day 0 | 21.57 (15.76) | 20.90 (15.14) | 0.840 |
| Day 1 | 18.90 (14.96) | 17.03 (14.23) | 0.532 |
| Day 2 | 14.17 (15.45) | 13.90 (13.87) | 0.935 |
| Day 7 | 3.67 (10.15) | 3.60 (8.25) | 0.971 |
| Number of analgesic taken | |||
| Day 1 | 1.87 (1.04) | 1.77 (0.97) | 0.682 |
| Day 2 | 2.67 (1.82) | 2.53 (1.59) | 0.742 |
| Day 3 | 1.47 (1.47) | 1.10 (1.29) | 0.304 |
| Data evaluation | 8 mg dexamethasone | P value | |
|---|---|---|---|
| Pterygomandibular space injection, mean (SD) | Sublingual space injection, mean (SD) | ||
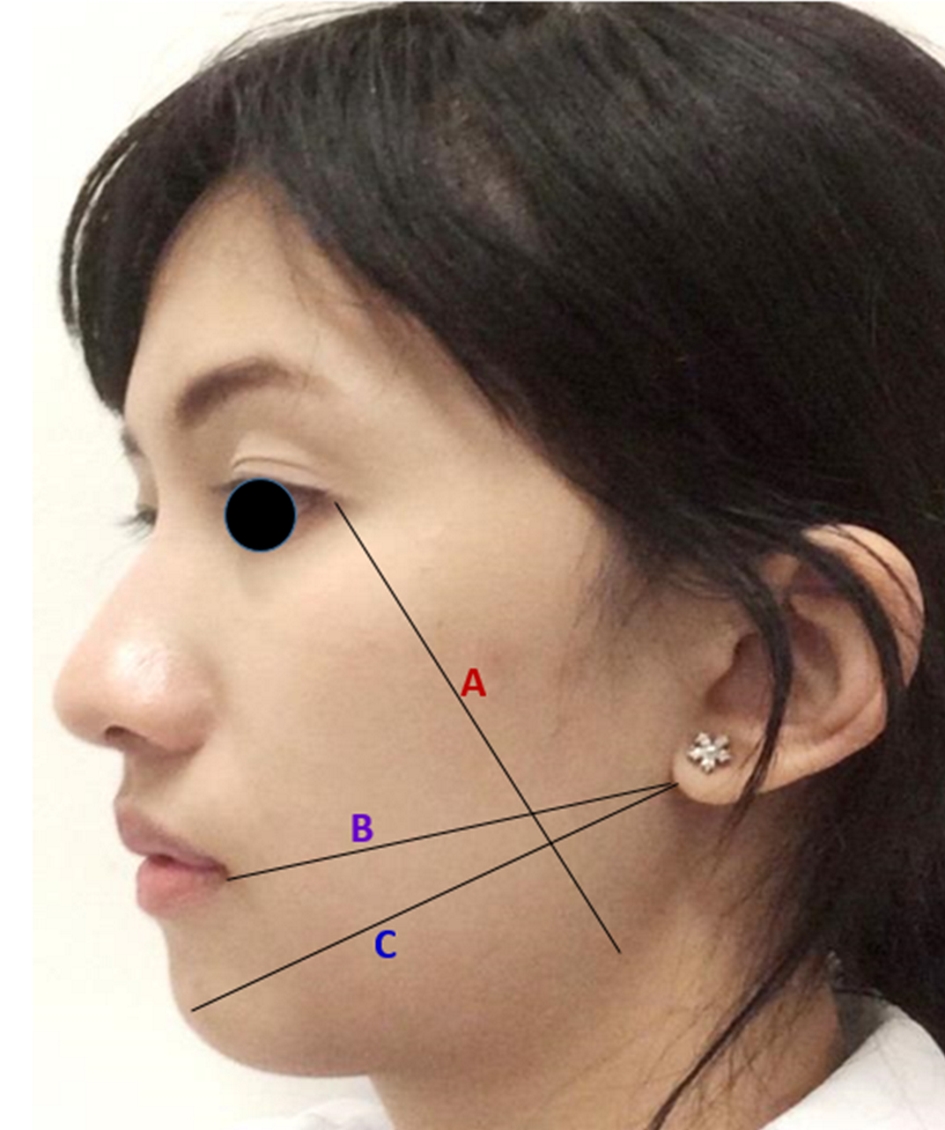
| Tr-Com: tragus-commissure of mouth; Tr-Pog: tragus-pogonion; Gn-Lc: goial angle-lateral canthal of eye. | |||
| Tr-Com | |||
| Baseline | 115.10 (6.05) | 115.03 (6.12) | 0.326 |
| Second day | 118.83 (6.84) | 118.66 (6.01) | 0.724 |
| Seventh day | 116.56 (6.85) | 116.53 (6.22) | 0.926 |
| Differences | |||
| Second day-baseline | 3.73 (2.42) | 3.63 (2.44) | 0.835 |
| Seventh day-baseline | 1.46 (2.14) | 1.50 (1.50) | 0.926 |
| Tr-Pog | |||
| Baseline | 115.10 (6.05) | 115.03 (6.12) | 0.326 |
| Second day | 118.83 (6.84) | 118.66 (6.01) | 0.724 |
| Seventh day | 116.56 (6.85) | 116.53 (6.22) | 0.926 |
| Differences | |||
| Second day-baseline | 3.73 (2.42) | 3.63 (2.44) | 0.835 |
| Seventh day-baseline | 1.46 (2.14) | 1.50 (1.50) | 0.926 |
| Gn-Lc | |||
| Baseline | 106.86 (8.52) | 105.6 (7.92) | 0.99 |
| Second day | 110.83 (8.02) | 109.53 (7.65) | 0.177 |
| Seventh day | 108.56 (8.16) | 107.26 (7.77) | 0.115 |
| Differences | |||
| Second day-baseline | 3.96 (2.57) | 3.93 (2.11) | 0.954 |
| Seventh day-baseline | 1.7 (2.56) | 1.6 (2.13) | 0.897 |
| Maximum incisal distance | 8 mg dexamethasone | P value | |
|---|---|---|---|
| Pterygomandibular space injection (SD) | Sublingual space injection (SD) | ||
| *P < 0.01. | |||
| Baseline | 46.36 (5.03) | 46.36 (5.03) | 1 |
| Second day | 33.83 (7.68) | 36.96 (5.49) | 0.004* |
| Seventh day | 42.61 (5.11) | 43.23 (4.88) | 0.293 |
| Differences | |||
| Baseline-second day | 12.53 (6.62) | 9.40 (5.11) | 0.004* |
| Baseline-seventh day | 3.75 (3.74) | 3.13 (2.62) | 0.293 |
| Authors | Year | Type of steroid | Administration | Parameter measurement | Results |
|---|---|---|---|---|---|
| NA: unknown; HRQOL: health-related quality of life; IV: intravenous. | |||||
| Baxendale et al [15] | 1993 | 8 mg dexamethasone | Oral | Post-operative pain: 4 h post-operative reduction | Significant reduction in pain 4 h post-operatively |
