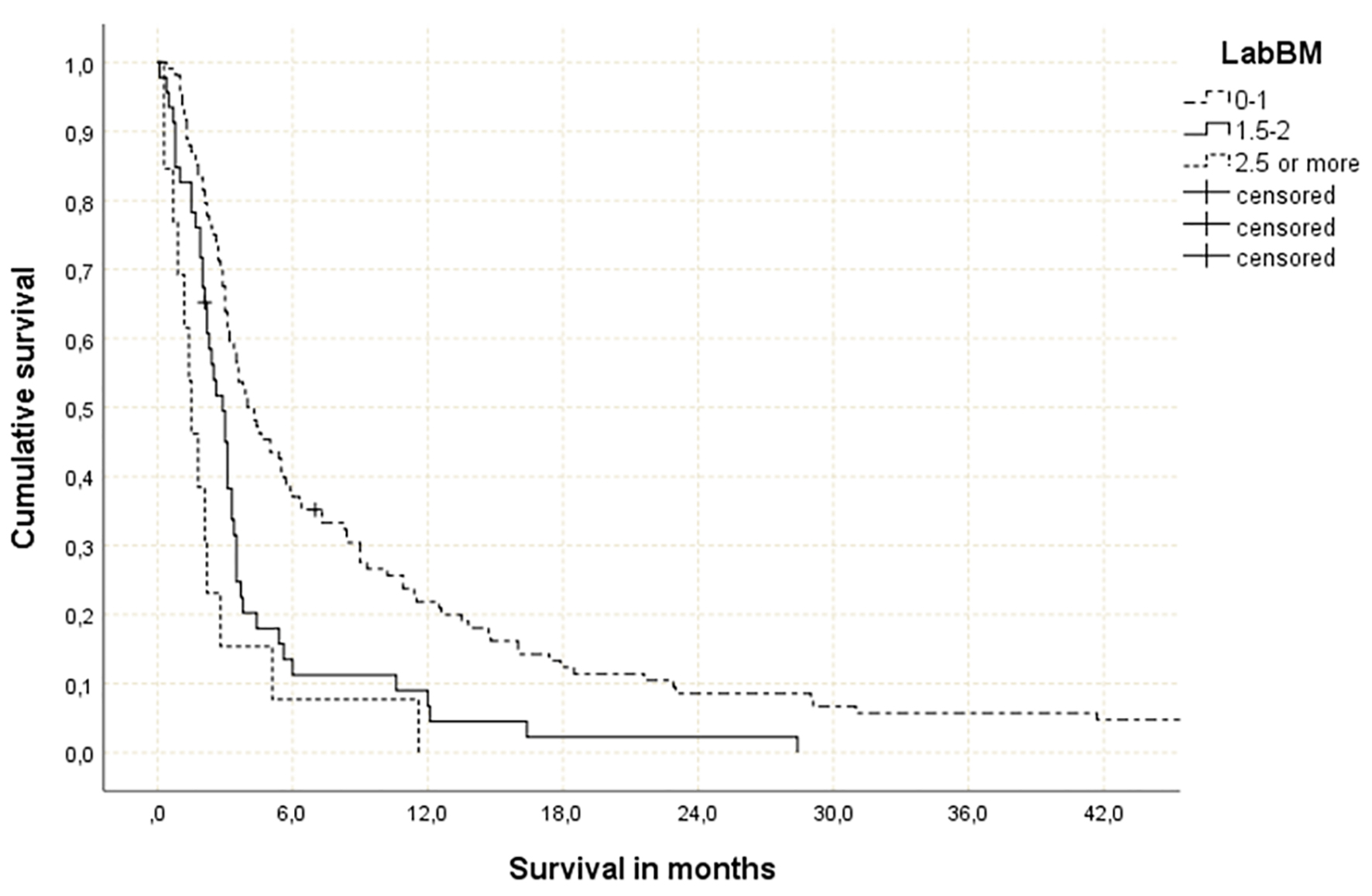
Figure 1. Actuarial overall survival stratified by LabBM score (0 - 1 point: median 4.0 months; 1.5 - 2 points: median 2.9 months; > 2 points: median 1.5 months; P = 0.0001 (best vs. intermediate and best vs. worst group), P = 0.046 (intermediate vs. worst group)).