| Tiwana et al [12] | 2005 | NA corticosteroids and without antibiotics, no corticosteroids | Intravenous before surgery | Effect on health-related quality of life outcomes | IV corticosteroid administration had a limited, but beneficial effect on HRQOL outcomes |
| Grossi et al [6] | 2007 | 4 and 8 mg dexamethasone | Submucosal injection before surgery | Post-operative edema | Significant reduction when 4 mg dexamethasone was given, but 8 mg provided no further benefit |
| Filho et al [2] | 2008 | 4 and 8 mg dexamethasone | The consumption of 4 mg versus 8 mg before surgery | Swelling, trismus, pain | Better for swelling and trismus but not effective for pain |
| Mojsa et al [8] | 2011 | 4 mg dexamethasone | 4 mg injection, the “before” group, placebo group, the “after” group | Facial swelling, post-operative pain, trismus | Better control of pain, swelling, trismus |
| Antonio et al [9] | 2011 | 8 mg dexamethasone | The oral administration, local injection in the masseter muscle | Pain, edema, limited mouth opening | Reducing post-operative pain, edema, trismus |
| Tiigimae-Saar et al [11] | 2011 | Single dose of 30 mg prednisolone, 120 mg etorikoxib | Prednisolone immediate before operation, etorikoxib 30 min before operation | Pain, facial swelling, trismus | Well-suited for treatment of post-operative pain, trismus, swelling, diminishing post-operative swelling of soft tissues |
| Chaurand-Lara and Facio-Umana [10] | 2013 | 20 mg of methylprednisolone | Intramuscular in masseter and no administer | Swelling, pain | Decrease and an effective therapeutic reduction of swelling and pain |
| Bauer et al [14] | 2013 | NA ibuprofen or placebo, NA ibuprofen + dexamethasone, placebo | Pre-emptive analgesia | Post-operative pain | Pre-emptive analgesia insufficient to inhibit central sensitization, association with dexamethasone more effective |
| Li et al [16] | 2013 | 4 mg and 8 mg dexamethasone | Injection of 4 - 5 mg in pericoronal injection | Post-operative swelling, trismus | Control facial swelling, trismus |
| Latt et al [17] | 2016 | Dexamethasone 8 mg, saline group | Pterygomandibular space injection | Post-operative pain | Decreased post-operative pain |
| Gozali et al [18] | 2017 | 8 mg dexamethasone, saline group | Injection into sublingual area | Post-operative pain | Decreased post-operative pain |